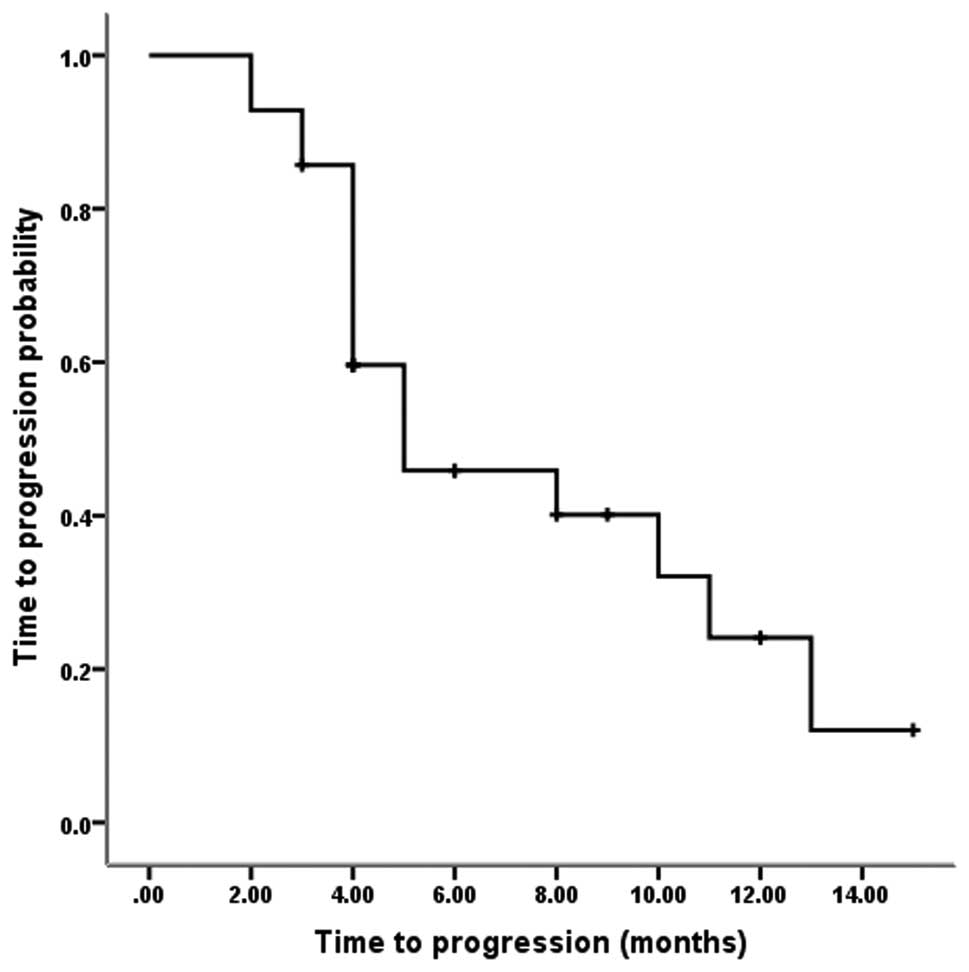
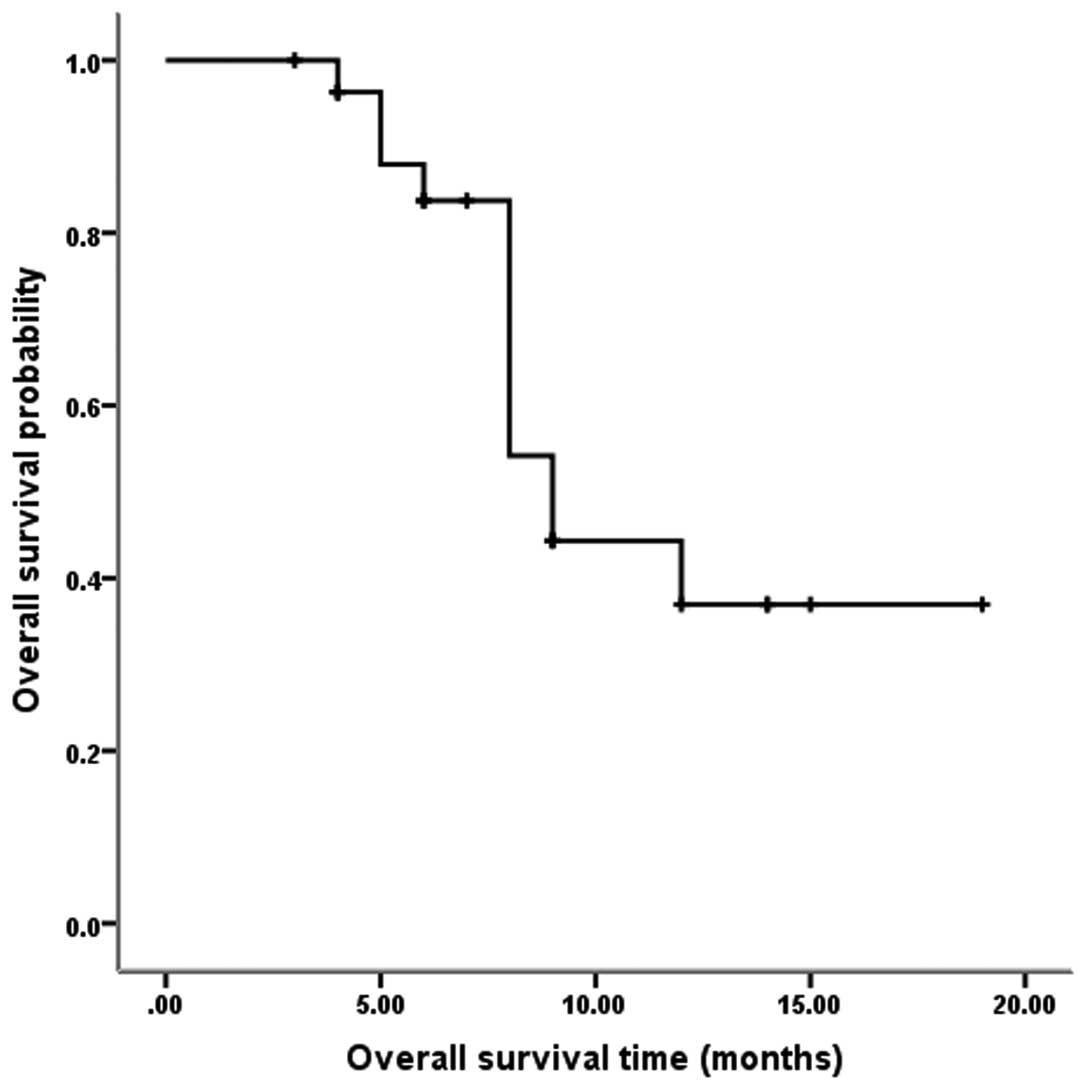
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Zhan YQ, Sun XW, Li W, et al: Multivariate
prognostic analysis in gastric carcinoma patients after radical
operation. Ai Zheng. 24:596–9. 2005.(In Chinese). PubMed/NCBI
|
|
3
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 10(Suppl 3): 49–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: a
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JH, Son SY, Lee CM, Ahn SH, Park DJ
and Kim HH: Factors predicting peritoneal recurrence in advanced
gastric cancer: implication for adjuvant intraperitoneal
chemotherapy. Gastric Cancer. 17:529–536. 2014. View Article : Google Scholar
|
|
6
|
Bijelic L and Sugarbaker PH: The role of
intraperitoneal chemotherapy in the treatment of patients with
advanced gastric cancer. Ann Ital Chir. 83:224–231. 2012.PubMed/NCBI
|
|
7
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
et al: V325 Study Group: Phase III study of docetaxel and cisplatin
plus fluorouracil compared with cisplatin and fluorouracil as
first-line therapy for advanced gastric cancer: a report of the
V325 Study Group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen XL, Chen XZ, Yang C, et al:
Docetaxel, cisplatin and fluorouracil (DCF) regimen compared with
non-taxane-containing palliative chemotherapy for gastric
carcinoma: a systematic review and meta-analysis. PLoS One.
8:e603202013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marchettini P, Stuart OA, Mohamed F, Yoo D
