Introduction
According to global cancer statistics, gastric
cancer is the fourth most common cancer and exhibits the second
highest mortality rate. China possesses a high incidence of gastric
cancer (1). Due to its atypical
symptoms, gastric cancer is often locally advanced or has undergone
distant metastasis at the time of clinical diagnosis, leading to a
poor prognosis. Even with radical surgery, the five-year survival
rates for stage III and IV gastric cancers are only 28.0 and 18.4%,
respectively (2). Although there is
no globally accepted standard regimen for the treatment of gastric
cancer, systemic chemotherapy is superior to the best supportive
care (3,4).
Studies have revealed that the local recurrence rate
for locally advanced gastric cancer remains as high as 30.4%, even
subsequent to D2 radical surgery. The peritoneum is the first site
of recurrence, accounting for up to 58.8% of recurrences.
Therefore, it has been recommended that, for postoperative adjuvant
therapy, intraperitoneal (IP) chemotherapy should be considered
(5). The extent of the local
regional spread is an important component of the natural history of
gastric cancer. Thus, even if there is no clinical evidence of
peritoneal dissemination, strong reasons for local-regional
treatment remain. IP chemotherapy has become increasingly popular
for use in clinical practice and has achieved a certain amount of
success. However, no standards exist regarding drug choices and
administration approaches (6).
V325, the phase III randomized controlled trial,
confirmed the significance of docetaxel in treating advanced
gastric cancer, and the overall response rate (ORR), time to
progression (TTP) and overall survival (OS) of the docetaxel,
cisplatin (DDP) and fluorouracil (FU) (DCF) regimen were
significantly improved compared to the DDP and FU (CF) regimen
(7). A previous meta-analysis
confirmed the superiority of docetaxel in treating gastric cancer
(8). Notably, the DCF regimen has
been reported to exhibit strong toxicity, with 69% of patients
experiencing treatment-associated grade III/IV adverse reactions,
and the rate of grade III/IV neutropenia reaching 82%, while that
of febrile and infectious neutropenia was 29%. Such serious adverse
reactions affect the application of the DCF regimen. In addition to
increasing the IP concentrations of chemotherapy drugs, IP
chemotherapy can also reduce the systemic toxicity (5). Additionally, the unique
pharmacokinetic characteristics of docetaxel are suitable for
application via IP perfusion (9,10).
Theoretically, a DCF IP and intravenous (IV) dual chemotherapy
regimen should be able to achieve a good treatment effect and
reduce systemic toxicity.
Studies using IP and IV dual chemotherapy as the
sole means of treatment for advanced gastric cancer are rare. To
the best of our knowledge, the present study is the first study to
report DCF dual IV and IP chemotherapy.
Materials and methods
Eligibility
The present study enrolled patients from North China
Petroleum Bureau General Hospital of Hebei Medical University
(Renqui, China) in the III–IV clinical stages of disease with
unresectable gastric cancer that was pathologically or
cytologically confirmed, locally-advanced, metastasized or
recurrent, and who possessed at least one evaluable lesion. The
inclusion criteria were as follows: 18–75 years old; Karnofsky
performance status (KPS) score ≥60 and expected survival of at
least three months; complete recovery from the toxicity of previous
treatment; and a period of at least four weeks since the previous
treatment. The bone marrow conditions were as follows: White blood
cell (WBC) count, ≥4.0 × 109/l; neutrophil count, ≥2.0 ×
109/l; platelet (PLT) count, ≥100 ×109/l; and
hemoglobin level, ≥100 g/l. The required blood creatinine level was
≤ 135 μmol/l, the alkaline phosphatase level was required to be
<1.5 times the upper limit of the normal level and the required
serum bilirubin level was ≤26 μmol/l. Other inclusion criteria
consisted of no significant gastrointestinal bleeding, normal heart
and lung function, no significant systemic infection and no other
serious visceral disease, no previous application of docetaxel,
good dependability, willing and able to comply with the regimen
during the study period and provided written informed consent.
Exclusion criteria
The following patients were excluded: Pregnant or
lactating women; patients with no consciousness or with
uncontrollable central nervous system metastasis and uncontrollable
seizures or who lost consciousness or judgment due to psychosis;
patients who had received other chemotherapy drugs or radiation
therapy over the past four weeks; patients with organ
transplantation; and patients with long-term use of
immunosuppressive agents and adrenocortical hormones.
Withdrawal criteria
The withdrawal criteria consisted of patients who
asked to withdraw; patients who had drug allergies or experienced
serious adverse reactions or events in the trial and; those who
experienced disease progression in the trial.
Assessment prior to treatment
Medical history, physical examination, KPS score
evaluation, a routine blood test, liver function and kidney
function tests, chest and abdominal computed tomography (CT) and
electrocardiography (ECG) were completed one week prior to
treatment.
Trial design
The present study was a prospective, open-label,
single-arm feasibility study. The main endpoint of this study was
to evaluate the tolerability of dual IP and IV DCF chemotherapy in
advanced gastric cancer. The secondary endpoint was to obtain the
ORR, TTP and OS of the chemotherapy.
