Introduction
Colorectal cancer (CRC) is the third most common
malignancy in Western countries and is one of the leading causes of
cancer-related mortality in China (1,2). In
total, approximately 10–25% of patients with CRC develop pulmonary
metastases (3). As no effective
chemotherapy regimen has been developed for the treatment of
pulmonary metastases of colorectal origin, surgery is the only
potentially curative treatment option. However, only 2–4% of
pulmonary metastases can be treated surgically and others require
external beam radiotherapy and chemotherapy (4). Increasing the therapeutic doses of
traditional external beam radiotherapy is challenging due to the
severe side-effects. Although three-dimensional conformal radiation
therapy (3D-CRT) and stereotactic external beam radiotherapy can
administer tumoricidal doses, the side-effect of lung tissue damage
remains a problem (5).
Percutaneous computed tomography (CT)-guided
radioactive 125I seed implantation (CTRISI) is a
minimally invasive modality. This brachytherapy is less time
consuming and less traumatic, compared with the aforementioned
treatments, and the side-effect of radiation damage is minimal
(6). Patients are also more likely
to accept this therapy due the minimally invasive nature of the
technique. CTRISI has been used for the treatment of non-small cell
lung cancer (NSCLC) (7,8). However, there have been few
radioactive seed implantations for pulmonary metastases following
resection of CRC and the efficiency of CTRISI has not been
determined. The present study reports the preliminary results of
six patients with pulmonary metastases following resection, who
could not tolerate a surgical procedure and therefore, underwent
CT-guided 125I brachytherapy.
Materials and methods
In total, six patients, three males and three
females, with an ages range of 68–86 years (mean ± standard
deviation, 76.0±7.6 years), with pulmonary metastases following
colon cancer resection, were treated with percutaneous CTRISI at
the Department of Thoracic Surgery, Second Hospital of Tianjin
Medical University (Tianjin, China) between November 2002 and May
2010. Informed consent was obtained from the subjects and the
present study was approved by the Ethics Committee of Tianjin
Medical University. The patient characteristics are shown in
Table I. Of the total 13 metastatic
lesions, eight were located in the left lung and five in the right
lung. In total, 10 were located in the lung and three were located
beneath the hilum of the lung. The average diameter was 2.8±1.5 cm
(range, 1–6 cm) and the average volume was 29.5±29.4
cm3. A complication of right supraclavicular lymph node
metastasis was observed in one case and subsequently received seed
implantation.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient | Gender | Age, years | Time to recurrence,
months | Adenocarcinoma
pathology | Number of
lesions | Location | Type | Diameter, cm | Volume,
cm3 | Reason for seed
implantation |
|---|
| 1 | Female | 71 | 4 |
Poorly-differentiated | 1 | Adjacent to aortic
arch | Central | 4 | 60 | Beside the aortic
arch |
| 2 | Female | 71 | 12 |
Well-differentiated | 1 | Lower right lung | Peripheral | 4 | 48 | Economic reasons |
| 3 | Male | 68 | 12 |
Well-differentiated | 2 | Upper right lung | Peripheral | 2 | 10 | Multiple pulmonary
metastases |
| | | | | | Upper left lung | Peripheral | 2 | 15 |
| 4 | Female | 75 | 6 |
Poorly-differentiated | 1 | Right pulmonary
hilum | Central | 5 | 80 | Lymph node
metastasis |
| 5 | Male | 85 | 36 |
Well-differentiated | 2 | Right pulmonary
hilum | Central | 6 | 96 | Multiple pulmonary
metastases |
| | | | | | Upper right lung | Peripheral | 2 | 10 | |
| | | | | 2 | Upper left lung | Peripheral | 2 | 15 | |
| | | | | | Upper left lung | Peripheral | 3 | 27 | |
| | | | | 1 | Lower left lung | Peripheral | 3 | 25 | |
| 6 | Male | 86 | 45 |
Well-differentiated | 3 | Upper left lung | Peripheral | 1 | 2 | Multiple pulmonary
metastases |
| | | | | | Upper left lung | Peripheral | 1 | 2 |
| | | | | | Upper left lung | Peripheral | 2 | 8 |
The CT-guided brachytherapy procedure was carried
out as previously described (9).
