Introduction
Globally, gastric cancer is one of the most common
cancers, the third most frequent malignancy and the second most
common cause of cancer-related mortality annually (1,2).
Gastric cancer is usually diagnosed in the later stages of the
disease and has an extremely poor prognosis, as patients have
unresectable, metastatic or recurrent gastric cancer with few
available therapeutic options (3).
Therefore, there is an urgent requirement to extensively
investigate the molecular mechanisms of gastric carcinogenesis, and
to develop novel therapeutic strategies for the control of gastric
cancer.
Previous studies (4–6) have
found that various methylation abnormalities can lead to gene
inactivation and gene silencing, promoting the development of
gastric cancer. The human hedgehog interacting protein (HHIP) gene
is located on chromosome 4q31.21 31.3. HHIP was identified via the
screening of a mouse cDNA expression library for proteins that bind
to Shh. HHIP binds all three hedgehog (Hh) proteins with an
affinity equal to that of Ptc-1 and thus, functions to negatively
regulate the Hh pathway. Abnormalities in the activation of the Hh
signaling pathway are one cause of the occurrence and development
of tumors; the HHIP gene is a negative feedback factor of this
pathway, which can directly inhibit the Hh pathway, and has been
shown to have a significant role in development (7–9). At
present, the expression of the HHIP gene in human gastric cancer
and its association with the CpG island methylation status of the
promoter has not been reported. The present study aimed to analyze
the methylation of the HHIP gene in patients with gastric
carcinoma.
Materials and methods
Clinical specimens
Surgical specimens from 60 patients with gastric
cancer and adjacent normal tissues were collected from the
Department of Surgery, Zhangjiagang First People’s Hospital
(Jiangsu, China) between 2009 and 2013. All surgically resected
tissue specimens were snap-frozen in liquid nitrogen until use.
These specimens were examined by at least two experienced
pathologists and tumor classification was made using the
tumor-node-metastasis (TNM) classification. The patients consisted
of 34 males and 26 females with an age range between 36 and 72
years (median, 60.82 years). According to the TNM staging system,
40 cases were stage II patients and 20 cases were stage III
patients. A total of 32 tumors were well- and
moderately-differentiated, while 28 were poorly-differentiated. A
total of 24 tumors exhibited lymph node metastasis, whereas 36
cases were without lymph node metastasis. The study was approved by
the ethics committee of Zhangjiagang First People’s Hospital.
Written informed consent was provided by all patients.
Cell culture and 5-aza-2′-deoxycytidine
(5-aza-dc) treatment
The gastric cancer AGS cell line was purchased from
the Shanghai Institute of Life Science Cell Information Center of
the Chinese Academy of Sciences (Shanghai, China) and cultured in
RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (FBS), 100 μg/ml streptomycin and 100 U/ml
penicillin at 37°C in a humidified atmosphere with 5%
CO2. Cells in the logarithmic growth phase were cultured
for ~24 h until they had reached 80% confluence, and then
5×106 mol/l 5-Aza-dc (Sigma, St. Louis, MO, USA) was
added. The liquid was changed once after 24h and then the cells
were harvested following 72 h of continuous treatment.
RNA isolation and reverse transcription
polymerase chain reaction (RT-PCR)
Evaluate of HHIP mRNA expression using RT-PCR. Total
RNA from the AGS gastric cancer cell line was isolated using TRIzol
reagent (Shanghai Jingmei Bioengineering Co., Ltd., Shanghai,
China) and converted into cDNA. The primer sequences of HHIP were
as follows: forward, 5′-CTGCTTCTGTATTCAGGAGGTT-3′ and reverse,
5′-GGGATGGAATGCGAGGCTTA-3′, with an amplified fragment length of
229 bp. The primer sequences of the internal control, β-actin, were
as follows: forward, 5′-AGAGCTACGAGCTGCCTGAC-3′ and reverse,
5′-AGCACTGTGTTGGCGTACAG-3′, with an amplified fragment length of
184 bp. The volume of the reaction agents was 20 μl, with reaction
conditions of 95°C for 5 sec, 55°C for 5 sec and 72°C for 30 sec.
