Introduction
The interactions between stromal cells and tumor
cells are important aspects of tumor growth and invasion. In
salivary gland tumors, myoepithelial cells have been implicated in
the regulation of the transition from in situ to invasive
neoplasia (1).
Myoepithelial cells exert inhibitory effects on
numerous neoplastic phenotypes, including tumor cell growth,
invasion and angiogenesis, and have been described as natural tumor
suppressors (2–5). Therefore, extracellular matrix-cell
interactions are essential not only for normal development, but
also for their role in tumorigenesis (6).
In vivo modification of the phenotype of
benign myoepithelial cells in in situ areas of carcinoma ex
pleomorphic adenoma (PA) induced by malignant transformation of
epithelial cells has been demonstrated, revealing crosstalk between
the myoepithelial and adenoma cells (7,8). Due
to these studies, an in vitro model was used to investigate
the role of myoepithelial cells and the tumor microenvironment in
salivary gland neoplasms (9). The
focus was the influence of extracellular matrix proteins, including
basement membrane matrix, type I collagen and fibronectin, on the
morphology and differentiation of benign myoepithelial cells from
PA that were cultured with medium obtained from the culture of
squamous cell carcinoma tumor cells (10). This demonstrated that the
extracellular matrix plays an important role in the morphology of
benign myoepithelial cells under the influence of squamous cell
carcinoma tumor medium, and also plays a role in inducing an
increase in the expression of fibroblast growth factor (FGF)-2 and
α-smooth muscle actin (α-SMA) in these cells, particularly in the
fibronectin substratum.
Considering the interaction between squamous cell
carcinoma and myoepithelial cells under the influence of the tumor
microenvironment (10), the present
in vitro study aimed to examine the role of
tumor-conditioned medium, obtained from melanoma and breast ductal
adenocarcinoma cells, in the morphological and phenotypic
alterations of neoplastic benign myoepithelial cells obtained from
PA under a fibronectin substratum.
Materials and methods
Cell culture
Benign myoepithelial cells were obtained from
explants of PA tumors from three different donors, according to the
methodology described in previous studies (8–10). The
present study was approved by the Ethics Committee of São Leopoldo
Mandic Institute and Dental Research Center (Campinas, Brazil;
Protocol 09/0014). All patients provided written informed
consent.
The cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 1% antimycotic-antibiotic solution (10,000 units
penicillin, 10 mg streptomycin and 25 μg/ml amphotericin B in 0.9%
sodium chloride; Sigma-Aldrich), supplemented with 10% donor calf
serum (Gibco Life Technologies, Carlsbad, CA, USA). The cells were
then plated in 60-mm diameter plastic culture dishes and incubated
under the standard cell culture conditions of 37°C, 100% humidity,
95% air and 5% CO2. Subsequent to reaching confluence,
the cells were detached using 0.05% trypsin and subcultured at a
density of 110 cells/mm2 in 20 μg/ml of fibronectin
substratum (Sigma-Aldrich). The cells were then placed in the
polystyrene plate or on 13-mm coverslips for the subsequent
experiments. The plated benign myoepithelial cells were cultured in
DMEM for 24 h prior to being supplemented with tumor-conditioned
medium, according to the methodology described by Martinez et
al (9).
For the in vitro induction with
tumor-conditioned medium, melanoma Hs 852.T and breast ductal
adenocarcinoma AU-565 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). The cell medium was changed
48 h prior to use. Benign myoepithelial cells cultured in DMEM for
24 h were then incubated for four days with the tumor-conditioned
medium, which was previously filtered using a 0.22 μm sterile
syringe filter (Corning, Inc., Corning, New York, NY, USA). The
analysis was also carried out using non-conditioned DMEM as a
control.
Immunofluorescence
The cells grown on coverslips in various substrata
were fixed in methanol for 6 min at −20°C, rinsed with
phosphate-buffered saline (PBS) and then blocked with 1% bovine
albumin in PBS for 30 min at room temperature. The primary
antibodies used were FGF-2 (1:50; polyclonal rabbit anti-human;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and α-SMA (1:50;
monoclonal mouse anti-human; Dako, Glostrup, Denmark). The control
staining reaction was performed using PBS in place of the primary
antibody. The secondary antibody used was biotinylated goat
anti-rabbit polyclonal immunoglobulin G (IgG) or goat anti-mouse
polyclonal IgG (Vector Laboratories, Inc., Burlingame, CA, USA).
