Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer, with >600,000 HCC cases developing
annually worldwide (1). HCC
commonly occurs in patients secondary to chronic hepatitis or
cirrhosis resulting from either hepatitis B or C virus infection,
or from non-virus-related causes, such as alcohol or aflatoxin
exposure (2–4). A persistent, non-specific and
ineffective immune system activation within the chronically
inflamed liver is hypothesized to induce carcinogenesis (2,3,5).
Current treatments for HCC include surgical resection, liver
transplantation and local ablative therapies, such as percutaneous
ethanol injection, thermal ablation and intra-arterial
chemoembolization (6). However,
>75% patients relapse within five years and the overall survival
for HCC patients remains poor (7,8).
Therefore, the development of more effective therapeutic tools and
strategies is required.
A number of studies have suggested that the tumor
microenvironment is important in tumor development, tumor control
and the response to treatment (9–14). In
breast, colorectal and lung cancer, as well as HCC, the status of
the stroma and the local adaptive immune response are superior
prognostic factors compared with tumor phenotype or clinical
staging (11–14). In clinicopathological practice,
intratumoral infiltration of CD4 or CD8 T cells was found to be
correlated with lower disease recurrence and improved survival
rates in HCC (14–16) and ovarian carcinoma (17). Furthermore, HCC tumor size was also
found to have prognostic significance (7,18,19).
In human colorectal tumors, the type, density and location of
tumor-infiltrating immune cells have been reported to be predictors
of clinical outcome (11). In a
transgenic mice model, the adoptive transfer of CD8-positive
cytotoxic T lymphocytes (CTLs) into immune-deficient mice markedly
reduced tumor growth and tumor diameter (20). However, in human HCC, the
association between tumor-infiltrating immune cells and tumor size
is less understood. In the present study, the association between
T-cell type, location and the biological behavior in human HCC
specimens was investigated, particularly focusing on CD4 and CD8 T
cells in the tumor parenchyma and stroma.
Materials and methods
HCC specimens
A total of 86 cases of HCC (61 males and 25 females)
were selected from medical records at Koseiren Takaoka Hospital
(Toyama, Japan). In each case, HCC was carefully diagnosed as
determined by macroscopic and histopathological findings. As a
control, corresponding pericancerous non-tumor liver tissues (at
least 3 cm away from the tumor site) were also analyzed. None of
the individuals had suffered metastasis or had received prior
treatment, such as percutaneous ethanol injection, thermal ablation
or intra-arterial chemoembolization, which may influence HCC
biological behavior, prior to surgery. The HCC samples were
classified into four groups according to the International Union
Against Cancer tumor-node-metastasis (TNM) classification (21). Pericancerous non-tumor liver tissues
were also classified into the following four groups according to
the modified histological activity index system (22): Non-chronic hepatitis (NCH), chronic
hepatitis (CH), chronic hepatitis with pre-cirrhotic changes
(pre-cirrhotic stage, PC) or cirrhosis (C). The detailed profiles
of all HCC cases (gender, age, tumor diameter, differentiation,
Edmondson staging (23), nodule
number, TNM staging, and infiltration into hepatic vein, portal
vein or capsule) are shown in Tables
I and II. This study was
approved by the ethics committee of Koseiren Takaoka Hospital
(Toyama, Japan) and written informed consent was obtained from all
patients.
 | Table IAssociation between the numbers of
CD4- and CD8-positive lymphocytes in hepatocellular carcinoma tumor
parenchymal tissues, and patient clinicopathological
characteristics. |
Table I
Association between the numbers of
CD4- and CD8-positive lymphocytes in hepatocellular carcinoma tumor
parenchymal tissues, and patient clinicopathological
characteristics.
| Clinicopathological
characteristic | No. | No. CD4 T cells, mean
± SEM | P-value(s) | No. CD8 T cells, mean
± SEM | P-value(s) |
|---|
| Age at diagnosis,
years |
| ≤60 | 19 | 7.2±1.7 | 0.623 | 15.2±4.3 | 0.911 |
| >60 | 67 | 7.6±1.7 | | 16.5±3.0 | |
| Gender |
| Male | 61 | 7.0±0.9 | 0.137 | 16.1±2.6 | 0.061 |
| Female | 25 | 6.4±1.8 | | 15.8±5.9 | |