The PCR products were subjected to 1.5% agarose gel electrophoresis
analysis.
Bisulfite conversion of DNA
Using phenol/chloroform, DNA was extracted, purified
and transformed by the EZ DNA Methylation-Gold kit (Beijing Tianmo
Technology Development Co., Ltd., Beijing, China).
Methylation-specific PCR (MSP) for
detection of HHIP gene methylation
The HHIP MSP primer was designed by ABI Methyl
Primer Express v1.0 software (Applied Biosystems, Foster City, CA,
USA). The methylation primer sequences of HHIP were as follows:
forward, 5′-GTAGTAGTCGGGTAGTTTCGGAATTTTC-3′ and reverse,
5′-AAAAACGACTAACCGCGACG-3′, with an amplified fragment length of
190 bp. The non-methylation primer sequences were as follows:
forward, 5′-AGTAGTTGGGTAGTTTTGGAATTTTTGG-3′ and reverse,
5′-AAAAACAACTAACCACAACA-3′, with an amplified fragment length of
188 bp. The volume of the reaction agents was 50 μl, with reaction
conditions of 94°C for 30 sec, 60°C for 40 sec and 72°C for 50 sec.
The PCR products were subjected to 1.5% agarose gel electrophoresis
analysis.
Bisulfite sequencing PCR (BSP) for
detection of HHIP gene methylation sites
The HHIP BSP primer was designed by ABI Methyl
Primer Express v1.0 software (Applied Biosystems). The primer
sequences of HHIP were as follows: forward,
5′-GGGGAGGAGAGAGGAGTTTG-3′ and reverse, 5′-CCCCACCACCTCCCTACTAC-3′,
with an amplified fragment length of 243 bp. The primer sequences
of the internal control, β-actin, were as follows: forward,
5′-AGAGCTACGAGCTGCCTGAC-3′ and reverse, 5′-AGCACTGTGTTGGCGTACAG-3′,
with an amplified fragment length of 184 bp. The volume of the