and Sugarbaker PH: Docetaxel: pharmacokinetics and tissue levels
after intraperitoneal and intravenous administration in a rat
model. Cancer Chemother Pharmacol. 49:499–503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fushida S, Kinoshita J, Yagi Y, et al:
Dual anti-cancer effects of weekly intraperitoneal docetaxel in
treatment of advanced gastric cancer patients with peritoneal
carcinomatosis: A feasibility and pharmacokinetic study. Oncol Rep.
19:1305–1310. 2008.PubMed/NCBI
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
12
|
Lai CL, Tsai CM, Chiu CH, Wang GS, Su WJ,
Chen YM and Perng RP: Phase II randomized trial of tri-weekly
versus days 1 and 8 weekly docetaxel as a second-line treatment of
advanced non-small cell lung cancer. Jpn J Clin Oncol. 35:700–706.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kunitoh H, Watanabe K, Onoshi T, Furuse K,
Niitani H and Taguchi T: Phase II trial of docetaxel in previously
untreated advanced non-small-cell lung cancer: a Japanese
cooperative study. J Clin Oncol. 14:1649–1655. 1996.PubMed/NCBI
|
|
14
|
Nakamura Y, Kunitoh H, Kubota K, Sekine I,
Yamamoto N, Tamura T, et al: Retrospective analysis of safety and
efficacy of low-dose docetaxel 60 mg/m2 in advanced
non-small cell lung cancer patients previously treated with
platinum-based chemotherapy. Am J Clin Oncol. 26:459–464. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin Q, Gao XS, Qiao XY, et al: Phase I
trial of escalating-dose cisplatin with 5-fluorouracil and
concurrent radiotherapy in Chinese patients with esophageal cancer.