| Tumor diameter,
cm |
| ≤5 | 56 | 7.8±1.3 | 0.604 | 18.1±3.3 | 0.037 |
| >5 | 30 | 6.2±1.3 | | 12.2±3.8 | |
| Differentiation
status |
| Well | 24 | 7.2±1.7 | 0.982 | 11.0±2.3 | 0.784 |
| | | 0.996 | | 0.255 |
| Moderately | 39 | 7.6±1.5 | 0.982 | 15.1±2.6 | 0.784 |
| | | 0.957 | | 0.507 |
| Poorly | 23 | 6.9±1.2 | 0.996 | 22.1±7.7 | 0.255 |
| | | 0.957 | | 0.507 |
| Edmondson stage |
| I–II | 70 | 7.2±0.9 | 0.310 | 16.4±3.3 | 0.225 |
| III–IV | 16 | 10.5±3.0 | | 14.0±3.6 | |
| Background |
| NCH | 6 | 6.4±2.8 | 0.998 | 24.5±16.1 | 0.821 |
| | | 1.000 | | 0.934 |
| | | 0.978 | | 0.751 |
| CH | 47 | 7.1±1.4 | 0.998 | 15.0±3.3 | 0.821 |
| | | 0.999 | | 0.996 |
| | | 0.980 | | 0.989 |
| PC | 11 | 7.0±1.9 | 1.000 | 16.9±6.2 | 0.934 |
| | | 0.999 | | 0.996 |
| | | 0.991 | | 0.973 |
| C | 22 | 8.1±1.8 | 0.978 | 12.9±4.1 | 0.751 |
| | | 0.980 | | 0.989 |
| | | 0.991 | | 0.973 |
| Nodule number |
| Single | 77 | 7.4±0.9 | 0.400 | 16.7±2.7 | 0.667 |
| Double | 9 | 6.6±2.8 | | 9.9±3.0 | |
| Portal vein
infiltration |
| Yes | 17 | 6.2±2.6 | 0.667 | 21.7±7.8 | 0.633 |
| No | 69 | 7.3±0.9 | | 14.5±2.4 | |
| Hepatic vein
invasion |
| Yes | 16 | 7.2±1.9 | 0.709 | 13.8±4.1 | 0.228 |
| No | 70 | 7.4±1.0 | | 16.7±3.0 | |
| Infiltration into
capsule |
| Yes | 29 | 7.4±1.4 | 0.608 | 17.7±5.2 | 0.812 |
| No | 57 | 7.2±1.1 | | 15.1±2.7 | |
| TNM stage |
| I–II | 54 | 7.7±1.2 | 0.364 | 16.3±3.1 | 0.301 |
| III–IV | 32 | 6.0±1.1 | | 13.2±3.7 | |
 | Table IIAssociation between the numbers of
CD4- and CD8-positive lymphocytes in hepatocellular carcinoma tumor
stromal tissues, and patient clinicopathological
characteristics. |
Table II
Association between the numbers of
CD4- and CD8-positive lymphocytes in hepatocellular carcinoma tumor
stromal tissues, and patient clinicopathological
characteristics.
| Clinicopathological
characteristic | No. | CD4 T cells, n,
mean ± SEM | P-value(s) | CD8 T cells, n,
mean ± SEM | P-value(s) |
|---|
| Age at diagnosis,
years |
| ≤60 | 12 | 23.9±10.6 | 0.400 | 33.3±10.0 | 0.842 |
| >60 | 39 | 21.8±3.8 | | 33.1±4.8 | |
| Gender |
| Male | 37 | 24.0±4.6 | 0.315 | 31.3±4.1 | 0.849 |
| Female | 14 | 17.2±5.9 | | 37.8±11.5 | |
| Tumor diameter,
cm |
| ≤5 | 40 | 23.1±4.4 | 0.519 | 36.5±4.8 | 0.022 |
| >5 | 11 | 19.4±7.2 | | 21.9±8.9 | |
| Differentiation
status |
| Well | 10 | 20.7±6.8 | 0.911 | 28.3±5.9 | 0.870 |
| | | 0.971 | | 0.886 |
| Moderately | 27 | 24.7±6.0 | 0.911 | 34.0±6.1 | 0.870 |
| | | 0.757 | | 0.999 |
| Poorly | 14 | 18.1±5.1 | 0.971 | 34.3±9.5 | 0.886 |
| | | 0.757 | | 0.999 |
| Edmondson
stage |
| I–II | 31 | 20.7±4.0 | 0.317 | 32.7±4.4 | 0.858 |
| III–IV | 20 | 27.4±8.6 | | 36.7±11.5 | |
| Background |
| NCH | 3 | 8.8±5.5 | 0.894 | 5.3±3.9 | 0.530 |
| | | 0.154 | | 0.149 |
| | | 0.946 | | 0.665 |
| CH | 33 | 20.9±4.4 | 0.894 | 32.1±5.5 | 0.530 |
| | | 0.117 | | 0.416 |
| | | 0.997 | | 0.996 |
| PC | 4 | 54.9±25.3 | 0.154 | 59.0±19.9 | 0.149 |
| | | 0.117 | | 0.416 |
| | | 0.141 | | 0.424 |
| C | 11 | 18.9±5.7 | 0.946 | 29.7±6.8 | 0.665 |
| | | 0.997 | | 0.996 |
| | | 0.141 | | 0.424 |
| Nodule number |
| Single | 43 | 22.2±4.1 | 0.728 | 32.7±4.8 | 0.368 |
| Double | 8 | 21.6±6.9 | | 36.9±7.6 | |
| Portal vein
infiltration |
| Yes | 9 | 24.1±8.0 | 0.652 | 43.2±16.4 | 0.942 |
| No | 42 | 22.0±4.1 | | 31.3±3.8 | |
| Hepatic vein
invasion |
| Yes | 15 | 22.5±6.2 | 0.879 | 42.0±10.5 | 0.558 |
| No | 36 | 22.5±4.6 | | 31.2±4.1 | |
| Infiltration into
capsule |
| Yes | 18 | 21.9±5.0 | 0.573 | 38.2±9.0 | 0.700 |
| No | 33 | 22.3±4.9 | | 29.0±3.5 | |
| TNM stage |
| I–II | 31 | 23.9±5.3 | 0.978 | 31.8±4.9 | 0.867 |
| III–IV | 20 | 20.3±4.7 | | 34.7±7.7 | |
Tissue microarray
Tissue microarrays were constructed as described
previously (24). Briefly, in each
case, hematoxylin and eosin-stained HCC sections and paired
pericancerous liver tissue sections (designated as tumor and
peritumor, respectively) were observed under a microscope (Olympus
SZX10; Olympus Corporation, Tokyo, Japan). Representative areas of
lymphocyte infiltration, away from the necrotic and hemorrhagic
areas, were marked and punched with a cylinder (3 mm in diameter)
followed by transferal to a recipient block. In total, 172 cores
were punched and distributed into 11 recipient blocks. The lesions
were placed in duplicate cores adjacent to one another. The blocks
were then embedded in paraffin for sectioning at 4 μm.