reaction agents was 50 μl, with reaction conditions of 94°C for 30
sec, 60°C for 40 sec and 72°C for 50 sec. The BSP products were
sent to Shanghai Shengong Biological Engineering Co., Ltd.
(Shanghai, China) for sequence analysis.
Statistical analysis
The data was analyzed using the χ2 test
and Student t-test, and correlation analysis was performed using
SPSS version 16.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of HHIP mRNA in human gastric
cancer tissues
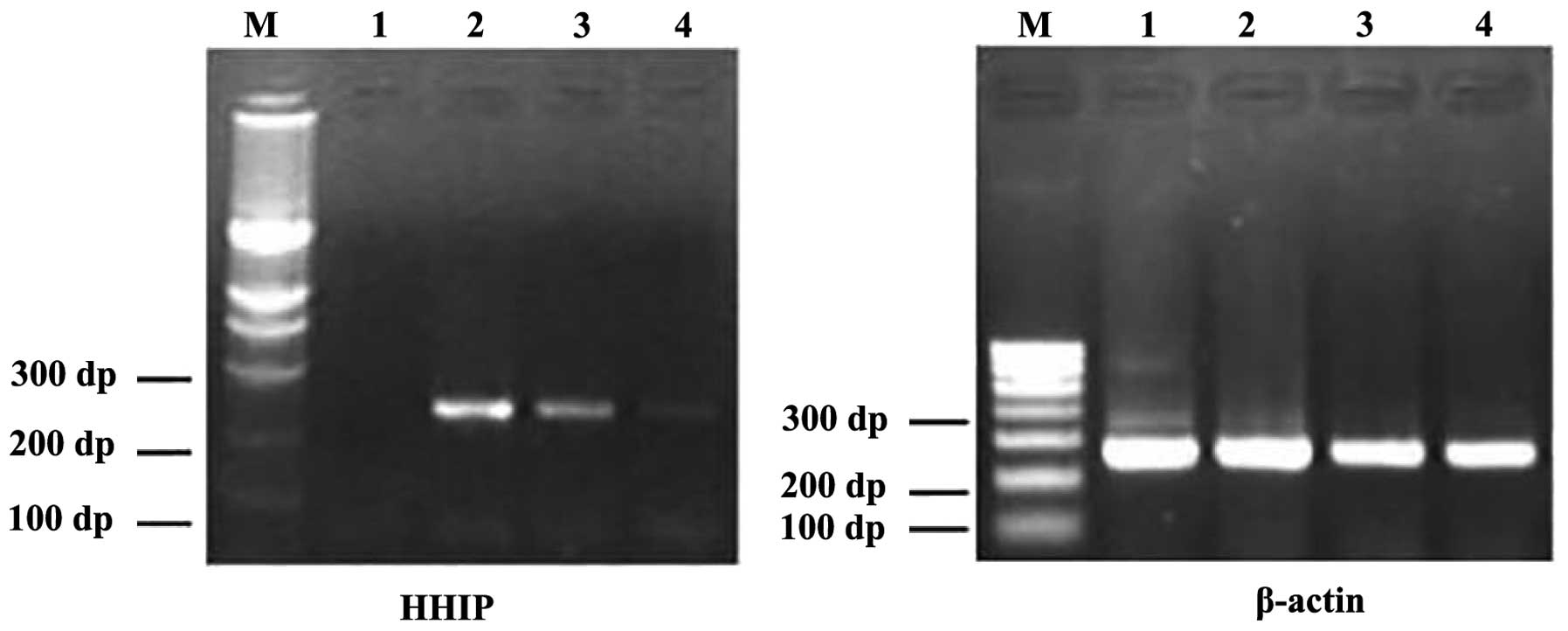
Based on the RT-PCR results, HHIP mRNA was found to
be expressed in the human gastric cancer tissues and adjacent
gastric tissues, and was found to have almost no expression in the
AGS cells (Fig. 1). The positive
rate of HHIP mRNA expression in the gastric cancer tissues was 30%
(18/60) compared with 66.67% in the adjacent normal tissues
(40/60). The relative expression in the gastric cancer tissues was
lower than that in the adjacent cancer tissues (0.8±0.38 vs.
1.6±0.26; P<0.001). No significant correlations were observed
between the expression of HHIP mRNA and age, gender, TNM stage,
differentiation degree and lymph node metastasis (P>0.05)
(Table I).
 | Table IAssociation between HHIP mRNA and the
clinical features. |
Table I
Association between HHIP mRNA and the
clinical features.
| | HHIP |
|---|
| |
|
|---|
| Clinical
features | n | Relative
expression | t-value | P-value |
|---|
| Gender |
| Male | 34 | 1.3±0.02 | 0.545 | 0.596 |
| Female | 26 | 1.3±0.01 | | |
| Age, years |
| <50 | 22 | 1.3±0.02 | 0.012 | 0.991 |
| ≥50 | 38 | 1.3±0.01 | | |
| TNM stage |
| II | 40 | 1.3±0.01 | −0.031 | 0.976 |