Streptavidin, fluorescein-conjugated (Vector Laboratories, Inc.)
was used for the second step. The preparations were washed and
mounted using DAPI-associated (4′-6-diamidino-2-phenylindole)
Vectashield (Vector Laboratories, Inc.) and assessed on a Zeiss
Axioskop 2 conventional fluorescence microscope (Carl Zeiss
MicroImaging GmbH, Jena, Germany) equipped with 63X Plan
Apochromatic 1.4NA and 100X Plan Apochromatic 1.4NA objectives in
standard conditions (Carl Zeiss, Oberköchen, Germany). To verify
the morphological changes of benign myoepithelial cells obtained
from PA cultured with tumor-conditioned medium, the cells were also
immunostained with vimentin (1:400; monoclonal mouse anti-human;
Dako).
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from PA myoepithelial cells
cultured in various conditions using Tri Reagent (Molecular
Research Center, Cincinnati, Ohio, USA). The RNA underwent reverse
transcription using the Superscript III First Strand cDNA Synthesis
kit (Invitrogen, Carlsbad, CA, USA), according to the
manufacturer’s instructions. The primer sets were as follows:
α-SMA, forward; 5′-ATGCTCCCAGGGCTGTTTT-3′ and reverse;
5′-GCTTCGTCACCCACGTAGCT-3′; FGF-2, forward,
5′-GTGCTAACCGTTACCTGGCTAT-3′ and reverse;
5′-CCAATCGTTCAAAAAAGAAACAC-3′; and for the internal gene reference
β-actin (ACTB), forward; 5′-AGGCCAACCGCGAGAAG-3′ and reverse;
5′-ACAGCC TGGATAGCAACGTACA-3′. qPCR was performed using a 7300 Real