Immunohistochemistry
Briefly, following deparaffinization, the sections
were subjected to antigen retrieval under microwave heating with
target retrieval solution (Dako Cytomation, Kyoto, Japan) for 15
min. Thereafter, the sections were immersed in 0.3%
H2O2 in methanol for 30 min to inhibit
endogenous peroxidase activity. The sections were then incubated
for 15 min with rabbit anti-human CD4 polyclonal antibodies (1:100;
Santa Cruz Biotechnology, Inc., CA, USA) and rabbit anti-human CD8
polyclonal antibodies (1:100; Santa Cruz Biotechnology, Inc.) in
phosphate-buffered saline containing 1% normal goat serum (Wako
Pure Chemical Industries, Ltd., Tokyo, Japan) and 1% bovine serum
albumin (Wako Pure Chemical Industries, Ltd.) under intermittent
microwave irradiation, as previously described (25,26).
Envision+ (Dako Cytomation) for rabbit immunoglobulin
was added and the sections were incubated under intermittent
microwave irradiation for 15 min. Positive reactions were
visualized with 3,3′-diaminobenzidine tetrahydrochloride.
Morphometrical analysis
CD4- and CD8-positive lymphocytes were classified
into the following three groups according to cell distribution:
Tumor parenchyma lymphocytes, which were located within a cancer
cell nest; tumor stroma lymphocytes, with lymphocytes located in
the stroma contacting the cancer cells; and peritumor parenchyma
lymphocytes, which were located in the pericancerous liver
parenchyma. Morphometrical analysis was performed according to
methods described in a previous study (27), for semi-quantitative evaluation of
the immunohistochemical findings by two investigators without prior
knowledge. Briefly, in each case, using an Olympus SZX10 microscope
(Olympus Corporation), 15 independent and intact high power
microscopic areas (magnification, ×400) with the most abundant
lymphocyte infiltrations were selected (five tumor parenchyma, five
tumor stroma and five peritumor parenchyma areas), and the numbers
of CD4 and CD8 T cells were counted in each microscopic field. The
