Introduction
Lung cancer is one of the leading causes of
cancer-related mortalities worldwide (1). The incidence rate of lung cancer is
increasing in Asia, particularly in China. However, despite chemo-
and radiation therapy producing survival benefits in patients with
advanced non-small cell lung cancer (NSCLC), the survival rate of
lung cancer remains particularly low. Therefore, there is a clear
requirement for novel and more effective control strategies for
lung cancer. Thus, inhibition of epidermal growth factor receptor
(EGFR) tyrosine kinase has emerged as a novel therapeutic option
for the treatment of NSCLC. Gefitinib, an oral EGFR tyrosine kinase
inhibitor (TKI), is a leading agent in this class of novel
therapeutic agents. Two major phase II trials (2,3), large
expanded access programs across the world (4–6), as
well as other studies (7,8) have demonstrated a higher objective
response rate and prolonged survival time in females and
never-smoking adenocarcinoma patients of East-Asian origin.
Furthermore, a prospective trial, which administered gefitinib as a
first-line therapy for advanced lung adenocarcinoma patients that
were never-smokers, was conducted in South Korea and was found to
be highly efficacious (9).
Molecularly, NSCLC cells demonstrate mutation
(10–12) and amplification (13,14) of
the EGFR gene. Treatment with gefitinib has demonstrated that NSCLC
patients with such mutations or amplifications, as well as
expression of phosphorylated Akt (15) and ErbB3 (16) are associated with an improved
outcome (17–23). However, it is often difficult to
obtain the tumor tissue of patients to detect gene status.
Therefore, it is necessary to investigate the correlation between
the clinical features of NSCLC and the prognosis that is determined
in the clinical setting. The current study was conducted to
investigate such correlations in Chinese lung adenocarcinoma
patients using gefitinib-induced disease controls.
Patients and methods
Patient population
A total of 52 patients were recruited between
October 2004 and August 2008 at the Zhejiang Cancer Hospital
(Hangzhou, China). The clinical characteristics of the patients are
summarized in Table I. All patients
were histologically or cytologically diagnosed with lung
adenocarcinoma. The interval between the final cycle of
chemotherapy and administration of the gefitinib treatment was ≥30
days. The present study was approved by the Institutional Review
Board of Zhejiang Cancer Hospital and performed in accordance with
the recommendations of the Declaration of Helsinki with regard to
the biomedical research involving human subjects. Written informed
consent was obtained from all patients.
 | Table ICharacteristics of 52 patients (median
age, 65 years; range, 34–84 years) with lung adenocarcinoma. |
Table I
Characteristics of 52 patients (median
age, 65 years; range, 34–84 years) with lung adenocarcinoma.
| Characteristic | Patients, n | % |
|---|
| Age, years |
| <65 | 25 | 48.1 |
| ≥65 | 27 | 51.9 |
| Gender |
| Male | 19 | 36.5 |
| Female | 33 | 63.5 |
| Cigarettes per
year |
| ≥400 | 16 | 30.8 |
| <400 | 4 | 7.7 |
| Never-smoker | 32 | 61.5 |
| ECOG PS |
| 0–1 | 20 | 38.5 |
| ≥2 | 32 | 61.5 |
| Therapy |
| No previous
chemotherapy regimens received | 10 | 19.2 |
| 1 previous
chemotherapy regimen | 28 | 53.8 |
| ≥2 previous
chemotherapy regimens | 9 | 17.3 |
| Radiation
therapy | 5 | 9.6 |
Treatment schedule
Gefitinib (ZD1839; AstraZeneca, Wilmington, DE, USA)
was administered orally at a dosage of 250 mg/day until disease
progression, an unacceptable type of toxicity or withdrawal of
patient consent. No other chemotherapeutic agents were administered
during the course of the study. Medications for symptomatic relief,
such as analgesics and bisphosphonates were permitted. Twenty-three
patients with brain metastases received whole brain radiotherapy
(WBRT) during the gefitinib treatment period. Seventeen patients
with symptomatic bone metastases received palliative radiotherapy.