Time PCR system (Applied Biosystems, Foster City, CA, USA) with
SYBR Green as detection dye. The cycling conditions were 10 min at
95°C followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
The quantification data were analyzed using the SDS System Software
(Applied Biosystems) and the relative expression levels were
calculated according to the comparative Ct method, as
2−ΔΔCt. Each qPCR experiment was repeated three
times.
Statistics
The results are expressed as the mean ± standard
deviation. In order to compare the results between the various
conditions, two-way analysis of variance and post hoc Bonferroni
test were performed, with a significance level of 0.05.
Results
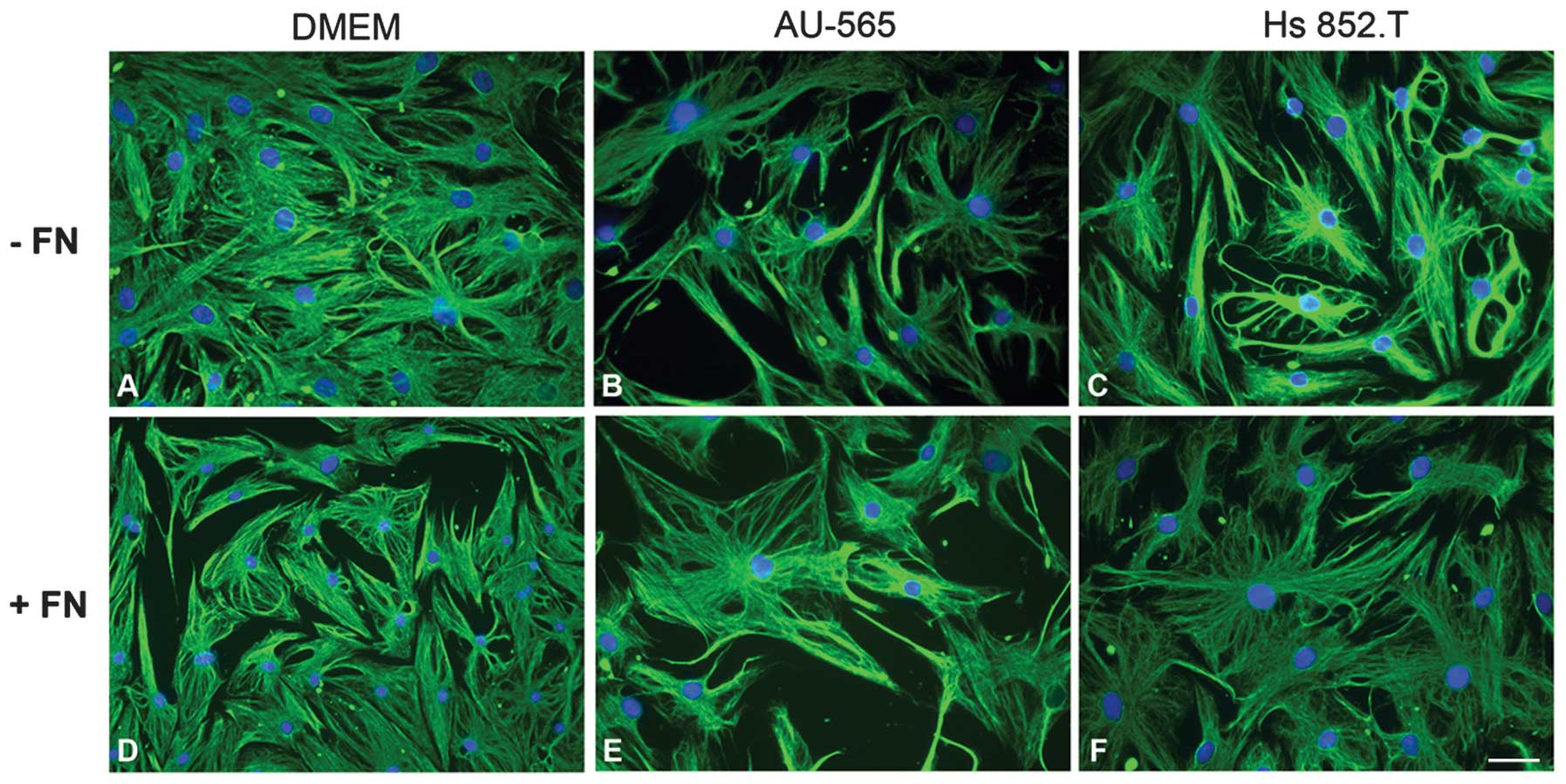
Myoepithelial cell morphology
In order to verify whether the tumor-conditioned
media from breast ductal adenocarcinoma AU-565 and melanoma Hs
852.T cells altered myoepithelial cell morphology, the cells were
examined using indirect immunofluorescence for vimentin (Fig. 1). There was no change in the
morphology of myoepithelial cells plated with fibronectin
substratum in vitro. In all the studied conditions, the
cells exhibited stellate and polyhedral morphology, even when
supplemented with the tumor-conditioned media.
Myoepithelial cell immunophenotype
The immunophenotype of the myoepithelial cells alone
or supplemented with the various tumor-conditioned media was
assessed using indirect immunofluorescence supported by qPCR
analysis (Figs. 2 and 3).