average numbers of CD4 and CD8 T cells in the five selected
microscopic fields signified the CD4 and CD8 expression levels in
each HCC or pericancerous liver tissue specimen. For the evaluation
of CD4 and CD8 immunoreactions in the tumor stroma, 35 cases were
omitted since distinguishing the carcinoma stroma from the
surrounding carcinoma parenchyma in these cases was difficult.
Statistical analysis
The mean and standard error of the mean were
calculated for all parameters determined in this study. Statistical
analysis was performed using the nonparametric Mann-Whitney U test,
one-factor analysis of variance or Spearman’s correlation
coefficient by rank test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Lymphocyte distribution
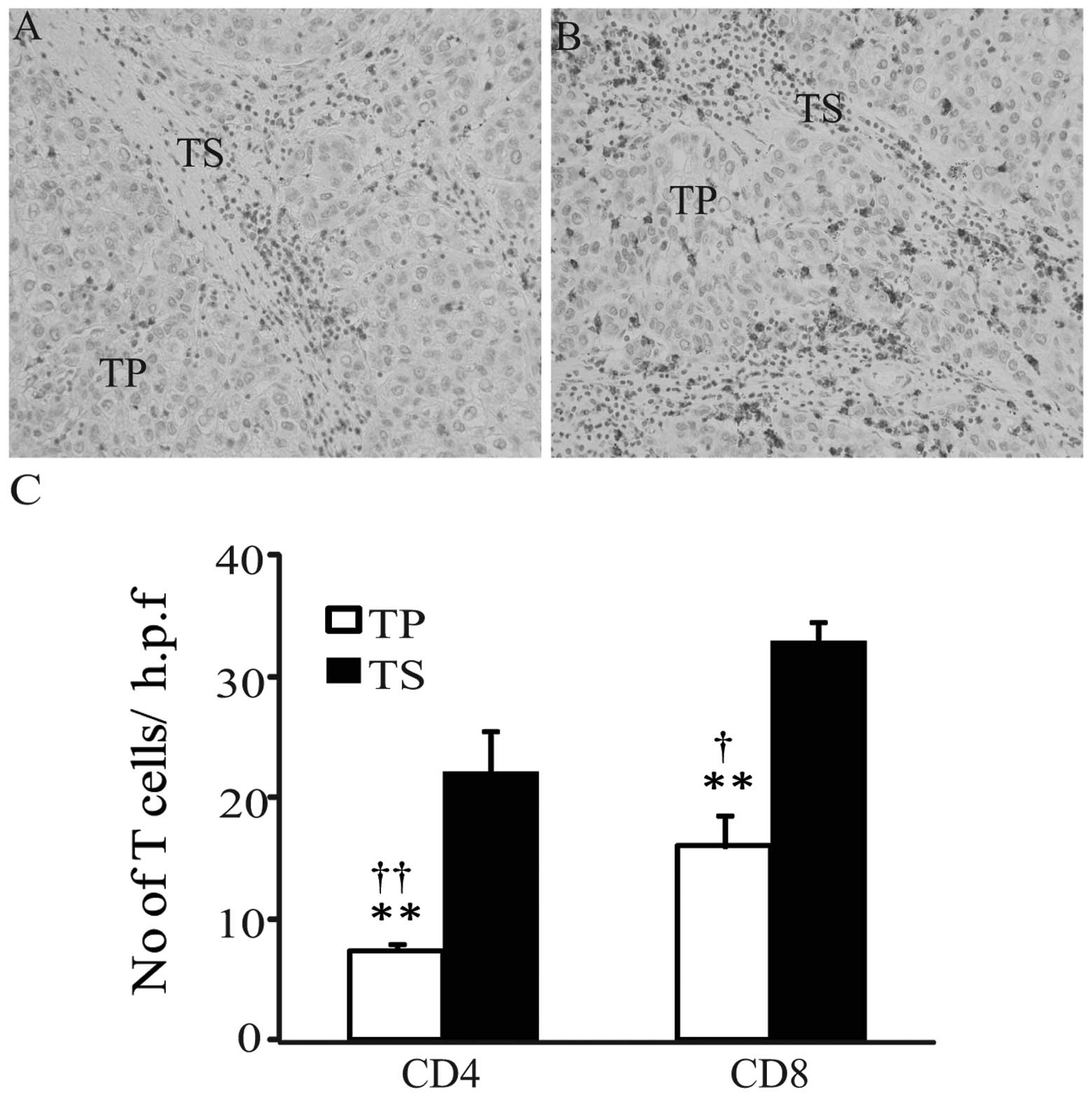
In the HCC samples, CD4 and CD8 T cells were
observed in the tumor parenchyma and tumor stroma (Fig. 1A and B), and the intensity of CD4 or
CD8 immunoreactivity was homogeneous in all samples examined. The
numbers of CD4- and CD8-positive T cells appeared fewer in the
tumor parenchyma, compared with those in tumor stroma. In order to
semi-quantitatively evaluate the immunohistochemical findings,
morphometrical analysis was performed. As shown in Fig. 1C, the average numbers of CD4-and
CD8-positive T cells were significantly increased in the tumor
stroma, compared with those in the tumor parenchyma (tumor stroma
versus tumor parenchyma: CD4, 22±3.6 versus 7.4±0.9; CD8, 32.8±4.2
versus 16±2.5; both P<0.01). Furthermore, the average numbers of
CD8-positive T cells in tumor parenchyma and tumor stroma were
significantly increased, compared with the numbers of CD4-positive
cells (CD8 versus CD4: tumor parenchyma, 16±2.5 versus 7.4±0.9,
P<0.01; tumor stroma, 32.8±4.2 versus 22±3.6, P<0.05). This
observation suggests that CD8 T cells were predominant in the host
anticancer cellular immunity.
Association between CD4 and CD8
expression and HCC behavior
In the tumor parenchyma and stroma, no significant