Prior to the procedure, a treatment plan was prepared for each
patient using a computerized treatment planning system (TPS;
Prowess Panther, Prowess Inc., Concord, CA, USA) based on the CT
images of the patients. The TPS generated a dose-volume histogram
(DVH) and isodose curves of various percentages, and calculated the
position (coordinates) of the brachytherapy applicator, dose and
number of implanted seeds (Table
II). Under local anesthesia, interstitial needles (Medical
Device Technologies, Inc., Gainesville, FL, USA) were inserted into
the tumor at ~0.5 cm intervals. Each 125I seed was
implanted within an average of 1 cm3 of the tumor. The
average planning target volume (PTV) of the 13 metastatic lesions
was 32.1±30.1 cm3, and the average number of implanted
seeds was 28±14.4 seeds. The average dose of the target area was
157.3±11.6 Gy, with a median dose of 152.4 Gy. The dose covered 90%
of the volume (D90), 88.4±7.3 Gy, and the volume that
received >90% of the prescribed dose (V90) was
31.5±29.5 cm3. Once the implant was completed, a CT scan
was performed to verify the position and intensity of the
125I seeds according to TPS. The six-month
post-procedural follow-up was complemented by CT examination. The
follow-up was completed in May 2010 (Table III).
 | Table IISeed implant characteristics. |
Table II
Seed implant characteristics.
| Patient | Implantation time
(yyyy/mm) | Location | PTV,
cm3 | Planned number | Number implanted | Average dose,
cGy | D90,
cGy | V90,
cm3 | Complication |
|---|
| 1 | 2002.11 | Upper left lung
beside aortic arch | 64.6 | 35 | 35 | 14921.4 | 8880 | 63.3 | Pneumothorax |
| 2 | 2004.3 | Lower right lung | 53.1 | 34 | 35 | 16635.1 | 9200 | 50.8 | |
| 3 | 2005.3 | Upper right lung | 11.9 | 12 | 15 | 16923.3 | 9760 | 11.8 | |
| | Upper left lung | 17.1 | 15 | 20 | 16390.9 | 9280 | 16.7 | |
| 4 | 2005.7 | Right pulmonary
hilum | 76.9 | 43 | 50 | 17659.8 | 9920 | 76.1 | Hemoptysis |
| 5 | 2007.8 | Right pulmonary
hilum | 93.1 | 43 | 50 | 16532.1 | 8960 | 90.4 | Pneumothorax |
| | Upper right lung | 10.8 | 8 | 10 | 15238.4 | 8206 | 10.3 | |
| | Upper left lung | 16.8 | 13 | 13 | 16253.9 | 8240 | 16.1 | |
| | Upper left lung | 32.1 | 28 | 27 | 15231.7 | 8170 | 31.8 | |
| | Lower left lung | 30.9 | 25 | 25 | 15085.5 | 7920 | 30.2 | |
| 6 | 2008.5 | Upper left lung | 1.7 | 4 | 4 | 13822.4 | 8320 | 1.6 | |
| | Upper left
lung | 1.7 | 4 | 4 | 13822.4 | 8320 | 1.6 | |
| | Upper left
lung | 8.4 | 10 | 10 | 16003.0 | 9920 | 8.4 | |
 | Table IIIFollow-up and outcome of the
disease. |
Table III
Follow-up and outcome of the
disease.
| Patient | Number of
lesions | Location | Lesion status at
six-month follow-up | Date of death
(month/year) | Survival time,
months |
|---|
| 1 | 1 | Adjacent to aortic
arch | Decreased by
50% | 04/2005 | 29 |
| 2 | 1 | Lower right
lung | Decreased by
50% | 04/2008 | 49 |
| 3 | 2 | Upper right
lung | Disappeared | 08/2009 | 53 |
| | Upper left
lung | Disappeared | | |
| 4 | 1 | Right pulmonary
hilum | Enlarged | 03/2006 | 8 |
| 5 | 2 | Right pulmonary
hilum | Decreased by
50% | 05/2010 | 33 |
| | Upper right
lung | Decreased by
50% | | |
| 2 | Upper left
lung | Decreased by
50% | | 33 |
| | Upper left
lung | Decreased by
50% | | |
| 1 | Lower left
lung | Decreased by
50% | | |
| 6 | 3 | Upper left
lung | Disappeared | 05/2010 | 24 |
| | Upper left
lung | Disappeared | | |
| | Upper left
lung | Decreased by
50% | | |
Results
The brachytherapy catheters and 125I
seeds were satisfactorily placed in all patients. Of the six
patients, three developed pneumothorax during the procedure. These
patients subsequently received chest-tube drainage as a curative
treatment for the pneumothorax, and two to three days following
this, the condition was resolved. Hemoptysis (~20 ml) was observed
in one patient; this ceased two days following the oral
administration of carbazochrome salicylate (5 mg three times a day,
for three days).