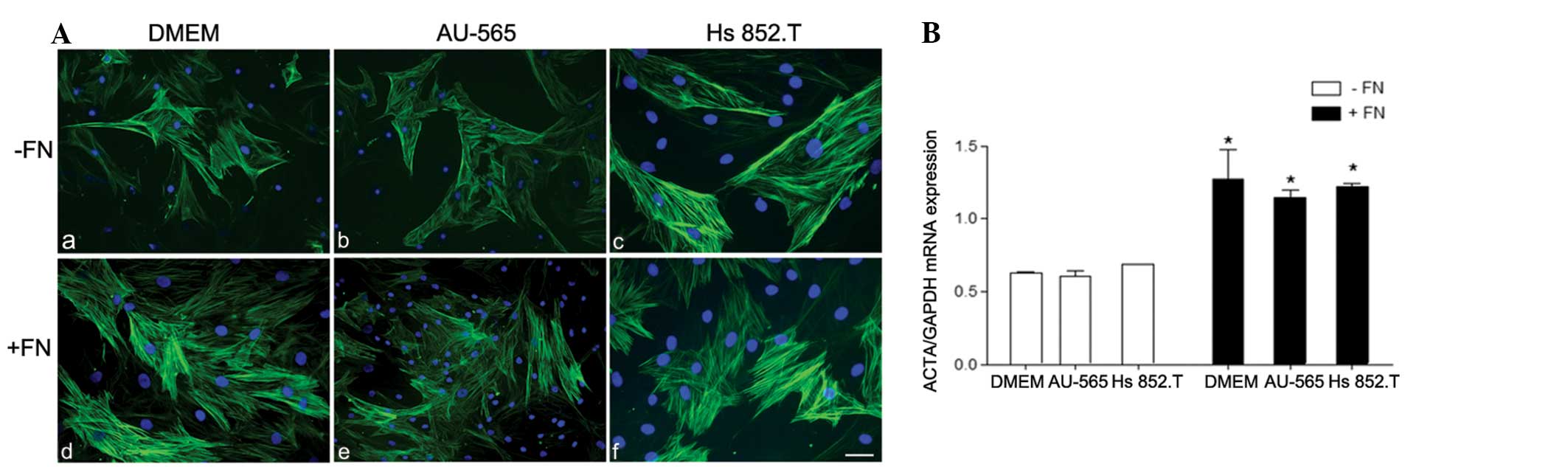 | Figure 2(A) Immunostaining for α-smooth muscle
actin (α-SMA) in myoepithelial cells from pleomorphic adenoma on
(a–c) polystyrene (−FN) and (d–f) fibronectin substratum (+FN).
α-SMA was heterogeneously immunoexpressed in the myoepithelial
cells in all the studied conditions. However, in the +FN cells,
there was an increase in α-SMA immunostaining. The nuclei stained
with DAPI appear in blue. Scale bars: A, B and E, 50 μm;
magnification, ×200; and C, D and F, 100 μm; magnification, ×400.
(B) Relative α-SMA mRNA expression. The expression of α-SMA was
significantly upregulated in all +FN conditions. *+FN
vs. −FN, P<0.05. Medium: a and d, Dulbecco’s modified Eagle’s
medium (DMEM); b and e, breast ductal adenocarcinoma AU-565
cell-conditioned medium; and c and f, melanoma Hs 852.T
cell-conditioned medium. |
The results demonstrated that only α-SMA was
upregulated in benign PA myoepithelial cells in tumor-conditioned
media from breast ductal adenocarcinoma and melanoma cells in the
fibronectin substratum (Fig. 2). As
previously demonstrated (10),
α-SMA was also heterogeneously immunoexpressed in myoepithelial
cells. No α-SMA immunophenotypical differences or differences in
mRNA expression were observed independent of the studied conditions
of tumor-conditioned media stimulation and DMEM.
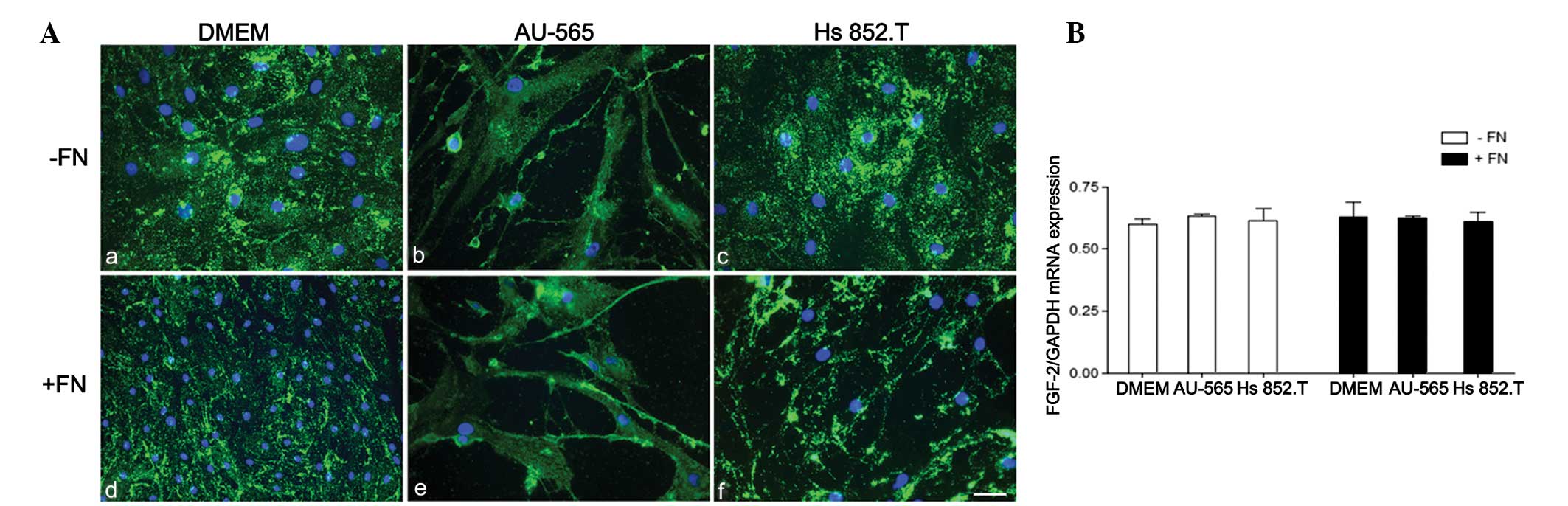
FGF-2 was immunoexpressed in the myoepithelial cells
as punctuate deposits throughout the cytoplasm in all studied
conditions (Fig. 3). No difference
in FGF-2 mRNA expression was detected when the cells were cultured
either in the tumor-conditioned medium or in the fibronectin
substratum.
Discussion
The interaction between cells and the surrounding
extracellular matrix is an important component of the development
and function of numerous biological events, including normal
development and tumorigenesis (11). Although the tumor microenvironment
has been extensively studied, including the important role of the
extracellular matrix associated with the secretion of numerous
molecules and the stromal cells, the process remains unclear. An
in vitro model that mimics an in situ scenario of
carcinoma ex PA (CXPA) has been developed (9) in order to investigate the crosstalk
between myoepithelial and cancer cells. A squamous cell carcinoma
cell line is used as the source of the tumor-conditioned medium
based on the CXPA characteristics. In this type of tumor,
epithelial cells can undergo malignant change and present features
similar to those of a squamous cell carcinoma (12). In the present study, various
tumor-conditioned media were used in order to investigate the