differences in the CD4 immunoreactions were observed between
patients with tumor diameters ≤5 cm and patients with tumor
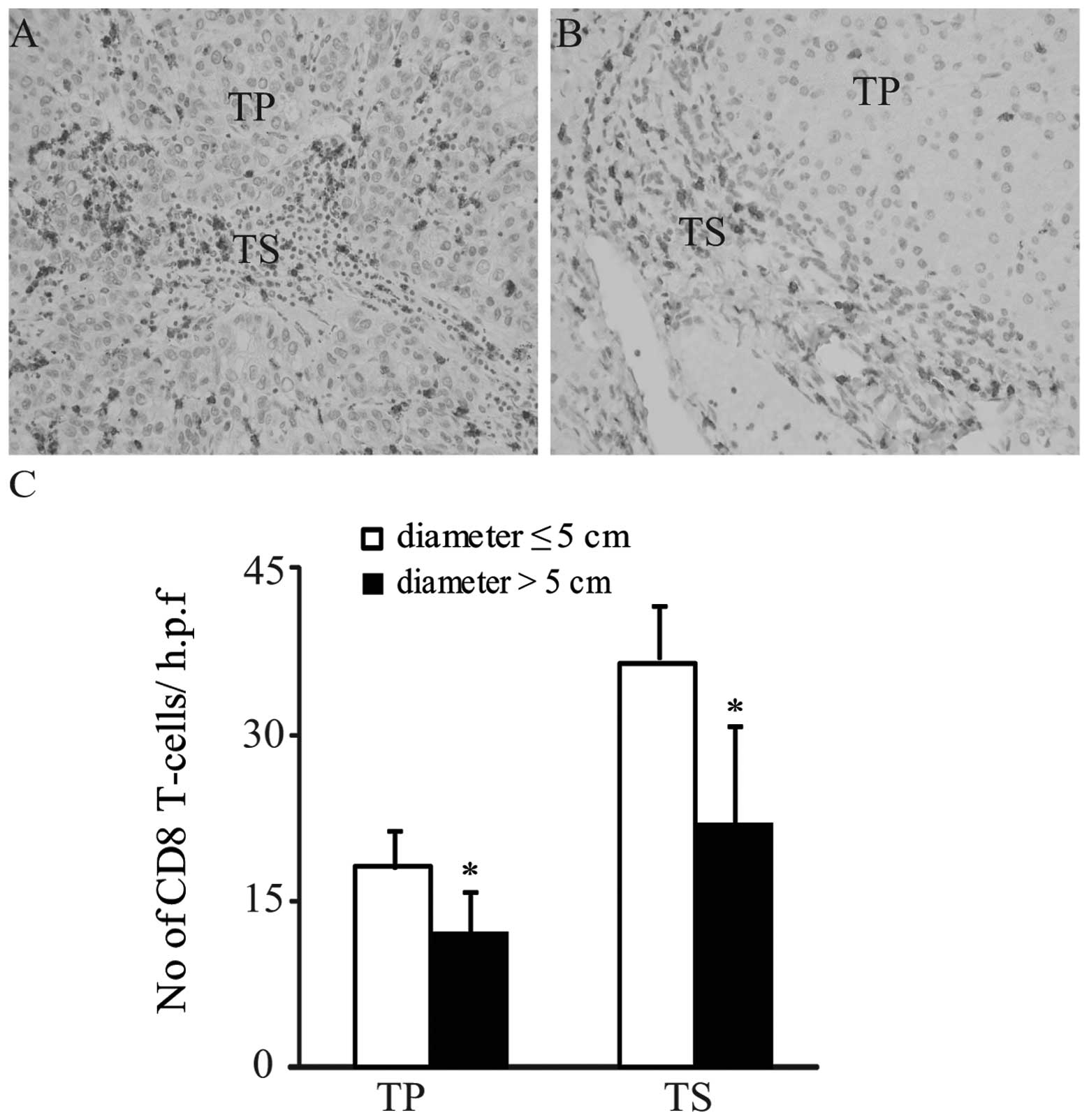
diameters >5 cm (both P>0.05; Tables I and II). By contrast, the average numbers of
CD8 T cells in the tumor parenchyma and tumor stroma were
significantly increased in patients with tumor diameters ≤5 cm
compared with patients with tumor diameters >5 cm (diameter ≤5
cm versus diameter >5 cm: tumor parenchyma, 18.1±3.3 versus
12.2±3.8; tumor stroma, 36.5±4.8 versus 21.9±8.9; both P<0.05;
Tables I and II; Fig.
2A–C). Furthermore, in the tumor parenchyma and stroma, no
significant differences in either CD4 or CD8 immunoreactivity were
detected between age, gender, differentiation, Edmondson staging
(23), liver disease background,
number of nodules, TNM stage or infiltration into the portal vein,
hepatic vein or the capsule variables (Tables I and II). These observations suggest that the
numbers of CD8 T cells in HCC parenchyma and stroma may not be
correlated with tumor progression or metastasis, but may be
correlated with tumor volume.
Association between CD4 and CD8
expression and background hepatic disease
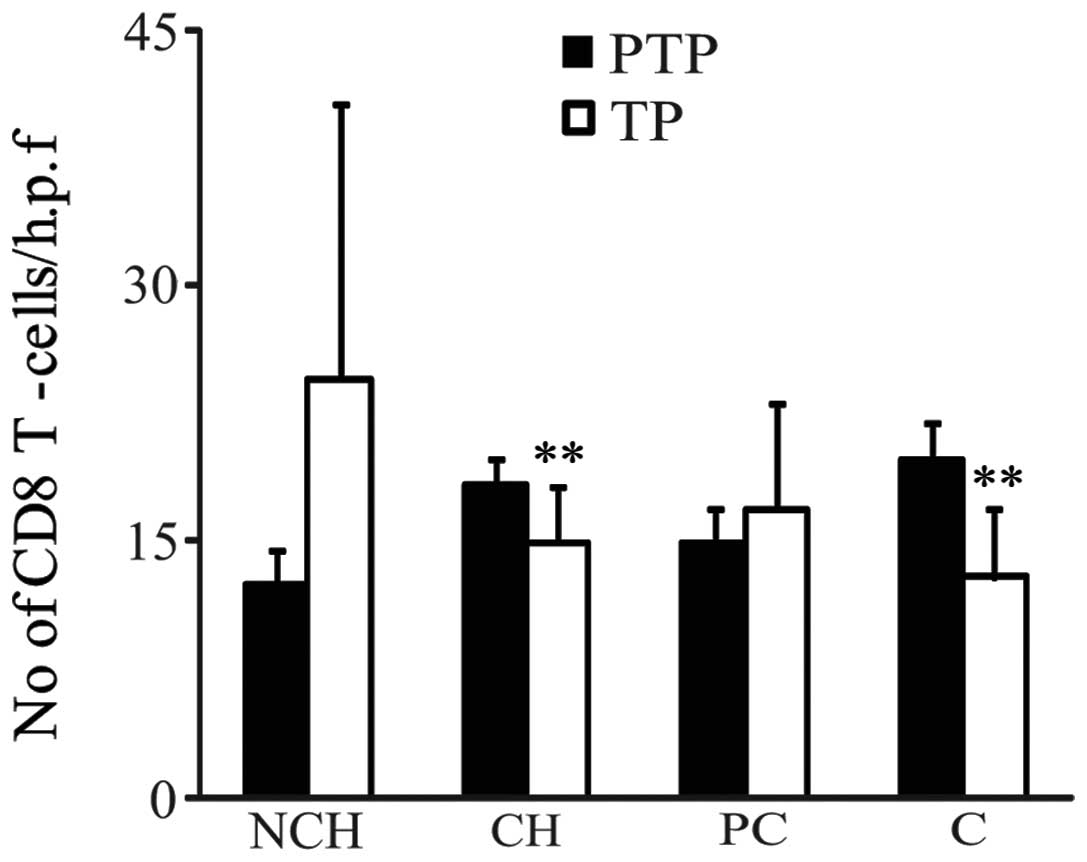
As shown in Fig. 3,
in the CH and C background groups, CD8 expression levels in the
peritumor parenchymas were significantly higher than those in the
paired tumor parenchymas (peritumor parenchyma vs. tumor
parenchyma: CH background, 18.4±1.4 vs. 15.0±3.3; C background,
19.8±2.2 vs. 12.9±4.1; both P<0.01). By contrast, in the NCH and
PC background groups, no significant differences in CD8 expression
were detected between the tumor parenchyma and peritumor parenchyma
(Fig. 3). Furthermore, in HCC and
pericancerous liver tissues from all background groups, no
significant differences in the CD4 T cells between the tumor
parenchyma and peritumor parenchyma (peritumor parenchyma versus
tumor parenchyma: NCH background, 4.1±1.4 vs. 6.4±2.8; CH
background, 7.9±1.8 vs. 7.1±1.4; PC background, 10.0±3.3 vs.