| III | 20 | 1.3±0.01 | | |
| Differentiation |
| Well and
moderate | 32 | 1.3±0.02 | 0.972 | 0.394 |
| Poor | 28 | 1.3±0.01 | | |
| Lymph node
metastasis |
| Yes | 24 | 1.3±0.02 | 0.162 | 0.875 |
| No | 36 | 1.3±0.01 | | |
Detection of HHIP gene promoter
methylation in gastric cancer tissues
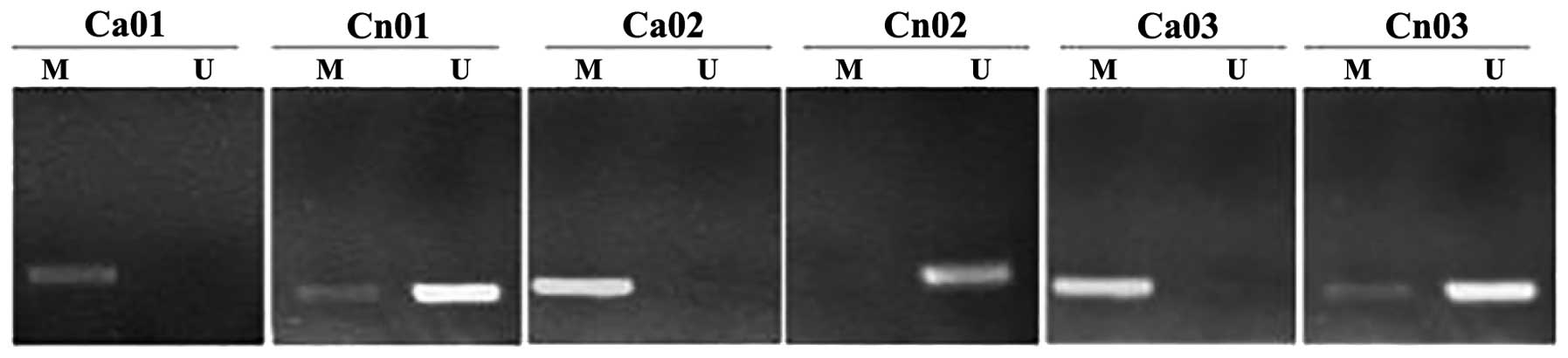
Based on the results of the amplification procedure
performed using MSP, a number of gastric cancer tissues were shown
to exhibit methylated HHIP gene promoters compared with the
adjacent normal tissues, as shown in Fig. 2. No significant difference in HHIP
gene promoter region methylation was observed in the gastric cancer
tissues and AGS cells (P>0.05), however, the HHIP gene promoter
region methylation level was significantly lower in the adjacent
normal tissues compared with the gastric cancer tissues and AGS
cells (17.7±3.59 vs. 62.9±6.14 and 99.7±0.67%; all P<0.05).
Effects of 5-aza-dc on the expression of
HHIP mRNA and promoter methylation
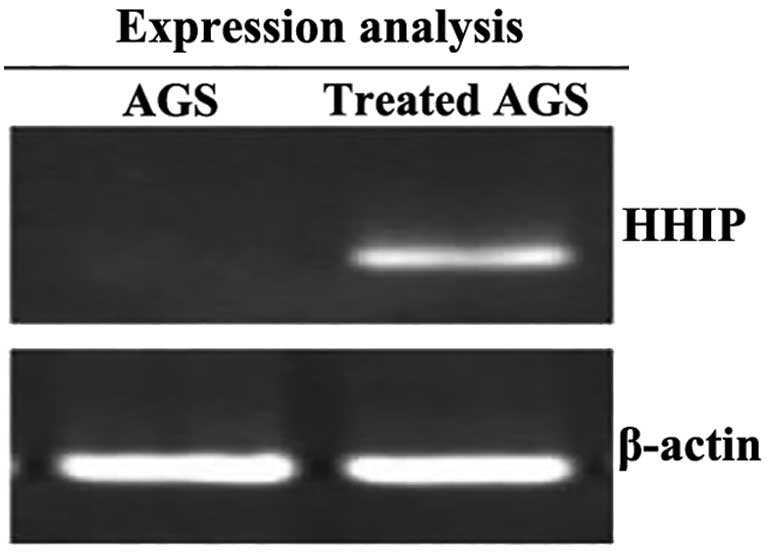
Using RT-PCR, it was found that the AGS cells were
activated following the intervention with 5-aza-dC; the expression
of HHIP mRNA was significantly increased (0.21±0.12 vs. 4.68±0.22;
P<0.01) (Fig. 3). Based on the
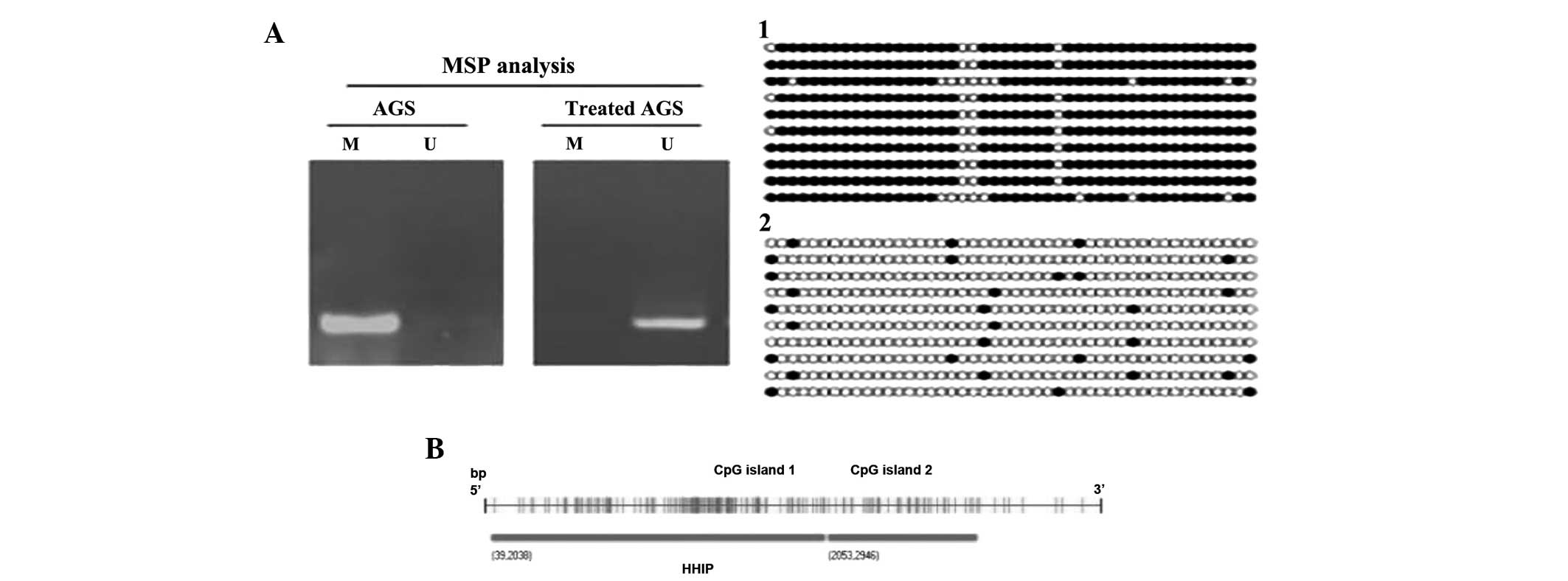
results from the amplification procedure using MSP, the methylation
level was shown to be significantly decreased following 5-aza-dC
treatment (90.2±0.67 vs. 10.1±0.21%; P<0.01), as shown in
Fig. 3. Spearman’s correlation
analysis showed that HHIP gene promoter methylation was negatively