effects of the secretory factors released by carcinoma cells on
myoepithelial cells, which may be important to determine tumor
behavior. Two malignant cell lines were studied, the breast ductal
adenocarcinoma AU-565 cell line, which exhibits similarities to the
salivary gland neoplasm (13,14)
and the melanoma Hs 852.T cell line, due to cytokine and growth
factor production-associated aggressiveness (15).
The present results revealed that, despite the
several growth factors these cell lines secrete and using the
proposed in vitro model, no alteration in myoepithelial cell
morphology was detected. These findings are in line with those of a
previous study (10) that used
squamous cell carcinoma-conditioned medium, in which the
myoepithelial cells exhibited a polyhedral and stellate morphology
in the fibronectin substratum, forming sites of adhesion and focal
contact with the matrix, a fundamental event for malignant cell
colonization. In salivary gland neoplasms, unlike tenascin,
fibronectin is not present in the tumor invasion front (16,17).
In breast tissue, however, the fibronectin in the stromal
extracellular matrix may assist the process of tumorigenesis
(18). Furthermore, the
extracellular matrix may influence intracellular signaling and cell
cycle control, thus contributing to tumor cell migration and
invasion (19). Based on the
results obtained in a previous study, the morphological aspect of
the myoepithelial cells in fibronectin, regardless of the malignant
in situ condition, appeared to have impaired their tumor
suppressor function.
The present data revealed that the expression of
α-SMA was upregulated in benign myoepithelial cells from PA in
fibronectin. This finding corroborates those from a previous study
(10), thus highlighting the
importance of this extracellular matrix protein in triggering
cell-matrix interactions and subsequent changes in actin
cytoskeleton, which are essential for the control of directional
cell migration and invasion (11).
Notably, no difference was observed between the
morphology of the studied groups, indicating that the
tumor-conditioned media from breast ductal adenocarcinoma and
melanoma cells did not influence myoepithelial cell
differentiation. de Araújo et al (7) demonstrated that the myoepithelial
cells surrounding regions of malignant transformation were
phenotypically different from the benign myoepithelial cells of the
PA. In addition, the former expressed a higher level of α-SMA. It
is of note that the extracellular matrix molecules, mainly
described for breast tumors, have emerged as an important cell
regulator, which may affect tumor cell behavior (16,17).