7.0±1.9; cirrhosis background, 7.6±2.5 vs 8.1±1.8, all P>0.05)
were identified.
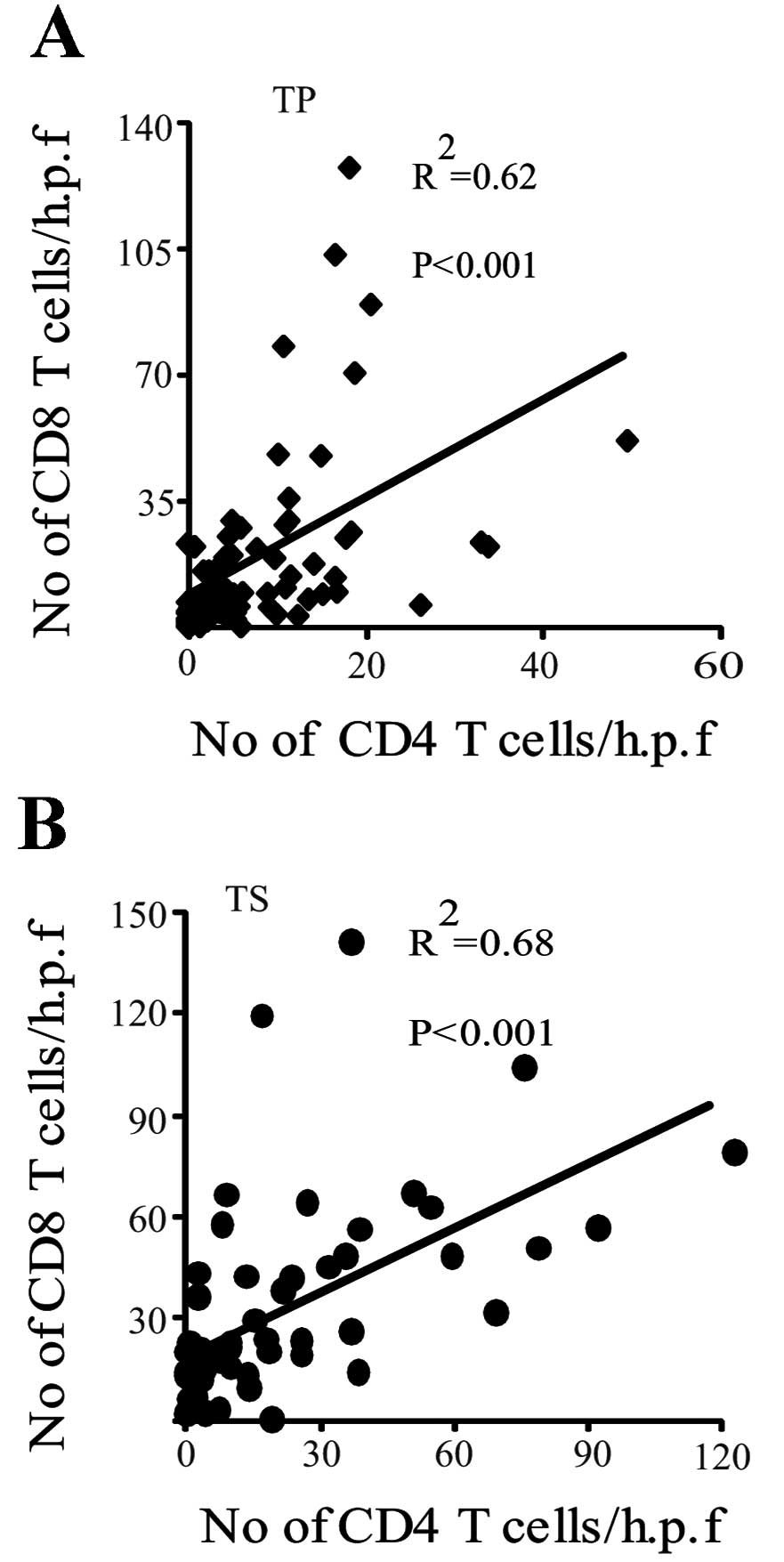
Correlation between CD4 and CD8
expression in HCC
Spearman’s correlation analysis revealed that CD8
expression was positively correlated with CD4 expression in the
tumor parenchyma and tumor stroma (correlation coefficient = 0.62
in tumor parenchyma; correlation coefficient = 0.68 in tumor
stroma; both P<0.001; Fig. 4A and
B).