correlated with mRNA expression (r=−0.693; P<0.001). With regard
to the detection of the methylation status in the promoter region
by the BSP method, the level of methylation significantly decreased
following treatment, as shown in Fig.
4. Using CpG analysis (ABI Methyl Primer Express v1.0
software), the HHIP promoter region was determined to have 2 CpG
islands; the first island is located from +39 bp to +2038 bp, the
second island is located from +2053 to +2946 bp (Fig. 4B) The first island was used to
design the HHIP primer sequence. It was found that the number of
HHIP gene promoter CpG island methylation loci was significantly
reduced following 5-aza-dC treatment.
Discussion
Methylation of tumor suppressor genes has been found
to cause numerous cancers and has been a focus of tumor research in
recent years. DNA methylation is a form of chemical modification,
which changes genetic expression without altering the DNA sequence.
In a variety of human tumor genes, the DNA methylation level is low
and a degree of regional hypermethylation coexists. Previous
studies (10–12) have shown that 5′-CpG island
hypermethylation leads to partial inactivation of tumor suppressor
genes, which is a significant cause of the malignant transformation
of cells. As a result treatment with demethylation drugs, the gene
expresses a tumor suppressor function. It has been found that when
using 5-azacytidine to act on promoter hypermethylation, the
corresponding mRNA and protein expression can be restored in the
tumor cells; this confirmed the fact that promoter methylation is
the main cause of the inhibition of gene expression (13–15).