Baseline evaluations were performed within the week prior to
enrollment, including a complete medical history and physical
examination, laboratory tests (whole blood counts, and liver and
renal function), electrocardiograms, thorax computed tomography
(CT), ultrasonography of the abdomen, bone scintigraphy and brain
CT or magnetic resonance imaging (MRI). Furthermore, blood counts,
and liver and renal function tests were performed prior to each
30-day treatment cycle. Follow-up data after gefitinib treatment
(for example, recurrence, metastasis, vitals status, mortality and
cause of mortality) were obtained from the patient records.
Response assessment and evaluation of
toxicity
Response to the treatment was evaluated by CT, MRI
and ultrasonography of the abdomen, as well as bone scintigraphy.
The response rate was recorded according to the Response Evaluation
Criteria In Solid Tumors (24). For
data analysis, complete response (CR) and partial response (PR)
were combined and termed responders. CR and PR refer to a sustained
response over a period of four weeks or longer, however, stable
disease (SD) refers to a response persisting for eight weeks or
more. The type of toxicity was evaluated according to the National
Cancer Institute Common Toxicity Criteria (25) and the worst scores obtained during
treatment were recorded.
Statistical analysis
Progression-free survival (PFS) and overall survival
(OS) were measured from the date of initiation of gefitinib
treatment until disease progression or mortality, respectively. The
survival curves were calculated using the Kaplan-Meier method.
Multivariate survival analysis was performed using a Cox
proportional hazard regression model with a backward stepwise
procedure. The considered variables included age, gender,
cigarettes per year, Eastern Cooperative Oncology Group performance
status (ECOG PS) score, type of metastatic lesion and whether
gefitinib is the first-line treatment. Statistical analysis was
performed using SPSS software, version 13.0 (SPSS Inc., Chicago,
IL, USA). All probability values were two-tailed and P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical significance of gefitinib
treatment in patients with NSCLC
The clinical characteristics of 52 patients are
summarized in Table I. Of the 52
patients who participated in the present study, CR and PR rates
were 23.1% (12/52) and 57.7% (30/52), respectively. An additional
19.2% (10/52) of patients demonstrated SD.
Table II shows the
response of primary and various metastatic lesions to gefitinib
treatment. For example, the primary tumor size was reduced by
54.8±19.8% (mean ± standard deviation) in the five assessable