Ethics
The procedures were approved by the Ethics Committee
of Hebei Medical University (Shijiazhuang, Hebei, China) and were
performed in accordance with the ethics standards of human
experimentation and with the Helsinki Declaration of 1975, as
revised in 2000. The patients provided written informed
consent.
Chemotherapy
IP chemotherapy
A peritoneal catheter was implanted and, in the
presence of ascites, drainage was performed to remove as much of
the ascites as possible. A total of 1,000 ml normal saline, 10 mg
dexamethasone and 20 ml 5% lidocaine were injected through the
catheter, and the chemotherapy drugs were then injected. Based on
the peritoneal conditions of the patient, 1,000–1,500 ml normal
saline was injected again. All perfusion liquids were at room
temperature. Following the perfusion, the patients were asked to
sequentially take right lateral decubitus, left lateral decubitus,
prone and supine positions, each for 15 minutes, to allow the drugs
to be distributed in the abdominal cavity as uniformly as
possible.
First dose level (Level I)
Level I consisted of the full-dose DCF regimen:
Docetaxel at 40 mg/m2 via IP perfusion on day one and 35
mg/m2 via IV infusion on day eight; DDP (DDP) at 75
mg/m2 via IP perfusion on day two; FU (FU) at 750
mg/m2 via continuous IV infusion once per day for five
days.
Second dose level (Level II)
The Level II DCF regimen consisted of a reduced
dosage, as the first six patients experienced intolerable adverse
reactions. It was reasoned that the full dose of the DCF regimen
resulted in excessive toxicity. Thus, from the seventh patient
onward, the chemotherapy dosages of all three drugs were reduced by
20%. This provided the doses of docetaxel at 30 mg/m2
via IP perfusion on day one and 30 mg/m2 via IV infusion
on day eight; DDP at 60 mg/m2 via IP perfusion on day
two; and FU at 600 mg/m2 via continuous IV infusion once
per day for five days.
Prophylactic anti-allergy treatment was applied with
10 mg of dexamethasone twice per day one day prior to treatment and
on days one and two, for three consecutive days. The treatment was
repeated every 28 days until disease progression or the occurrence
of intolerable toxicity. The maximum number of treatment cycles was
six. During the treatment, all patients were given 5-HT3 receptor
antagonists for antiemetic prophylactic treatment. To ensure the
continuity of chemotherapy, recombinant human granulocyte
colony-stimulating factor was administered for supportive treatment
when the WBC count was <4.0 × 109/l or the absolute
neutrophil count (ANC) was <2.0 × 109/l, and
interleukin-11 treatment was applied when PLT was <75 ×
109/l. Appropriate supportive treatments, including oral
drug administration for the enhancement of WBCs and PLTs, anemia
correction and IV rehydration, were applied when indicated.
Evaluation standards
Assessment of adverse reactions was based on the
Common Terminology Criteria for Adverse Events v3.0. RECIST1.1 was
used for the evaluation of short-term efficacy (11). Efficacy was evaluated for patients
who completed two or more cycles of chemotherapy. The time point
for efficacy evaluation was the eighth week after the initiation of
the treatment. Efficacy evaluation was divided into complete
response (CR), partial response (PR), stable disease (SD) and
progressive disease (PD). The response rate (R) was calculated as
CR + PR. The main imaging evidence for the evaluation was from
CT/magnetic resonance imaging (MRI), and superficial lymph nodes
were examined by B-ultrasonography.
Follow-up
Following completion of the treatment, follow-up
studies were conducted once every two months in the first six
months and then once every three months, subsequently. Each
follow-up study included medical history, physical examination,
routine blood tests, comprehensive biochemical examinations, chest
and abdominal CT and superficial lymph node B-ultrasonography. All
patients were followed up via re-examinations in the outpatient
clinic and by telephone, and all patients were followed up until
mortality due to any reason or loss of follow-up.
Statistical analysis
SPSS 19.0 software (IBM, Armonk, NY, USA) was used
for data analysis, and the Kaplan-Meier method was used to
calculate the TTP and OS of the patients.
Results
Patient characteristics
Between July 2010 and June 2013, a total of 32
advanced gastric cancer patients who all possessed unresectable
lesions were enrolled in the present study. In total, 59.4% (19/32)
of patients possessed ascites and 53.1% (17/32) possessed visceral
metastases. There were 19 males and 13 females, with 17 cases
receiving treatment for the first time, eight cases being retreated
and seven cases being treated for recurrence or metastasis. The age
range of the patients was 39–75 years, with a median of 65 years.
The median KPS score was 70 (range, 60–90), with seven cases
receiving scores of 60, 18 cases receiving scores of 70, six cases
receiving scores of 80 and one case receiving a score of 90
(Table I). The body surface area
ranged between 1.52–1.89 m2, with a median of 1.74
m2. There were six stage IIIB cases and 26 stage IV
cases. In total, 28 cases exhibited evaluable efficacy, and all 32
cases had evaluable adverse reactions.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Value |
|---|
| Gender, n |
| Male | 19 |
| Female | 13 |
| Age, years |
| Range | 39–75 |
| Median | 65 |
| Stage, n |
| IIIB | 6 |
| IV | 26 |
| KPS |
| Range | 60–90 |
| Median | 70 |
As of 10 December 2013, there was loss of follow-up
in two cases, resulting in a follow-up rate of 93.8%.