The present results revealed that the extracellular matrix protein
fibronectin alone was able to upregulate α-SMA expression. The
effect of α-SMA upregulation on benign myoepithelial cells as
observed in the present in vitro model is, however,
unclear.
Furthermore, no difference in FGF-2 mRNA expression
was detected when the cells were cultured in the tumor-conditioned
medium, despite the presence of fibronectin substratum. An
upregulation in FGF-2 expression has previously been observed in
benign myoepithelial cells when using a squamous cell carcinoma
cell line, suggesting that the excessive release of FGF-2 favored
malignant cell growth. The same finding was observed when the cells
were cultured in fibronectin substratum (10), emphasizing the importance of this
extracellular matrix protein in modifying myoepithelial cell
function. The tumor media from breast carcinoma and melanoma used
in the present study did not influence FGF-2 mRNA regulation. This
finding may indicate that there is no correlation between FGF-2
secretion and the quantity of cytokines and growth factors released
by these tumors in the microenvironment, which could otherwise
promote tumor progression and dissemination (20,21).
In this context, using squamous cell carcinoma in the proposed
in vitro model, the myoepithelial cells appeared to have
favored tumor growth via the production of IL-6 and IL-10
stimulated by the malignant cells, in a paracrine way (22). This suggests that the neoplastic
benign myoepithelial cells from PA are most likely tumor-dependent,
which does not corroborate the finding from previous studies that
the myoepithelial cells from PA, albeit transformed, can be used as
a surrogate for normal mammary myoepithelial cells (23–25).
The present data resulted in the conclusion that the
tumor-conditioned medium obtained from breast ductal adenocarcinoma
and melanoma cells did not act on myoepithelial cell
differentiation and function, which was revealed by the lack of
increase in α-SMA and FGF-2 expression, respectively. Additionally,
in the case of the aforementioned malignant tumors, other factors
that were not the focus of the present investigation may be playing
a role in myoepithelial cell behavior.
Acknowledgements
The authors wish to thank Mrs Pollyanna Tombini
Montaldi for the excellent technical expertise and assistance. This
study was supported by grants from FAPESP/Brazil (nos. 2008/58721-7
and 2011/14053-3).
References
|
1
|
Barsky SH and Karlin NJ: Mechanisms of
disease: breast tumor pathogenesis and the role of the
myoepithelial cell. Nat Clin Pract Oncol. 3:138–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen M, Lee MC, Wang JL, Tomlinson JS,
Shao ZM, Alpaugh ML and Barsky SH: The human myoepithelial cell
displays a multifaceted anti-angiogenic phenotype. Oncogene.
19:3449–3459. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deugnier MA, Teulière J, Faraldo MM,
Thiery JP and Glukhova MA: The importance of being a myoepithelial
cell. Breast Cancer Res. 4:224–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Polyak K and Hu M: Do myoepithelial cells
hold the key for breast tumor progression? J Mammary Gland Biol
Neoplasia. 10:231–247. 2005. View Article : Google Scholar
|
|
5
|
da Silva A, Silva CA, Montalli VA,
Martinez EF, de Araújo VC and Furuse C: In vitro evaluation of the
suppressor potential of conditioned medium from benign
myoepithelial cells from pleomorphic adenoma in malignant cell
invasion. J Oral Pathol Med. 41:610–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bissell MJ, Kenny PA and Radisky DC:
Microenvironmental regulators of tissue structure and function also
regulate tumor induction and progression: the role of extracellular
matrix and its degrading enzymes. Cold Spring Harb Symp Quant Biol.