patients. Prior to gefitinib administration, 18 patients exhibited
intrapulmonary metastases, 17 of which regressed with gefitinib
treatment. Intrapulmonary metastases in 11 patients were assessed
as the target lesions and the extent of the reduction in size was
79.1±21.6%. Twenty-three patients exhibited brain metastases, 18 of
which regressed as a result of gefitinib administration. The brain
metastases in 21 patients were assessed as the target lesions and
the shrinkage of these was 68.5±27.9%. The response of lymph node
and liver metastases, and pleural effusion to gefitinib was also
favorable. Bone metastases was initially detected in 27 patients
and remained unchanged following gefitinib treatment, as identified
by bone scintigraphy.
 | Table IIResponse of primary and metastatic
lesions to gefitinib in the responders (partial and complete
response groups combined). |
Table II
Response of primary and metastatic
lesions to gefitinib in the responders (partial and complete
response groups combined).
| Site | Patients, n | Improved patients,
n | Patients with a
target lesion, n | Tumor shrinkage of
the target lesion, % (mean ± standard deviation) |
|---|
| Primary lesion | 5 | 5 | 5 | 54.8±19.8 |
| Metastastic
lesions |
|
Intrapulmonary | 18 | 17 | 11 | 79.1±21.6 |
| Brain | 23 | 18 | 21 | 68.5±27.9 |
| Lymph nodes | 12 | 10 | 12 | 47.5±10.5 |
| Bone | 27 | 0 | | |
| Pleura | 13 | 12 | | |
| Other | 6 | 5 | 3 | 41.7±9.7 |
One-year PFS and OS rates were 74.8 and 78.0%,
respectively. Multivariate analysis revealed that female patients
had significantly longer survival rates when compared with male
patients. Other factors, such as age, smoking status, ECOG PS,
metastatic lesions and gefitinib as first-line treatment, did not
exhibit a significant association with longer survival time
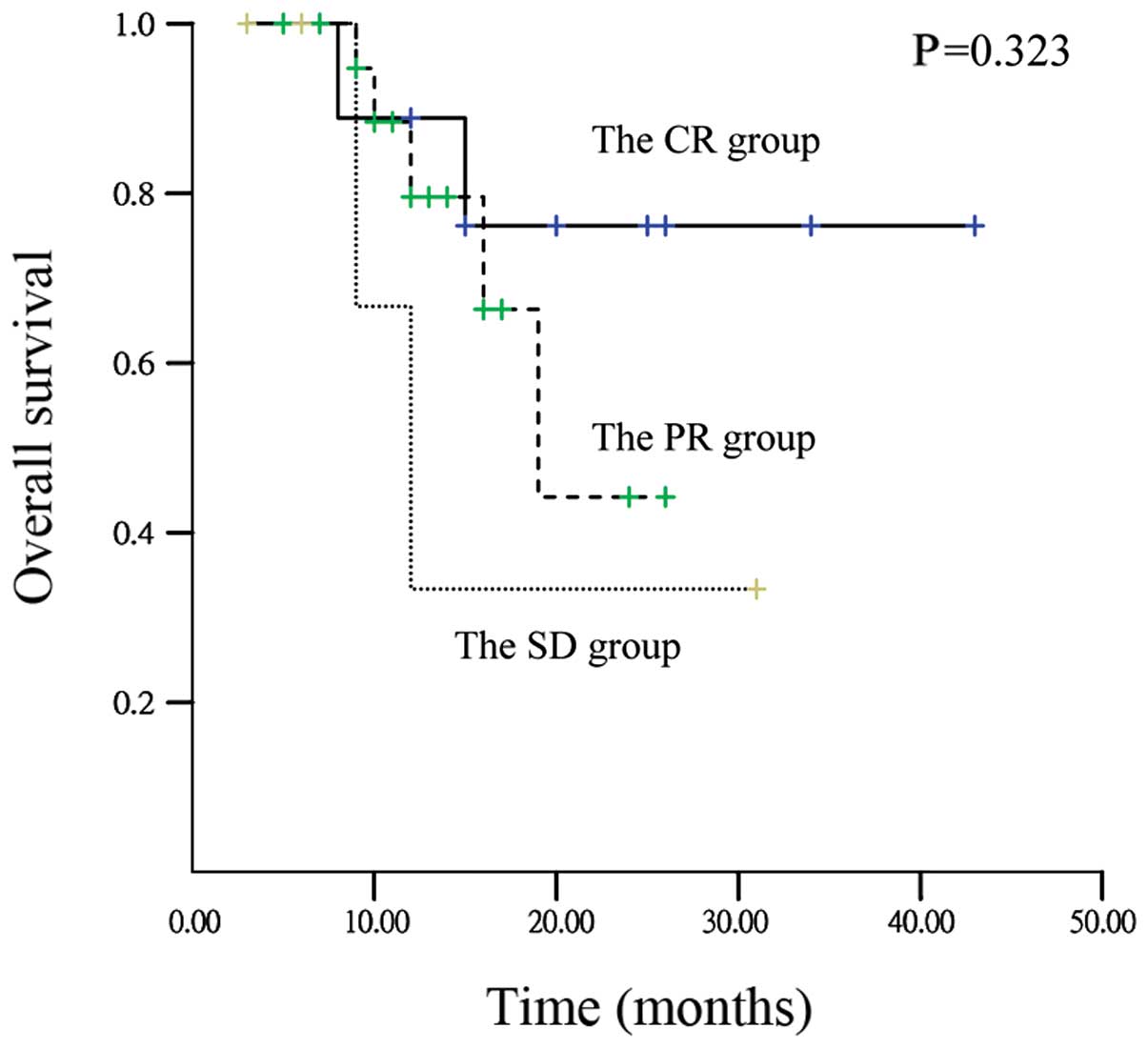
(Table III). One-year OS rates in
CR, PR and SD patients were 89.2, 79.8 and 33.7%, respectively.
One-year PFS rates in CR, PR, and SD patients were 77.8, 73.9 and
33.3%, respectively; however, there were no statistically
significant differences detected in OS (P=0.323) and PFS (P=0.379)
among patients with CR, PR and SD. Fig.
1 shows the OS curves of patients with CR, PR and SD. Median OS
of the SD patients was 12 months (95% confidence interval [CI],
7.2–16.8 months).
 | Table IIIFactors associated with overall
survival according to multivariate analysis. |
Table III
Factors associated with overall
survival according to multivariate analysis.
| Parameter | Hazard ratio | 95% CI | P-value |
|---|
| Age (<65 vs. ≥65
years) | 0.000 | 0.000–7.823 | 0.978 |
| Gender (Male vs.