Compared with the CT images captured prior to the
procedure, the CT images obtained at the six-month follow-up
revealed that four masses had been completely removed by the
treatment and eight masses had been reduced in size by >50%. The
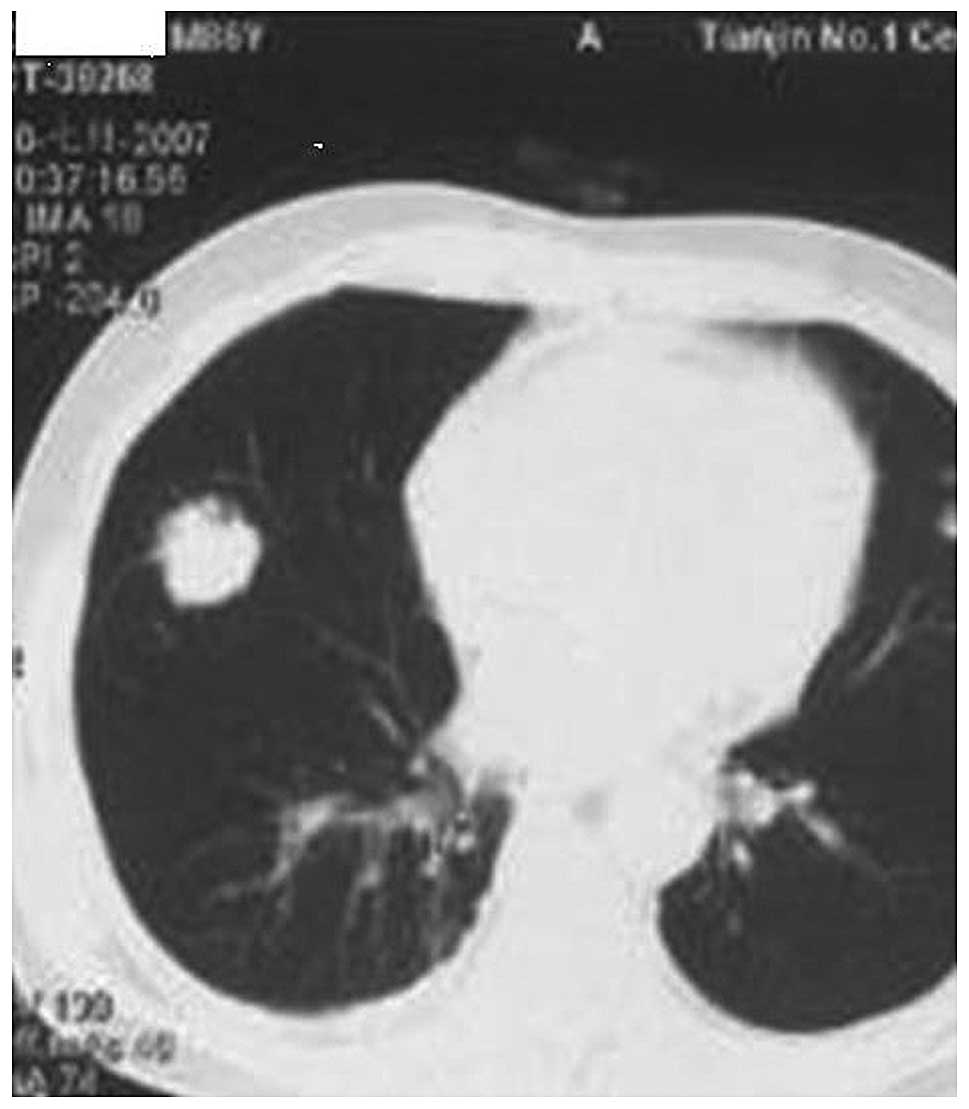
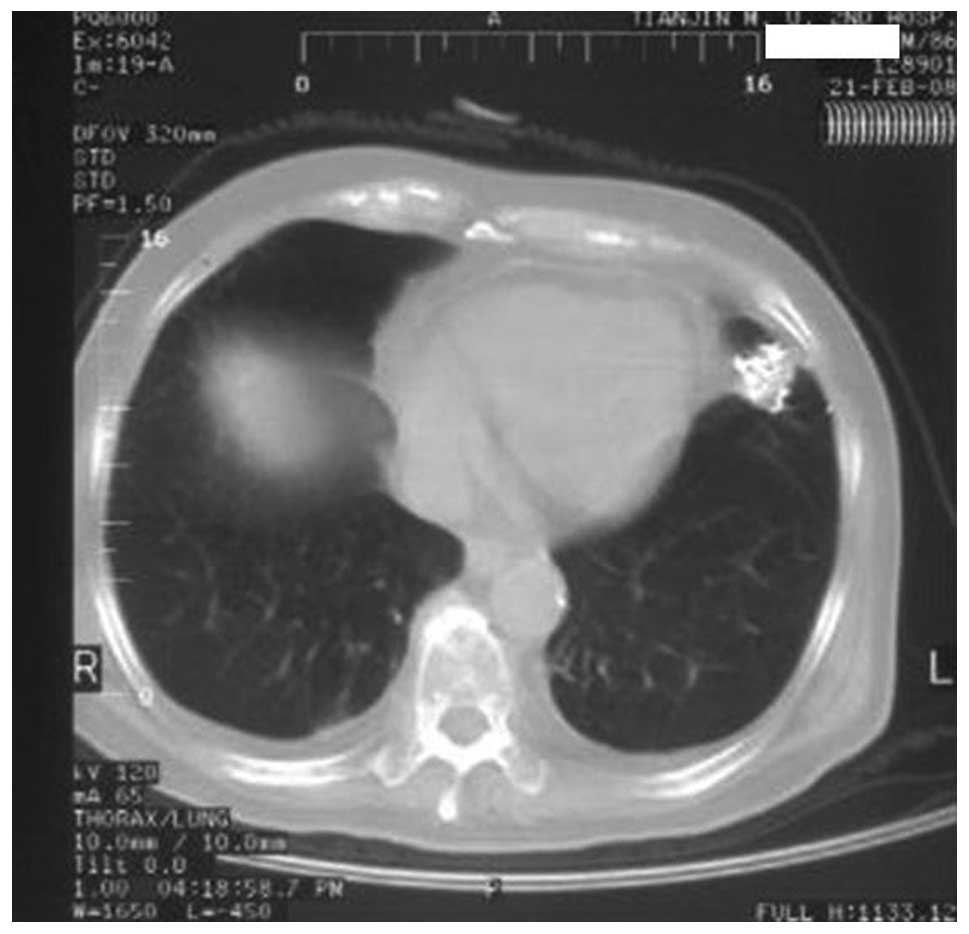
CT images of one 86-year-old male patient prior to and following
the procedure are shown in Figs.
1–5. Overall, only one mass was
enlarged, indicating that the local control rate was 92.3% (12/13).
None of the patients developed radioactive pneumonia or reduction
in peripheral-blood granulocytes. Following a median follow-up
period of 31 months (32.7±16.6 months; range, 8–53 months), no
local recurrence was observed for the 12 metastatic lesions. Of the
two patients with poorly-differentiated adenocarcinoma, one
suffered from pulmonary metastases, complicated by right
supraclavicular lymph node metastasis six months following radical
resection. One of these patients succumbed to the disease eight
months following brachytherapy and the other succumbed 29 months
following brachytherapy. The four patients with well-differentiated
adenocarcinoma succumbed to the disease 49, 53, 33 and 24 months
following brachytherapy. The mean survival time was 32.7 months and
median survival time was 31 months.
Discussion
The present study demonstrates that percutaneous
CTRISI is a feasible and promising, minimally invasive modality for
controlling the growth of pulmonary metastases following CRC
resection, particularly in the 12 months following surgery.