One study has even proposed the establishment of a pattern of DNA
methylation for multiple tumor-associated genes, to facilitate the
diagnosis and differential diagnosis of early (16).
The HH signaling pathway is a vital signal
transduction pathway that aids in regulating embryonic development.
HHIP was first identified by screening a mouse cDNA expression
library for proteins that bind to sonic hedgehog (Shh). HHIP binds
all three Hh proteins with an affinity equal to that of Ptc-1, and
functions to negatively regulate the Hh pathway. The expression of
the HHIP gene, a negative regulator of Hh signaling, has been shown
to be reduced in gastric cancer tissues, but retained in normal
gastric tissues or atypical hyperplasia (17–19).
The present study also found that the expression of HHIP mRNA in
the gastric cancer tissues was significantly lower than that in the
adjacent normal tissues (P<0.05), supporting the aforementioned
results.
Accumulating evidence has shown that DNA methylation
is closely associated with gastric cancer. Studies have found that
hypermethylation exists in each stage of gastric cancer, even at
the precancerous lesion stage. Lee et al (20) found that the p16 gene methylation
level had positive correlation with gastric carcinogenesis. The
level of p16 methylation may increase in intestinal metaplasioa,
chronic gastritis, polypoid adenoma and adenocarcinoma. Berman
et al (21) reported that,
in 2003, 81% of digestive tract cancer cells, including esophageal,
gastric, biliary and pancreatic cancer- derived cell lines, exhibit
the expression of SHH and its receptor, PTCH. A study by Shahi
et al (22) found that HHIP
hypermethylated in pancreatic cancer cell lines. These data suggest
that the Hh pathway is involved in the occurrence and development
of gastric cancer. There is currently a lack of studies with regard
to HHIP gene defects and mutations in gastric cancer. The number of
studies analyzing HHIP gene methylation is even less.
This study analyzed the occurrence of HHIP gene CpG
island methylation in gastric cancer. The study found that the
level of HHIP gene promoter methylation in peritumoral tissues
(17.7±3.59%) was significantly lower than that in gastric cancer
tissues (62.9±6.14%) and AGS cells (99.7±0.67%) (P<0.05). The
level of HHIP methylation increased significantly in normal gastric
mucosa, gastric cancer and gastric cancer cell lines. Following
intervention with 5-aza-dc, the methylation level of the AGS cell
line decreased significantly and non-methylation of the HHIP
promoter region was observed. The CpG methylation status was
significantly reduced, whereas the HHIP gene was activated and the
mRNA expression was significantly increased. Analysis showed that
the mRNA expression level was negatively correlated with the
methylation level. Therefore, HHIP gene CpG island hypermethylation
may decrease the expression of HHIP, which maybe participate in the
carcinogenesis of gastric cancer, therefore methylation in HHIP
gene CpG islands may be a good detection index for gastric
cancer.
In conclusion, HHIP gene promoter CpG island
methylation may be associated with the carcinogenesis of gastric
cancer, so the detection of the HHIP gene methylation level may be
a novel clinical marker for the early diagnosis of gastric cancer.
HHIP and the specific mechanism of gastric cancer require further
study.
References
|
1
|
de Martel C, Forman D and Plummer M:
Gastric cancer: epidemiology and risk factors. Gastroenterol Clin
North Am. 42:219–240. 2013.
|
|
2
|
Fock KM: Review article: the epidemiology
and prevention of gastric cancer. Aliment Pharmacol There.
40:250–260. 2014.
|
|
3
|
Bollschweiler E, Berlth F, Baltin C, et
al: Treatment of early gastric cancer in the Western World. World J
Gastroenterol. 20:5672–5678. 2014.
|
|
4
|
Sheikh A, Alvi AA, Aslam HM and Haseeb A:
Hedgehog pathway inhibitors - current status and future propects.