Female) | 0.077 | 0.007–0.830 | 0.035 |
| Cigarettes per year
(<400 vs. ≥400) | 8.238 | 0.763–88.984 | 0.082 |
| Gefitinib as a
first-line therapy (Yes vs. No) | 0.815 | 0.102–6.519 | 0.847 |
| Metastatic lesions
(Intrapulmonary vs. brain vs. lymph nodes vs. bone vs. pleura vs.
other) | 0.392 | 0.041–3.795 | 0.419 |
| ECOG PS (0–1
vs.≥2) | 4.970 | 0.682–36.216 | 0.114 |
Toxicity and safety issues
Treatment with gefitinib was generally well
tolerated. The most common types of toxicity were rashes (88%) and
diarrhea (48%; Table IV). Grade II
diarrhea was well controlled by supportive care and grade III
diarrhea occurred in six patients (12%). Two patients suffered from
hand-foot syndrome. However, none of the patients refused
continuous treatment with gefitinib.
 | Table IVToxicity profile of the 52 patients
following treatment with gefitinib. |
Table IV
Toxicity profile of the 52 patients
following treatment with gefitinib.
| % (number of
patients) |
|---|
|
|
|---|
| Complication | Grade 0 | Grade I | Grade II | Grade III | Grades
I+II+III |
|---|
| Rash | 12 (6) | 19 (10) | 65 (34) | 4 (2) | 88 (46) |
| Diarrhea | 52 (27) | 19 (10) | 17 (9) | 12 (6) | 48 (25) |
| Mucositis | 72 (37) | 13 (7) | 15 (8) | 0 (0) | 28 (15) |
| Liver
dysfunction | 87 (45) | 12 (6) | 2 (1) | 0 (0) | 13 (7) |
| Neutropenia | 92 (48) | 8 (4) | 0 (0) | 0 (0) | 8 (4) |
| Lung toxicity | 100 (52) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hand-foot
syndrome | 96 (50) | 4 (2) | 0 (0) | 0 (0) | 0 (0) |
Disease progression following
gefitinib-induced disease control
At the time of data analysis, with a median
follow-up time of 21 months, a total of 25 (48.1%) patients
exhibited disease progression and their treatment was discontinued.
The sites of initial disease progression following
gefitinib-induced disease control among the 52 patients are
summarized in Table V. The disease
progression sites included the primary lesion (3/52; 5.8%), the
intrapulmonary (5/52; 9.6%), brain (12/52; 23.1%) and bone (7/52;
13.5%) lesions, pleural effusion (5/52; 9.6%), and the peritoneum
(2/52; 3.8%) and liver lesions (2/52; 3.8%).
 | Table VSites of initial disease progression
following gefitinib-induced disease control. |
Table V
Sites of initial disease progression
following gefitinib-induced disease control.
| Site | Patients, n | % |
|---|
| Primary lesion | 3 | 5.8 |
| Metastatic
lesion |
|
Intrapulmonary | 5 | 9.6 |
| Brain | 12 | 23.1 |
| Bone | 7 | 13.5 |
| Pleura | 5 | 9.6 |
| Peritoneum | 2 | 3.8 |
| Liver | 2 | 3.8 |
Discussion
In the present retrospective study, the response and
disease progression of primary and metastatic lesions was analyzed
in lung adenocarcinoma patients who achieved PR, CR or SD following
three months of treatment with gefitinib. A more positive outcome
was observed in the present study compared with previous studies
(21,26,27).
The CR and PR rates were 23.1% (12/52) and 57.7% (30/52),
respectively. An additional 19.2% (10/52) of patients achieved SD.
One-year PFS and OS rates were 74.8 and 78.0%, respectively.
Multivariate analysis showed that female patients had significantly
longer survival times when compared with male patients. Fukuoka
et al (2) reported that the
PR and SD rates were 18.5 and 35.9%, respectively, with gefitinib
administered at a dosage of 250 mg/day. In patients with either CR
or PR, the median OS was reported as 13.3 months for the 250-mg/day
group and 10.6 months for the 500-mg/day group. Mok et al
(28) demonstrated that gefitinib
was superior to chemotherapy as an initial treatment modality for
lung adenocarcinoma among non-smokers or former light smokers in
East Asia, despite the one-year PFS rate of 24.9%. Therefore,
gefitinib treatment for patients with lung adenocarcinoma resulted
in a marked survival benefit.