Acta Med Okayama. 62:37–44. 2008.PubMed/NCBI
|
|
16
|
Lin Q, Liu YE, Chang CL, et al: Phase I
trial of dose escalation of capecitabine combined with fixed
docetaxel in previously treated patients with non-small cell lung
cancer. Chin-Ger J Clin Oncol. 11:6–10. 2012. View Article : Google Scholar
|
|
17
|
Wu Y and Wu W: Suggestions of improving
diagnostic level for gastric cancer early stage in China. China
Cancer. 18:738–740. 2009.
|
|
18
|
Kim HS, Kim HJ, Kim SY, et al: Second-line
chemotherapy versus supportive cancer treatment in advanced gastric
cancer: a meta-analysis. Ann Oncol. 24:2850–2854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lordick F, Lorenzen S, Yamada Y and Ilson
D: Optimal chemotherapy for advanced gastric cancer: is there a
global consensus? Gastric Cancer. 17:213–225. 2014. View Article : Google Scholar
|
|
20
|
GASTRIC (Global Advanced/Adjuvant Stomach
Tumor Research International Collaboration) Group. Oba K, Paoletti
X, Bang YJ, et al: Role of chemotherapy for advanced/recurrent
gastric cancer: an individual-patient-data meta-analysis. Eur J
Cancer. 49:1565–1577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coccolini F, Cotte E, Glehen O, et al:
Intraperitoneal chemotherapy in advanced gastric cancer.
Meta-analysis of randomized trials. Eur J Surg Oncol. 40:12–26.
2014. View Article : Google Scholar
|
|
22
|
Shi C, Yang B, Chen Q, Yang J and Fan N:
Retrospective analysis of adjuvant intraperitoneal chemotherapy
effect prognosis of resectable gastric cancer. Oncology.
80:289–295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue SL, Su HF, Hu XQ, Deng X, Hu ML and
Xie CY: Adjuvant combined systemic chemotherapy and intraperitoneal
chemotherapy for locally advanced gastric cancer. Oncol Lett.
4:1309–1314. 2012.PubMed/NCBI
|
|
24
|
Fushida S, Kinoshita J, Kaji M, et al:
Society for Study of Peritoneal Carcinomatosis in Gastric Cancer:
Phase I/II study of intraperitoneal docetaxel plus S-1 for the
gastric cancer patients with peritoneal carcinomatosis. Cancer
Chemother Pharmacol. 71:1265–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishigami H, Kitayama J, Kaisaki S, et al:
Phase II study of weekly intravenous and intraperitoneal paclitaxel
combined with S-1 for advanced gastric cancer with peritoneal
metastasis. Ann Oncol. 21:67–70. 2010. View Article : Google Scholar
|
|
26
|
Fujiwara Y, Takiguchi S, Nakajima K, et
al: Intraperitoneal docetaxel combined with S-1 for advanced
gastric cancer with peritoneal dissemination. J Surg Oncol.
105:38–42. 2012. View Article : Google Scholar
|
|
27
|
Fujiwara Y, Nishida T, Takiguchi S, et al:
Feasibility study of S-1 and intraperitoneal docetaxel combination
chemotherapy for gastric cancer with peritoneal dissemination.
Anticancer Res. 30:1335–1339. 2010.PubMed/NCBI
|
|
28
|
Matharu G, Tucker O and Alderson D:
Systematic review of intraperitoneal chemotherapy for gastric
cancer. Br J Surg. 98:1225–1235. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Di Lauro L, Vici P, Belli F, et al:
Docetaxel, oxaliplatin, and capecitabine combination chemotherapy
for metastatic gastric cancer. Gastric Cancer. 17:718–724. 2014.
View Article : Google Scholar
|
|
30
|
Al-Batran SE, Hartmann JT, Hofheinz R, et
al: Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel
(FLOT) for patients with metastatic adenocarcinoma of the stomach
or esophagogastric junction: a phase II trial of the
Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol.
19:1882–1887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Cutsem E, Boni C, Tabernero J, et al:
Randomized phase II study (GATE study) of docetaxel plus
oxaliplatin with or without fluorouracil or capecitabine in
metastatic or locally recurrent gastric cancer. J Clin Oncol.
29(Suppl 15): 40182011.
|