Although one patient experienced hemoptysis and three patients
suffered pneumothorax, these side-effects could be controlled,
indicating that CTRISI remains a safe treatment method in this
patient population.
The target area may tolerate sustained, closer
high-dose irradiation through the conformal implantation of seeds
into the interior of the tumor, overcoming target volume motion, so
that the local control rate can be elevated. Independent or
separate entity radiotherapy studies have demonstrated that local
control of the tumor body is likely to be markedly intensified
following irradiation with a bioeffect dose of 90–100 Gy.
Martínez-Monge et al used CT-guided permanent brachytherapy
to treat seven patients with early-stage T1N0M0 NSCLC. The median
dose was 144 Gy. This study found that one patient developed a
focal pneumonitis three months following the treatment, and no
patients developed local or regional failure within a 13 month
follow-up period (7). In the
present study, the mean tolerance dose of PTV (gross tumor volume +
0.5 cm) was 157.3 Gy, with a median dose of 152.4 Gy. The lesions
were irradiated with a dose of approximately twice the PD and a
local control rate of 92.3% was achieved, which is similar to the
effective rate of 93.8% (10) for
seed implantation for pulmonary metastases and 87% (11) for 3D conformal external beam
radiotherapy, as previously reported in the literature,
demonstrating good therapeutic effects. This may be associated with
the high-dose irradiation of the small target area as well as with
the sensitivity of well-differentiated adenocarcinoma to γ-ray
radiation.