Discussion
Solid tumors are composed of parenchyma (neoplastic
cells) and stroma. Neoplastic cells are also usually dispersed
within the stroma, which is composed of fibroblasts, endothelial
cells and a variety of immune cells (28,29).
These stromal cells are key in tumor development, tumor control and
the response to treatment (9–14). In
the present study, the distribution of tumor-infiltrating
lymphocytes (TIL) within the tumor parenchyma or tumor stroma was
investigated in order to more accurately evaluate the respective
impacts of these TILs on the biological behavior of HCC. To the
best of our knowledge, no studies have been conducted with regard
to the TIL expression in different areas of tumors in association
with HCC clinicopathological parameters. The results of the present
study revealed significant differences in the intratumoral
expression of CD8, but not CD4.
In the present study, a difference in the number of
CD8 T cells between the tumor parenchyma and stroma in HCC (tumor
parenchyma < tumor stroma) was detected. This may be explained
by the evidence from a previous study that tumor microenvironments
are rich in immune-cell-derived chemokines (10).
CD8 T cells exert a central role in the immune
defense against cancer. For example, CD8-positive CTLs directly
contact and kill tumor cells by releasing membrane-lytic granules,
such as perforin and granzyme. Indeed, the presence of tumor
antigen-specific CD8 T cells has been observed in HCC patients
(30). CD8-positive CTLs also kill
tumor stroma cells that cross-present antigens. In addition,
CTL-derived cytokines, including tumor necrosis factor α,
interleukin 4 (IL-4) and IL-10, contribute to tumor rejection by
inhibition of tumor stroma formation (20,31–33).
In the present study, the average numbers of CD8 T cells in the
tumor parenchyma and stroma were higher in patients with tumor
diameters ≤5 cm than in patients with tumor diameters >5 cm. In
concurrence with this finding, Gao et al (16) demonstrated that primary tumor size
was inversely correlated with the presence of CD8 T cells in HCC,
although no distinction was made regarding the precise location of
the T cells. Additionally, in the center (CT) and the invasive
margin (IM) of colorectal cancer tumors, CD3, CD8, GZMB (a marker
for CD8-positive CTLs) and CD45RO (a marker for memory T cells)
expression levels in each tumor region (CT and IM) were negatively
correlated with tumor recurrence. High CD8 density, and CD45RO and
GZMB expression were correlated with longer overall survival times
(11). A study conducted by Chew
et al (14) further
confirmed and complemented these findings; NK and CD8+ T
cells were observed to be the main proliferating lymphocytes in
human HCC. The presence of NK and CD8+ T cells was
associated with longer survival times, which is concurrent with the
finding from another previous study that host anticancer cellular
immunity is mainly attributable to CD8-positive CTLs (15). Collectively, these observations
suggest that an increased number of CD8 T cells in HCC is
associated with longer overall survival times and improved
prognosis.
Another finding in the present study was that CD8
expression was significantly increased in the peritumor chronic
hepatitis and cirrhotic parenchymas, compared with those in paired
tumor parenchymas. This finding is concurrent with the results of a
study revealing that the proportion of immune-suppressed regulatory
T cells was significantly higher in HCC than that in the
non-tumorous liver (34).
The results from the present study demonstrate that
CD8-positive T cells are not only important in tumor size control
but may also be a valuable prognostic factor. However, the present
study did not take account of factors such as survival analysis,
phenotypic characterizations (naïve, activated or regulated) and
cytotoxic function. Therefore, further studies are required,
particularly those that use human HCC specimens with known survival
times following HCC resection.
The present study demonstrated that elevated CD8
expression in tumor parenchyma and tumor stroma was correlated with
reduced tumor diameter. Therefore, tumor parenchyma and tumor
stroma infiltrating CD8 T cells were shown to be involved in HCC
diameter control.
Acknowledgements
The authors would like to thank Mr. Tokimasa Kumada
and Mr. Hideki Hatta for aid and technical assistance.
References
|
1
|
Schütte K, Bornschein J and Malfertheiner
P: Hepatocellular carcinoma - epidemiological trends and risk
factors. Dig Dis. 27:80–92. 2009.
|
|
2
|
Kremsdorf D, Soussan P, Paterlini-Brechot
P and Brechot C: Hepatitis B virus-related hepatocellular
carcinoma: paradigms for viral-related human carcinogenesis.
Oncogene. 25:3823–3833. 2006.
|
|
3
|
Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler
P and Chisari FV: Immune pathogenesis of hepatocellular carcinoma.
J Exp Med. 188:341–350. 1998.
|
|
4
|
Fattovich G and Llovet JM: Risk factors
for hepatocellular carcinoma in HCV-cirrhosis: what we know and
what is missing. J Hepatol. 44:1013–1016. 2006.
|
|
5
|
Naugler WE, Sakurai T, Kim S, et al:
Gender disparity in liver cancer due to sex differences in
MyD88-dependent IL-6 production. Science. 317:121–124. 2007.
|
|
6
|
Parmiani G and Anichini A: T cell
infiltration and prognosis in HCC patients. J Hepatol. 45:178–181.
2006.
|
|
7
|
Tobe T, Uchino J, Endo Y, Oto M, Okamoto
E, Kojiro M, et al: Predictive factors for long term prognosis
after partial hepatectomy for patients with hepatocellular
carcinoma in Japan. The Liver Cancer Study Group of Japan. Cancer.