Previous studies have demonstrated that gefitinib
produced a higher objective response rate and prolonged survival
time in females and never-smoking adenocarcinoma patients of East
Asian origin (7,8,29). In
the current study, multivariate analysis revealed that female
patients had a statistically significant association with longer
survival time when compared with male patients, whereas other
patient parameters, such as age, smoking status, ECOG PS, tumor
metastasis or gefitinib as a first-line treatment were not
associated with prolonged survival. The results from the current
study were similar to previous reports (7,8). It is
hypothesized that female patients demonstrate an improved response
to gefitinib as a results of EGFR mutations, which occur more
frequently in females (30).
The current study demonstrated that one-year PFS and
OS rates in CR, PR and SD patients were 77.8, 73.9, 33.3%, and
89.2, 79.8 and 33.7%, respectively, although neither difference was
identified to be statistically significant. However, a previous
study has indicated that patients obtaining SD following gefitinib
treatment had significantly longer OS than those with progressive
disease (31). In addition, Yang
et al (32) reported that
the PFS times in dramatic, gradual, and local progression groups,
following gefitinib treatment, were 9.3, 12.9 and 9.2 months,
respectively (P=0.007). Furthermore, the OS for these groups was
17.1, 39.4, and 23.1 months, respectively (P<0.001). TKI
continuation was identified to be superior to switching the type of
chemotherapy in a subsequent setting for gradual progression (39.4
months vs. 17.8 months; P=0.02) (32). The above-mentioned findings indicate
that patients achieving SD or gradual progression following
gefitinib treatment may achieve long-term survival.
In the current study, brain metastases (23.1%) was
the major site of disease progression after treatment with
gefitinib. Similarly, Omuro et al (33) reported that the central nervous
system (CNS) was the most frequent site of disease progression in
patients with NSCLC after an initial response to gefitinib. This
may be due to the presence of the intact blood-brain barrier, which
gefitinib could not penetrate despite its low molecular weight.
Fukuhara et al (34)
reported that the concentration of gefitinib in the patient’s
cerebrospinal fluid (CSF; 0.9 nM) was <1% of the serum
concentration (117 and 132 nM, prior to and 2 h following drug
re-administration, respectively) when treated with 250 mg/day
gefitinib. In another study, Jackman et al (35)reported that increasing doses of
gefitinib resulted in increasing concentrations of gefitinib in the
CSF, with the concentration of gefitinib in the patients’ CSF
varying from 6.2 to 18 nM, following a 500-mg dose, and reaching 42
nM following a 1,000-mg dose. Following administration of that
regimen, the patients’ carcinomatous meningitis was controlled for
~four months (35). Conversely, our
previous phase II study demonstrated that a concomitant treatment
with gefitinib and WBRT in patients with brain metastases from
NSCLC resulted in a favorable prognosis (36). Thus, further molecular studies are
required to investigate the efficacy of gefitinib in penetrating
the blood-brain barrier. The approach was previously investigated
in China by those that conducted the ZhejiangCH06 trial,
(NCT01158170) (37).
In conclusion, lung adenocarcinoma patients treated
with gefitinib-induced disease control showed marked survival
benefits. Furthermore, patients achieving SD with gefitinib
treatment may achieve long-term survival. Further studies are
required to analyze the efficacy of gefitinib in penetrating the
blood-brain barrier.