Completion of treatment
The 32 patients completed a total of 113 cycles of
chemotherapy, with a median of four chemotherapy cycles (range,
1–6). Among these patients, four cases completed one cycle, two
cases completed two cycles, six cases completed three cycles, 15
cases completed four cycles, one case completed five cycles and
four cases completed six cycles.
Adverse reactions from Level I
Table II describes
the hematological toxicity. The six cases treated using Level I
developed severe bone marrow suppression. In particular, the
incidence rates of grade III/IV neutropenia and leukopenia were
83.3%, while the incidence rate of febrile leukopenia was 33.3%.
The rates of grade III/IV anemia and thrombocytopenia were 33.3%.
In addition, this group also experienced severe nonhematological
toxicity, as shown in Table III.
All six patients experienced abdominal pain, and grade III/IV pain
was present in 66.7% of cases. Due to their abdominal pain, three
patients received opioid analgesics, two of these three cases only
completed one cycle of chemotherapy prior to requesting to withdraw
from the trial as the pain was not tolerable. The rates for grade
III/IV decreased appetite, fatigue, nausea and vomiting were 66.7,
50, 50 and 33.3%, respectively. Therefore, the full dose of the DCF
regimen was considered to be too strong for Asian populations.
Based on studies from East Asia (12–14)
and the results of previous chemotherapy dose studies (15,16), a
DCF regimen with a 20% dosage reduction was applied in the
subsequent treatment.
 | Table IIHematological toxicities. |
Table II
Hematological toxicities.
| Level I (6
cases) | Level II (26
cases) | Total (32
cases) |
|---|
|
|
|
|
|---|
| Cases, n | Cases, % | Cases, n | Cases, % | Cases, n | Cases, % |
|---|
| Leukopenia |
| I–II | 1 | 16.7 | 12 | 46.2 | 13 | 40.6 |
| III–IV | 5 | 83.3 | 14 | 53.8 | 19 | 59.4 |
| Neutropenia |
| I–II | 1 | 16.7 | 10 | 38.5 | 11 | 34.4 |
| III–IV | 5 | 83.3 | 16 | 61.5 | 21 | 65.6 |
| Febrile
neutropenia | 2 | 33.3 | 5 | 19.2 | 7 | 21.9 |
| Anaemia |
| I–II | 2 | 33.3 | 12 | 46.2 | 14 | 43.8 |
| III–IV | 2 | 33.3 | 4 | 15.4 | 6 | 18.8 |
|
Thrombocytopenia |
| I–II | 1 | 16.7 | 9 | 34.6 | 10 | 31.3 |
| III–IV | 2 | 33.3 | 3 | 11.5 | 5 | 15.6 |
 | Table IIINon-hematological toxicity. |
Table III
Non-hematological toxicity.
| Level I (6
cases) | Level II (26
cases) | Total (32
cases) |
|---|
|
|
|
|
|---|
| Cases, n | Cases, % | Cases, n | Cases, % | Cases, n | Cases, % |
|---|
| Abdominal pain |
| I–II | 2 | 33.3 | 18 | 69.2 | 20 | 62.5 |
| III–IV | 4 | 66.7 | 8 | 30.8 | 12 | 37.5 |
| Anorexia |
| I–II | 2 | 33.3 | 13 | 50.0 | 15 | 46.9 |
| III–IV | 4 | 66.7 | 3 | 11.5 | 7 | 21.9 |
| Fatigue |
| I–II | 3 | 50.0 | 20 | 76.9 | 23 | 71.9 |
| III–IV | 3 | 50.0 | 4 | 15.4 | 7 | 21.9 |
| Nausea |
| I–II | 3 | 50.0 | 13 | 50.0 | 16 | 50.0 |
| III–IV | 3 | 50.0 | 4 | 15.4 | 7 | 21.9 |
| Vomiting |
| I–II | 4 | 66.7 | 10 | 38.5 | 14 | 43.8 |
| III–IV | 2 | 33.3 | 2 | 7.7 | 4 | 12.5 |
| Diarrhea |
| I–II | 1 | 16.7 | 9 | 34.6 | 10 | 31.3 |
| III–IV | 2 | 33.3 | 3 | 11.5 | 5 | 15.6 |
| Abdominal
distension |
| I–II | 3 | 50.0 | 14 | 53.8 | 17 | 53.1 |
| III | 2 | 33.3 | 4 | 15.4 | 6 | 18.8 |
| Neurosensory |
| I–II | 1 | 16.7 | 4 | 15.4 | 5 | 15.6 |
| III–IV | 0 | 0.0 | 2 | 7.7 | 2 | 6.3 |
Adverse reactions from Level II
Following a 20% reduction in DCF dosages, the
incidence of bone marrow suppression was significantly reduced, and
the rates of grade III/IV neutropenia, leukopenia and febrile
neutropenia were 61.5, 53.8 and 19.2%, respectively. The rates of
grade III/IV anemia and thrombocytopenia were 19.2 and 15.4%,
respectively. Although the incidence rate of abdominal pain
remained at 100%, the rate for severe grade III/IV pain was 30.8%,
and only 7.7% (2/26) of patients terminated the treatment
subsequent to the completion of one cycle of chemotherapy due to
abdominal pain. Overall, sensory neuropathy was not common, with an
incidence of 21.9%, with 6.3% of patients experiencing grade III
sensory neuropathy, and no sensory neuropathy cases at grade
IV.