70:343–356. 2005. View Article : Google Scholar
|
|
7
|
de Araújo VC, Altemani A, Furuse C,
Martins MT and de Araújo NS: Immunoprofile of reatctive salivary
myoepithelial cells in intraductal areas of carcinoma
ex-pleomorphic adenoma. Oral Oncol. 42:1011–1016. 2006. View Article : Google Scholar
|
|
8
|
Martinez EF, Demasi AP, Miguita L,
Altemani A, de Araújo NS and de Araújo VC: FGF-2 is overexpressed
in myoepithelial cells of carcinoma ex-pleomorphic adenoma in situ
structures. Oncol Rep. 24:155–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martinez EF, Demasi AP, Napimoga MH,
Arana-Chavez VE, Altemani A, de Araújo NS and de Araújo VC: In
vitro influence of the extracellular matrix in myoepithelial cells
stimulated by malignant conditioned medium. Oral Oncol. 48:102–109.
2012. View Article : Google Scholar
|
|
10
|
Martinez EF, Montaldi PT, de Araújo NS,
Altemani A and de Araújo VC: A proposal of an in vitro model which
mimics in situ areas of carcinoma. J Cell Comun Signal. 6:107–109.
2012. View Article : Google Scholar
|
|
11
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Altemani A, Martins MT, Freitas L, Soares
F, Araújo NS and Araújo VC: Carcinoma ex pleomorphic adenoma
(CXPA): immunoprofile of the cells involved in carcinomatous
progression. Histophatol. 46:635–641. 2005. View Article : Google Scholar
|
|
13
|
Pia-Foschini M, Reis-Filho JS, Eusebi V
and Lakhani SR: Salivary gland-like tumors of the breast: surgical
and molecular pathology. J Clin Pathol. 56:497–506. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gudjonsson T, Adriance MC, Sternlicht MD,
Petersen OW and Bissel MJ: Myoepithelial cells: their origin and
function in breast morphogenesis and neoplasia. J Mammary Gland
Biol Neoplasia. 10:261–272. 2005. View Article : Google Scholar
|
|
15
|
Kang KH, Ling TY, Liou HH, Huang YK, Hour
MJ, Liou HC and Fu WM: Enhancement role of host 12/15-lipoxygenase
in melanoma progression. Eur J Cancer. 49:2747–2759. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Araújo VC, Furuse C, Cury PR, Altemani A,
Alves VA and de Araújo NS: Tenascin and fibronectin expression in
carcinoma ex pleomorphic adenoma. Appl Immunohistochem Mol Morphol.
16:48–53. 2008.
|
|
17
|
Araújo VC, Demasi AP, Furuse C, Altemani
A, Alves VA, Freitas LL and Araújo NS: Collagen type I may
influence the expression of E-cadherin and beta-catenin in
carcinoma ex-pleomorphic adenoma. Appl Immunohistochem Mol Morphol.
17:312–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park J and Schwarzbauer JE: Mammary
epithelial cell interactions with fibronectin stimulate
epithelial-mesenchymal transition. Oncogene. 27:1649–1657. 2014.
View Article : Google Scholar
|
|
19
|
Gehler S, Ponik SM, Riching KM and Keely
PJ: Bi-directional signaling: extracellular matrix and integrin
regulation of breast tumor progression. Crit Rev Eukaryot Gene
Expr. 23:139–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whiteside TL: The role of death receptor
ligands in shaping tumor microenvironment. Immunol Invest.
36:25–46. 2007. View Article : Google Scholar
|
|
21
|
Bianchi G, Borgonovo G, Pistoia V and
Raffaghello L: Immunosuppressive cells and tumour microenvironment:
focus on mesenchymal stem cells and myeloid derived suppressor
cells. Histol Histopathol. 26:941–951. 2011.PubMed/NCBI
|
|
22
|
Martinez EF, Napimoga MH, Montalli VA, de
Araújo NS and de Araújo VC: In vitro cytokine expression in in
situ-like areas of malignant neoplasia. Arch Oral Biol. 58:552–557.
2013. View Article : Google Scholar
|
|
23
|
Sternlicht MD, Kedeshian P, Shao ZM,
Safarians S and Barsky SH: The human myoepithelial cell is a
natural tumor suppressor. Clin Cancer Res. 3:1949–1958. 1997.
|
|
24
|
Sternlicht MD and Barsky SH: The
myoepithelial defense: a host defense against cancer. Med
Hypotheses. 48:37–46. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barsky SH and Alpaugh ML: Myoepithelium:
methods of culture and study. Culture of Human Tumor Cells.
Freshney RI: Wiley-Liss; New Jersey: pp. 221–261. 2004
|