74:2772–2780. 1994.
|
|
8
|
Levy I and Sherman M: Liver Cancer Study
Group of the University of Toronto: Staging of hepatocellular
carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging
systems in a cohort of 257 patients in Toronto. Gut. 50:881–885.
2002.
|
|
9
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001.
|
|
10
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006.
|
|
11
|
Galon J, Costes A, Sanchez-Cabo F, et al:
Type, density, and location of immune cells within human colorectal
tumors predict clinical outcome. Science. 313:1960–1964. 2006.
|
|
12
|
Dieu-Nosjean MC, Antoine M, Danel C, et
al: Long-term survival for patients with non-small-cell lung cancer
with intratumoral lymphoid structures. J Clin Oncol. 26:4410–4417.
2008.
|
|
13
|
Finak G, Bertos N, Pepin F, et al: Stromal
gene expression predicts clinical outcome in breast cancer. Nat
Med. 14:518–527. 2008.
|
|
14
|
Chew V, Tow C, Teo M, et al: Inflammatory
tumour microenvironment is associated with superior survival in
hepatocellular carcinoma patients. J Hepatol. 52:370–379. 2010.
|
|
15
|
Wada Y, Nakashima O, Kutami R, Yamamoto O
and Kojiro M: Clinicopathological study on hepatocellular carcinoma
with lymphocytic infiltration. Hepatology. 27:407–414. 1998.
|
|
16
|
Gao Q, Qiu SJ, Fan J, et al: Intratumoral
balance of regulatory and cytotoxic T cells is associated with
prognosis of hepatocellular carcinoma after resection. J Clin
Oncol. 25:2586–2593. 2007.
|
|
17
|
Zhang L, Conejo-Garcia JR, Katsaros D, et
al: Intratumoral T cells, recurrence, and survival in epithelial
ovarian cancer. N Engl J Med. 348:203–213. 2003.
|
|
18
|
Kashef E and Roberts JP: Transplantation
for hepatocellular carcinoma. Semin Oncol. 28:497–502. 2001.
|
|
19
|
Lu XY, Xi T, Lau WY, et al:
Pathobiological features of small hepatocellular carcinoma:
correlation between tumor size and biological behavior. J Cancer
Res Clin Oncol. 137:567–575. 2011.
|
|
20
|
Zhang B, Zhang Y, Bowerman NA, et al:
Equilibrium between host and cancer caused by effector T cells
killing tumor stroma. Cancer Res. 68:1563–1571. 2008.
|
|
21
|
The general rules for the clinical and
pathological study of primary liver cancer. Liver Cancer Study
Group of Japan. Jpn J Surg. 19:98–129. 1989.
|
|
22
|
Ishak K, Baptista A, Bianchi L, et al:
Histological grading and staging of chronic hepatitis. J Hepatol.
22:696–699. 1995.
|
|
23
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: a study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954.
|
|
24
|
Kononen J, Bubendorf L, Kallioniemi A, et
al: Tissue microarrays for high-throughput molecular profiling of
tumor specimens. Nat Med. 4:844–847. 1998.
|
|
25
|
Hatta H, Tsuneyama K, Kumada T, et al:
Freshly prepared immune complexes with intermittent microwave
irradiation result in rapid and high-quality immunostaining. Pathol
Res Pract. 202:439–445. 2006.
|
|
26
|
Kumada T, Tsuneyama K, Hatta H, Ishizawa S
and Takano Y: Improved 1-h rapid immunostaining method using
intermittent microwave irradiation: practicability based on 5 years
application in Toyama Medical and Pharmaceutical University
Hospital. Mod Pathol. 17:1141–1149. 2004.
|
|
27
|
Sato E, Olson SH, Ahn J, et al:
Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high
CD8+/regulatory T cell ratio are associated with favorable
prognosis in ovarian cancer. Proc Natl Acad Sci USA.
102:18538–18543. 2005.
|
|
28
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001.
|
|
29
|
Connolly JL, Schnitt SJ, Wang HH, Dvorak
AM and Dvorak HF: Principles of cancer pathology. Holland-Frei
Cancer Medicine. Bast RC Jr, Kufe DW, Pollock RE, Weichselbaum RR,
Holland JF and Frei E III: BC Decker, Inc.; Hamiton, ON, Canada:
pp. 384–399. 2000
|
|
30
|
Zerbini A, Pilli M, Soliani P, et al: Ex
vivo characterization of tumor-derived melanoma antigen encoding
gene-specific CD8+cells in patients with hepatocellular carcinoma.
J Hepatol. 40:102–109. 2004.
|
|
31
|
Spiotto MT and Schreiber H: Rapid
destruction of the tumor microenvironment by CTLs recognizing
cancer-specific antigens cross-presented by stromal cells. Cancer
Immun. 5:82005.
|
|
32
|
Singh S, Ross SR, Acena M, Rowley DA and
Schreiber H: Stroma is critical for preventing or permitting
immunological destruction of antigenic cancer cells. J Exp Med.
175:139–146. 1992.
|
|
33
|
Blankenstein T: The role of tumor stroma
in the interaction between tumor and immune system. Curr Opin
Immunol. 17:180–186. 2005.
|
|
34
|
Kobayashi N, Hiraoka N, Yamagami W, et al:
FOXP3+ regulatory T cells affect the development and progression of
hepatocarcinogenesis. Clin Cancer Res. 13:902–911. 2007.
|