Short-term efficacy
Among the 32 patients, 28 patients were evaluable.
There were no CR cases, while there were eight PR cases, 18 SD
cases and two PD cases among the 28 patients, providing the RR of
28.6% (8/28).
Survival analysis
The median follow-up time of eight months (range,
3–19 months) was relatively short, and the survival data are not
yet complete. Nevertheless, the preliminary survival data was
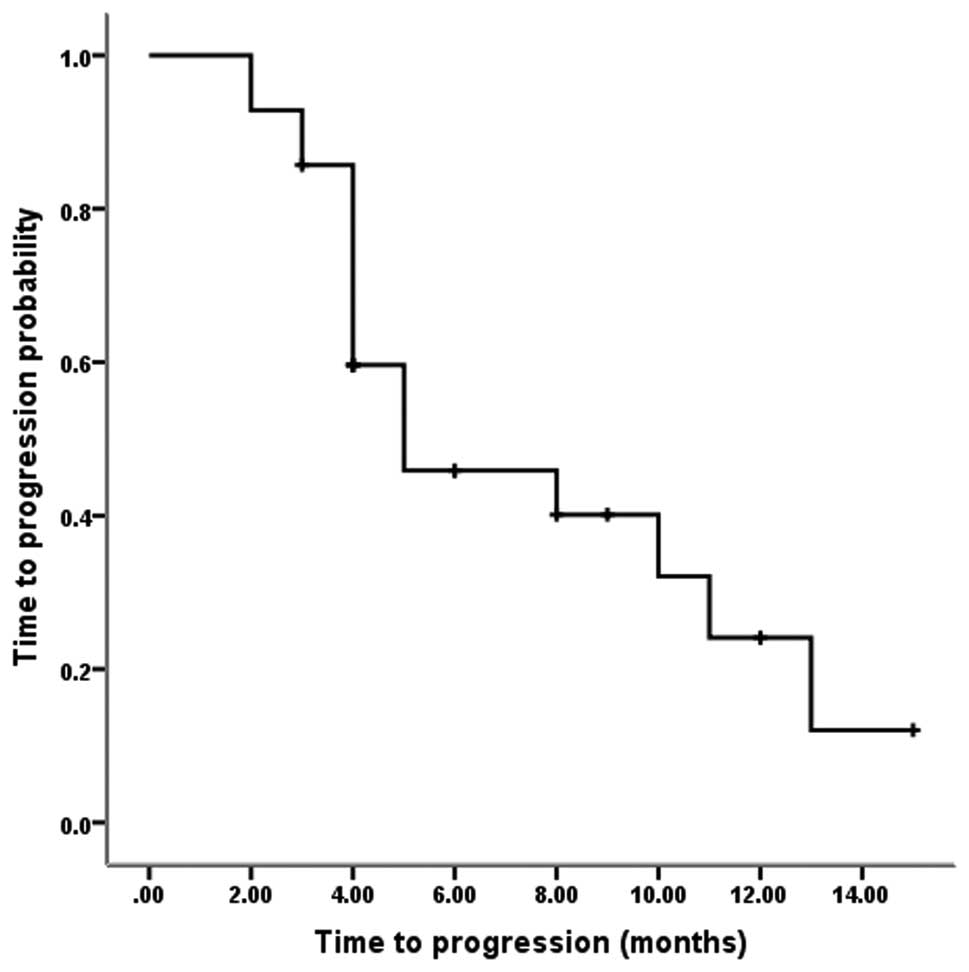
reported. Fig. 1 shows that among
the 28 evaluable patients, the median TTP was five months (95% CI,
1.0–9.0 months), and the one-year progression rate was 24.1%.