Infect Agent Cancer. 7:292012.
|
|
5
|
Gerardo Valadez J, Grover VK, Carter MD,
et al: Identification of Hedgehog pathway responsive glioblastomas
by isocitrate dehydrogenase mutation. Cancer Lett. 328:297–306.
2013.
|
|
6
|
Yun JI, Kim HR, Park H, et al: Small
molecule inhibitors of the hedgehog signaling pathway for the
treatment of cancer. Arch Pharm Res. 35:1317–1333. 2012.
|
|
7
|
Queiroz KC, Spek CA and Peppelenbosch MP:
Targeting Hedgehog signaling and understanding refractory response
to treatment with Hedgehog pathway inhibitors. Drug Resist Updat.
15:211–222. 2012.
|
|
8
|
Cobourne MT and Green JB: Hedgehog
signaling in development of the secondary palate. Front Oral Biol.
16:52–59. 2012.
|
|
9
|
Büller NV, Rosekrans SL, Westerlund J, et
al: Hedgehog signaling and maintenance of homeostasis in intestinal
epithelium. Physiology (Bethesda). 27:148–155. 2012.
|
|
10
|
Viatte S, Plant D and Raychaudhuri S:
Genetics and epigenetics of rheumatoid arthritis. Nat Rev
Rheumatol. 9:141–153. 2013.
|
|
11
|
Eising E, A Datson N, van den Maagdenberg
AM and Ferrari MD: Epigenetic mechanisms in migraine: a promising
avenue. BMC Med. 11:262013.
|
|
12
|
Hodes GE: Sex, stress, and epigenetics:
regulation of behavior in animal models of mood disorders. Biol Sex
Differ. 4:12013.
|
|
13
|
Toraño EG, Petrus S, Fernandez AF and
Fraga MF: Global DNA hypomethylation in cancer: review of validated
and clinical significance. Clin Chem Lab Med. 50:1733–1742.
2012.
|
|
14
|
Savio AJ, Lemire M, Mrkonjic M, et al:
MLH1 region polymorphisms show a significant association with CpG
island shore methylation in a large cohort of healthy individuals.
PLoS One. 7:e515312012.
|
|
15
|
Kim JG, Takeshima H, Niwa T, et al:
Comprehensive DNA methylation and extensive mutation analyses
reveal an association between the CpG island methylator phenotype
and oncogenic mutations in gastric cancers. Cancer Lett. 330:33–40.
2013.
|
|
16
|
Anastasiadou C, Malousi A, Maglaveras N
and Kouidou S: Human epigenome data reveal increased CpG
methylation in alternatively spliced sites and putative exonic
splicing enhancers. DNA Cell Biol. 30:267–275. 2011.
|
|
17
|
Lu JT, Zhao WD, He W and Wei W: Hedgehog
signaling pathway mediates invasion and metastasis of
hepatocellular carcinoma via ERK pathway. Acta Pharmacol Sin.
33:691–700. 2012.
|
|
18
|
Yun JI, Kim HR, Park H, et al: Small
molecule inhibitors of the hedgehog signaling pathway for the
treatment of cancer. Arch Pharm Res. 35:1317–1333. 2012.
|
|
19
|
Pan JY and Zhou SH: The hedgehog
signaling, a new therapeutic target for treatment of ischemic heart
disease. Pharmazie. 67:475–481. 2012.
|
|
20
|
Lee JH, Park SJ, Abraham SC, et al:
Frequent CpG island methylation in precursor lesions and early
gastric adenocarcinomas. Oncogene. 23:4646–4654. 2004.
|
|
21
|
Berman A, Mezey M, Kobayashi M, et al:
Gerontological nursing content in baccalaureate nursing programs:
comparison of findings from 1997 and 2003. J Prof Nurs. 21:268–275.
2005.
|
|
22
|
Shahi MH, Lorente A and Castresana JS:
Hedgehog signalling in medulloblas toma, glioblastoma and
neuroblastoma. Oncol Rep. 19:681–688. 2008.
|