The seeds can be accurately and evenly implanted
into the target area under CT guidance. Patients with the target
area located at the tumor center should undergo stereotactic
puncture following contrast-enhanced CT scanning of blood vessels.
The puncture needlepoint can be close to the heart and great
vessels without injury to them. In the present study,
D90 88.4 Gy (>PD) revealed a uniform and reasonable
dose distribution.
The 125I-ray seed ray decays in an
exponential manner with distance, which can reduce damage to
tissues around the target area. The occurrence rate of radioactive
pneumonia has been reported to be 44% (12) when the average therapeutic dose of
the 3D conformal radiotherapy is 60 Gy. In the present study, no
radioactive pneumonia was observed at PD 80 Gy, suggesting that
seed implantation causes less damage as a result of radioactivity
than conventional radiotherapy. Puncture-induced pneumothorax and
hemoptysis can be observed on CT imaging and can be treated using
conventional methods.
In conclusion, CTRISI is a safe and minimally
invasive treatment modality for metastases from CRC that may aid in
prolonging the survival rate in patients who cannot undergo
pulmonary resection for metastases. While these results are
promising, future studies including an increased number of cases,
are required to gain further information with regard to CTRISI for
the treatment of pulmonary metastases following CRC resection.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, et
al: Cancer incidence and mortality worldwide: sources, methods and
major patterns in GLOBOCAN 2012. Int J Cancer. Sep 13–2014.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Talton BM, Constable WC and Kersh CR:
Curative radiotherapy in non-small cell carcinoma of the lung. Int
J Radiat Oncol Biol Phys. 19:15–21. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rama N, Monteiro A, Bernardo JE, Eugénio L
and Antunes MJ: Lung metastases from colorectal cancer: surgical
resection and prognostic factors. Eur J Cardiothoracic Surg.
35:444–449. 2009. View Article : Google Scholar
|
|
4
|
Talton BM, Constable WC and Kersh CR:
Curative radiotherapy in non-small cell carcinoma of the lung. Int
J Radiat Oncol Biol Phys. 19:15–21. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belderbos JS, Heemsbergen WD, De Jaeger K,
Baas P and Lebesque JV: Final results of a Phase I/II dose
escalation trial in non-small-cell lung cancer using
three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol
Phys. 66:126–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cha CM, Potters L, Ashley R, et al:
Isotope selection for patients undergoing prostate brachytherapy.
Int J Radiat Oncol Biol Phys. 45:391–395. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martínez-Monge R, Pagola M, Vivas I and
López-Picazo JM: CT-guided permanent brachytherapy for patients
with medically inoperable early-stage non-small cell lung cancer
(NSCLC). Lung Cancer. 61:209–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang FJ, Li CX, Wu PH, et al: CT guided
radioactive 125I seed implantation in treating localized
advanced pulmonary carcinoma. Zhonghua Yi Xue Za Zhi. 87:3272–3275.
2007.(In Chinese).
|
|
9
|
Martínez-Monge R, Garrán C, Vivas I and
López-Picazo JM: Percutaneous CT-guided 103Pd implantation for the
medically inoperable patient with T1N0M0 non-small cell lung
cancer: a case report. Brachytherapy. 3:179–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bradley J, Graham MV, Winter K, et al:
Toxicity and outcome results of RTOG 9311: a phase I-II
dose-escalation study using three-dimensional conformal
radiotherapy in patients with inoperable non-small-cell lung
carcinoma. Int J Radiat Oncol Biol Phys. 61:318–328. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Timmerman R, McGarry R, Yiannoutsos C, et
al: Excessive toxicity when treating central tumors in a phase II
study of stereotactic body radiation therapy for medically
inoperable early-stage lung cancer. J Clin Oncol. 24:4833–4839.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baumann P, Nyman J, Hoyer M, et al:
Outcome in a prospective phase II trial of medically inoperable
stage I non-small cell lung cancer patients treated with
stereotactic body radiotherapy. J Clin Oncol. 27:3290–3296. 2009.
View Article : Google Scholar : PubMed/NCBI
|