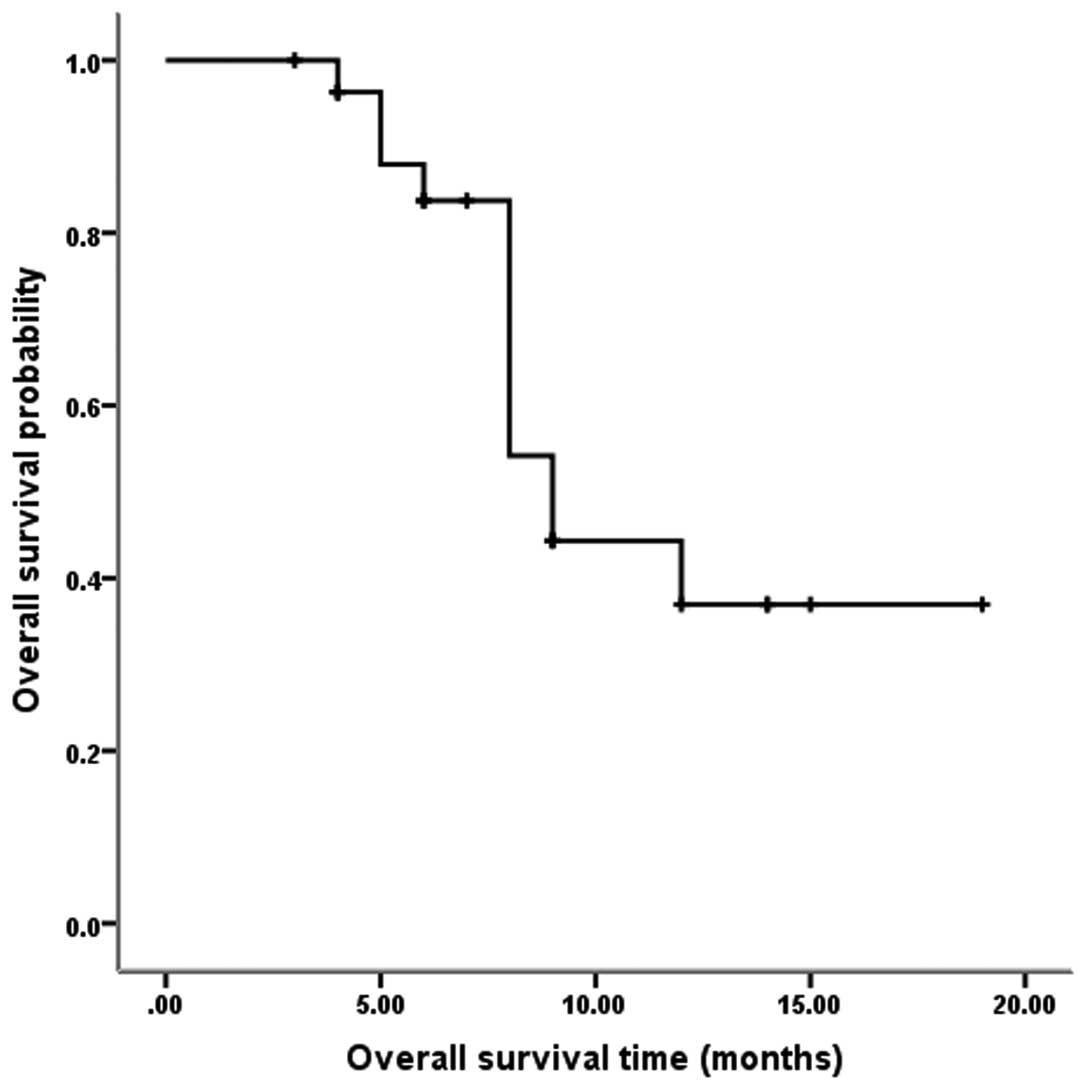
Fig. 2 shows that among the 28
evaluable patients, the median OS was nine months (95% CI, 7.4–10.6
months), and the one-year OS was 36.9%.
Discussion
Chinese gastric cancer patients account for almost
half of all gastric cancer patients worldwide (1). Likely due to cultural background and
economic reasons, a gastric cancer early screening system has not
been implemented in China, and patients are often diagnosed only
subsequent to exhibiting apparent clinical symptoms, when cancer
staging is often advanced and when radical surgical resection is an
option for only a small proportion of patients (17). Chemotherapy plays an important role
in advanced gastric cancer, as it has been demonstrated that
chemotherapy is superior to the best supportive care. In addition,
first- and second-line chemotherapy treatments can improve survival
(18,19). However, no standard chemotherapy
regimen has been established for gastric cancer (19,20).
Gastric cancer primarily spreads through the blood
and peritoneal fluid, with more cases of peritoneal fluid
dissemination than blood spread. Among gastric cancer patients, 40%
succumb to liver metastases, and 53–60% succumb to peritoneal
carcinomatosis (21). Therefore,
establishing a protocol to effectively eliminate peritoneal
carcinomatosis may become one of the key means of improving
treatment efficacy (6).
The use of IP chemotherapy as an adjuvant or
neoadjuvant treatment combined with surgery has become a current
hot topic (21–27). Post-operative adjuvant IP
chemotherapy has shown encouraging efficacy (21–23).
However, for patients with extremely advanced gastric cancer,
neoadjuvant IP chemotherapy combined with surgery requires a high
degree of selectivity. Such treatment not only requires patients to
be in a good physical condition and have no significant visceral
metastasis, but also requires precise, complex and expensive
staging and restaging means, such as one or more laparoscopic
examinations (24,25,26),
which are difficult to achieve under the current Chinese healthcare
resource allocation and economic levels. For patients with advanced
gastric cancer, who are in generally poor condition and often
possess massive ascites and organ metastases, such strong
comprehensive treatment is even more difficult to implement.
V325 performed a well-designed, randomized,
multinational phase III trial. V325 enrolled gastric cancer groups
with relatively poor prognoses, 97% possessed metastases, 81%
possessed metastases involving two or more organs and 57% had
experienced a weight loss of >5%. V325 also excluded all
patients who could potentially receive surgery. Even for such a
group of advanced gastric cancer, docetaxel combined with CF
significantly improved the OS and TTP compared with CF. The OS and
TTP of docetaxel combined with CF were 5.6 and 9.2 months, and
those of CF were 3.7 and 8.6 months, respectively (7). However, DCF resulted in serious
treatment-associated adverse events, with ≤82% neutropenia and 29%
febrile and infectious neutropenia, which significantly limits DCF
application (7). A previous
meta-analysis has confirmed that the two-year OS of the combination
of three drugs, including docetaxel, was significantly increased
compared with regimens without taxanes. However, the same study
also reported that the DCF regimen significantly increased the
incidence of febrile neutropenia, neutropenia, leukopenia and
diarrhea. Therefore, it is crucial to reduce the adverse reactions
of the DCF regimen (8).
IP chemotherapy possesses certain advantages
compared with systemic chemotherapy. IP chemotherapy is capable of
forming high drug concentrations in the peritoneal cavity, reducing
systemic toxicity and forming high concentrations in the portal
vein. Thus, IP chemotherapy exerts a good treatment effect on liver
metastasis, which is one of the main causes of gastric
cancer-associated death (28).
Docetaxel exhibits unique pharmacokinetic characteristics, making
it ideal for IP perfusion. Animal experiments have revealed that
the docetaxel concentration in the peritoneal fluid 90 min after IP
administration of docetaxel is >2500 times the level achieved by
IV administration. The area under the curve (AUC) of peritoneal
fluid was 976 times the AUC of the plasma, and the drug
concentrations in the abdominal wall, stomach and colon tissue
following IP administration were also significantly higher compared
with IV administration (9). In
human trials, the AUC of peritoneal fluid was 515 times greater
compared with the plasma subsequent to IP perfusion of docetaxel at
45 mg/m2 (10).
Additionally, DDP is one of the most common intraperitoneally
administered chemotherapy drugs (6). Therefore, DCF IP and IV dual
chemotherapy can theoretically achieve a good effect and result in
reduced toxicity compared with IV administration.
As demonstrated by the present exploratory
small-sample study, the six patients treated with the first dose
level experienced relatively serious bone marrow suppression, with
incidence rates ≤83.3% for grade III/IV neutropenia and leukopenia
and ≤33.3% for febrile leukopenia. In addition, the incidence rates
of grade III/IV anemia and thrombocytopenia also reached 33.3%, and
the rates of grade III/IV abdominal pain reached 66.7%. Two cases
completed only one cycle of chemotherapy prior to terminating the
treatment. The severe adverse reactions may be due to the
differences in physical conditions between eastern and western
populations. Certain studies from East Asia have reported that
Asians exhibit a lower chemotherapy tolerance compared with western
populations (12–16). Therefore, the DCF dosage was reduced
by 20% and the subsequent study was conducted using this second
dosage level. As a result, bone marrow suppression and
gastrointestinal symptoms were significantly reduced. The incidence
rates of grade III/IV neutropenia, leukopenia and febrile
neutropenia were 61.5, 53.8 and 19.2%, respectively. Following
supportive treatment, the majority of patients adhered to the
treatment. Only two cases terminated the treatment due to abdominal
pain following the completion of one cycle of chemotherapy.
Among the total 32 patients, four patients received
only one cycle of chemotherapy treatment due to adverse reactions.
The median number of chemotherapy cycles completed was four. During
the second-stage study using the modified dosage, the hematological
toxicity of the DCF IP and IV dual chemotherapy regimen was
significantly reduced compared with the V325 study, in which IV
administration of DCF was applied (7). However, abdominal pain increased
significantly, and grade III/IV pain reached an incidence of 30.8%
in the present study. By contrast, the V325 study did not report
significant abdominal pain, suggesting that the abdominal pain in
the present study was directly associated with IP chemotherapy.
Other gastrointestinal symptoms, consisting of anorexia, nausea and
vomiting, were comparable to the rates observed in the V325 study,
with incidences of 11.5, 15.9 and 7.7% in the present study vs. 10,
14 and 10% in the V325 study, whereas the incidence of diarrhea was
reduced in the present study (11.5 vs. 19%).
The incidence of abdominal pain in the present study
was significantly higher compared with other studies of docetaxel
IP perfusion. One of the studies reported a rate of 18.5%, with
pain at grade II or less (24), and
three other studies reported that IP treatment did not cause
significant pain (25–27). There are three possible reasons for
the abdominal pain in the present study. First, the two
chemotherapy drugs, docetaxel and DDP, were applied in perfusion,
thus increasing abdominal irritation and pain. Secondly, in the IP
approach, 2,000–2,500 ml of normal saline at room temperature was
infused so that the drugs could be evenly distributed in the
abdominal cavity, which increased abdominal bloating and pain.
Third, the enrolled patients possessed multi-organ metastasis and
poor physical conditions. These patients exhibited relatively poor
tolerance for the treatment and may already have experienced
symptoms of abdominal pain. However, pain control and other
symptomatic treatments were implemented so that the majority of
patients could adhere to the chemotherapy regimen.
The present study obtained a TTP of five months and
a median survival time (MST) of nine months, which is comparable to
the results of the V325 study and similar to the results of a
previous meta-analysis (8).
However, there appears to be a large gap between the present
survival results and those of other docetaxel peritoneal perfusion
studies (24–26). A study of 18 cases treated with
docetaxel IP chemotherapy plus oral administration of S-1 reported
an MST of 24.6 months. The 18 patients enrolled in this study were
able to receive radical surgery, and 88.9% (16/18) of them received
radical surgery following neoadjuvant therapy (26). The one-year OS rates reported by two
other docetaxel IP chemotherapy studies were 70.4 (24) and 78% (25), which are much higher compared with
the present rate of 36.9%. The two aforementioned studies mainly
enrolled gastric cancer patients who could potentially receive
radical surgery, and the patients experienced good physical
conditions, those with Eastern Cooperative Oncology Group (ECOG)
scores of 0 or 1 (equivalent to KPS scores of 100 and 90) accounted
for 100 and 95% of the patient populations in the two studies. The
organ metastasis rates in the two studies were 11.1 and 15%,
respectively, and subsequent to IP chemotherapy, 51.9 and 40% of
the patients, respectively, underwent radical surgery (24,25).
By contrast, all patients in the present study possessed
unresectable cancer, 59.4% of the patients possessed ascites, 53.1%
experienced visceral metastases, the KPS scores of 78.1% of
patients were in the range of 60–70, and only 3.1% possessed KPS
scores in the range of 90–100. The prognoses of the patients
enrolled in the present study were not comparable with the
prognoses of the patients enrolled in the aforementioned two
studies (24,25).
The current study contained three drawbacks First,
due to the small sample size, there may be bias in the present
study and it is difficult to conduct subgroup analyses to identify
specific populations that are more likely to benefit from DCF dual
chemotherapy. Second, as the data collection was not detailed
enough, it was not clarified whether the gastrointestinal symptoms
were caused by advanced gastric cancer itself or by IP treatment;
thus, the abdominal pain, bloating and other adverse reactions
associated with IP chemotherapy may have been overestimated.
Thirdly, DCF is an extremely strong chemotherapy regimen with high
toxicity. Improved DCF regimens with reduced toxicity have been
reported (29,30,31),
and the application of improved DCF regimens may be considered in
future dual chemotherapy studies.
In summary, when using a reduced-dosage DCF regimen,
patients with unresectable advanced gastric cancer could tolerate
IP and IV dual chemotherapy. This regimen achieved acceptable
survival results, including a TTP of five months and an MST of nine
months. Future directions of study include using more efficient and
less toxic chemotherapy drugs, such as oxaliplatin, capecitabine
and S-1, to further improve the treatment efficacy and to reduce
gastrointestinal side-effects, particularly abdominal pain.
Acknowledgements
This study was supported by the Health Department of
Hebei Province, China (grant no. 08420).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Zhan YQ, Sun XW, Li W, et al: Multivariate
prognostic analysis in gastric carcinoma patients after radical
operation. Ai Zheng. 24:596–9. 2005.(In Chinese). PubMed/NCBI
|
|
3
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 10(Suppl 3): 49–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: a
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JH, Son SY, Lee CM, Ahn SH, Park DJ
and Kim HH: Factors predicting peritoneal recurrence in advanced
gastric cancer: implication for adjuvant intraperitoneal
chemotherapy. Gastric Cancer. 17:529–536. 2014. View Article : Google Scholar
|
|
6
|
Bijelic L and Sugarbaker PH: The role of
intraperitoneal chemotherapy in the treatment of patients with
advanced gastric cancer. Ann Ital Chir. 83:224–231. 2012.PubMed/NCBI
|
|
7
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
et al: V325 Study Group: Phase III study of docetaxel and cisplatin
plus fluorouracil compared with cisplatin and fluorouracil as
first-line therapy for advanced gastric cancer: a report of the
V325 Study Group. J Clin Oncol. 24:4991–4997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen XL, Chen XZ, Yang C, et al:
Docetaxel, cisplatin and fluorouracil (DCF) regimen compared with
non-taxane-containing palliative chemotherapy for gastric
carcinoma: a systematic review and meta-analysis. PLoS One.
8:e603202013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marchettini P, Stuart OA, Mohamed F, Yoo D
and Sugarbaker PH: Docetaxel: pharmacokinetics and tissue levels
after intraperitoneal and intravenous administration in a rat
model. Cancer Chemother Pharmacol. 49:499–503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fushida S, Kinoshita J, Yagi Y, et al:
Dual anti-cancer effects of weekly intraperitoneal docetaxel in
treatment of advanced gastric cancer patients with peritoneal
carcinomatosis: A feasibility and pharmacokinetic study. Oncol Rep.
19:1305–1310. 2008.PubMed/NCBI
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
12
|
Lai CL, Tsai CM, Chiu CH, Wang GS, Su WJ,
Chen YM and Perng RP: Phase II randomized trial of tri-weekly
versus days 1 and 8 weekly docetaxel as a second-line treatment of
advanced non-small cell lung cancer. Jpn J Clin Oncol. 35:700–706.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kunitoh H, Watanabe K, Onoshi T, Furuse K,
Niitani H and Taguchi T: Phase II trial of docetaxel in previously
untreated advanced non-small-cell lung cancer: a Japanese
cooperative study. J Clin Oncol. 14:1649–1655. 1996.PubMed/NCBI
|
|
14
|
Nakamura Y, Kunitoh H, Kubota K, Sekine I,
Yamamoto N, Tamura T, et al: Retrospective analysis of safety and
efficacy of low-dose docetaxel 60 mg/m2 in advanced
non-small cell lung cancer patients previously treated with
platinum-based chemotherapy. Am J Clin Oncol. 26:459–464. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin Q, Gao XS, Qiao XY, et al: Phase I
trial of escalating-dose cisplatin with 5-fluorouracil and
concurrent radiotherapy in Chinese patients with esophageal cancer.
Acta Med Okayama. 62:37–44. 2008.PubMed/NCBI
|
|
16
|
Lin Q, Liu YE, Chang CL, et al: Phase I
trial of dose escalation of capecitabine combined with fixed
docetaxel in previously treated patients with non-small cell lung
cancer. Chin-Ger J Clin Oncol. 11:6–10. 2012. View Article : Google Scholar
|
|
17
|
Wu Y and Wu W: Suggestions of improving
diagnostic level for gastric cancer early stage in China. China
Cancer. 18:738–740. 2009.
|
|
18
|
Kim HS, Kim HJ, Kim SY, et al: Second-line
chemotherapy versus supportive cancer treatment in advanced gastric
cancer: a meta-analysis. Ann Oncol. 24:2850–2854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lordick F, Lorenzen S, Yamada Y and Ilson
D: Optimal chemotherapy for advanced gastric cancer: is there a
global consensus? Gastric Cancer. 17:213–225. 2014. View Article : Google Scholar
|
|
20
|
GASTRIC (Global Advanced/Adjuvant Stomach
Tumor Research International Collaboration) Group. Oba K, Paoletti
X, Bang YJ, et al: Role of chemotherapy for advanced/recurrent
gastric cancer: an individual-patient-data meta-analysis. Eur J
Cancer. 49:1565–1577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coccolini F, Cotte E, Glehen O, et al:
Intraperitoneal chemotherapy in advanced gastric cancer.
Meta-analysis of randomized trials. Eur J Surg Oncol. 40:12–26.
2014. View Article : Google Scholar
|
|
22
|
Shi C, Yang B, Chen Q, Yang J and Fan N:
Retrospective analysis of adjuvant intraperitoneal chemotherapy
effect prognosis of resectable gastric cancer. Oncology.
80:289–295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xue SL, Su HF, Hu XQ, Deng X, Hu ML and
Xie CY: Adjuvant combined systemic chemotherapy and intraperitoneal
chemotherapy for locally advanced gastric cancer. Oncol Lett.
4:1309–1314. 2012.PubMed/NCBI
|
|
24
|
Fushida S, Kinoshita J, Kaji M, et al:
Society for Study of Peritoneal Carcinomatosis in Gastric Cancer:
Phase I/II study of intraperitoneal docetaxel plus S-1 for the
gastric cancer patients with peritoneal carcinomatosis. Cancer
Chemother Pharmacol. 71:1265–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishigami H, Kitayama J, Kaisaki S, et al:
Phase II study of weekly intravenous and intraperitoneal paclitaxel
combined with S-1 for advanced gastric cancer with peritoneal
metastasis. Ann Oncol. 21:67–70. 2010. View Article : Google Scholar
|
|
26
|
Fujiwara Y, Takiguchi S, Nakajima K, et
al: Intraperitoneal docetaxel combined with S-1 for advanced
gastric cancer with peritoneal dissemination. J Surg Oncol.
105:38–42. 2012. View Article : Google Scholar
|
|
27
|
Fujiwara Y, Nishida T, Takiguchi S, et al:
Feasibility study of S-1 and intraperitoneal docetaxel combination
chemotherapy for gastric cancer with peritoneal dissemination.
Anticancer Res. 30:1335–1339. 2010.PubMed/NCBI
|
|
28
|
Matharu G, Tucker O and Alderson D:
Systematic review of intraperitoneal chemotherapy for gastric
cancer. Br J Surg. 98:1225–1235. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Di Lauro L, Vici P, Belli F, et al:
Docetaxel, oxaliplatin, and capecitabine combination chemotherapy
for metastatic gastric cancer. Gastric Cancer. 17:718–724. 2014.
View Article : Google Scholar
|
|
30
|
Al-Batran SE, Hartmann JT, Hofheinz R, et
al: Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel
(FLOT) for patients with metastatic adenocarcinoma of the stomach
or esophagogastric junction: a phase II trial of the
Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol.
19:1882–1887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Cutsem E, Boni C, Tabernero J, et al:
Randomized phase II study (GATE study) of docetaxel plus
oxaliplatin with or without fluorouracil or capecitabine in
metastatic or locally recurrent gastric cancer. J Clin Oncol.
29(Suppl 15): 40182011.
|