Acknowledgements
The present study was sponsored by the China Wu
Jieping Medical Foundation-EGFR targeted therapy basic research
projects (grant no. 08-ZH-0062), which was awarded to Professor
Ya-Ping Xu.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2009. CA Cancer J Clin. 59:225–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 21:2237–2246.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kris MG, Natale RB, Herbst RS, et al:
Efficacy of gefitinib, an inhibitor of the epidermal growth factor
receptor tyrosine kinase, in symptomatic patients with non-small
cell lung cancer: a randomized trial. JAMA. 290:2149–2158. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jänne PA, Gurubhagavatula S, Yeap BY, et
al: Outcomes of patients with advanced non-small cell lung cancer
treated with gefitinib (ZD1839, ‘Iressa’) on an expanded access
study. Lung Cancer. 44:221–230. 2004. View Article : Google Scholar
|
|
5
|
Santoro A, Cavina R, Latteri F, et al:
Activity of a specific inhibitor, gefitinib (Iressa, ZD1839), of
epidermal growth factor receptor in refractory non-small-cell lung
cancer. Ann Oncol. 15:33–37. 2004. View Article : Google Scholar
|
|
6
|
Park J, Park BB, Kim JY, et al: Gefitinib
(ZD1839) monotherapy as a salvage regimen for previously treated
advanced non-small cell lung cancer. Clin Cancer Res. 10:4383–4388.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konishi J, Yamazaki K, Kinoshita I, et al:
Analysis of the response and toxicity to gefitinib of non-small
cell lung cancer. Anticancer Res. 25:435–441. 2005.PubMed/NCBI
|
|
8
|
Simon GR, Ruckdeschel JC, Williams C, et
al: Gefitinib (ZD1839) in previously treated advanced
non-small-cell lung cancer: experience from a single institution.
Cancer Control. 10:388–395. 2003.PubMed/NCBI
|
|
9
|
Lee DH, Han JY, Lee HG, et al: Gefitinib
as a first-line therapy of advanced or metastatic adenocarcinoma of
the lung in never-smokers. Clin Cancer Res. 11:3032–3037. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paez JG, Jänne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okamoto I, Takahashi T, Okamoto H, et al:
Single-agent gefitinib with concurrent radiotherapy for locally
advanced non-small cell lung cancer harboring mutations of the
epidermal growth factor receptor. Lung Cancer. 72:199–204. 2011.
View Article : Google Scholar
|
|
13
|
Cappuzzo F, Hirsch FR, Rossi E, et al:
Epidermal growth factor receptor gene and protein and gefitinib
sensitivity in non-small-cell lung cancer. J Natl Cancer Inst.
97:643–655. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirsch FR, Herbst RS, Olsen C, et al:
Increased EGFR gene copy number detected by fluorescent in situ
hybridization predicts outcome in non-small-cell lung cancer
patients treated with cetuximab and chemotherapy. J Clin Oncol.
26:3351–3357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cappuzzo F, Magrini E, Ceresoli GL, et al:
Akt phosphorylation and gefitinib efficacy in patients with
advanced non-small-cell lung cancer. J Natl Cancer Inst.
96:1133–1141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Engelman JA, Jänne PA, Mermel C, et al:
ErbB-3 mediates phosphoinositide 3-kinase activity in
gefitinib-sensitive non-small cell lung cancer cell lines. Proc
Natl Acad Sci USA. 102:3788–3793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitsudomi T, Kosaka T, Endoh H, et al:
Mutations of the epidermal growth factor receptor gene predict
prolonged survival after gefitinib treatment in patients with
non-small-cell lung cancer with postoperative recurrence. J Clin
Oncol. 23:2513–2520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han SW, Kim TY, Hwang PG, et al:
Predictive and prognostic impact of epidermal growth factor
receptor mutation in non-small-cell lung cancer patients treated
with gefitinib. J Clin Oncol. 23:2493–2501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hirsch FR, Varella-Garcia M, McCoy J, et
al: Southwest Oncology Group: Increased epidermal growth factor
receptor gene copy number detected by fluorescence in situ
hybridization associates with increased sensitivity to gefitinib in
patients with bronchioloalveolar carcinoma subtypes: a Southwest
Oncology Group Study. J Clin Oncol. 23:6838–6845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tokumo M, Toyooka S, Kiura K, et al: The
relationship between epidermal growth factor receptor mutations and
clinicopathologic features in non-small cell lung cancers. Clin
Cancer Res. 11:1167–1173. 2005.PubMed/NCBI
|
|
21
|
Kim KS, Jeong JY, Kim YC, et al:
Predictors of the response to gefitinib in refractory non-small
cell lung cancer. Clin Cancer Res. 11:2244–2251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takano T, Ohe Y, Sakamoto H, et al:
Epidermal growth factor receptor gene mutations and increased copy
numbers predict gefitinib sensitivity in patients with recurrent
non-small-cell lung cancer. J Clin Oncol. 23:6829–6837. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riely GJ, Pao W, Pham D, et al: Clinical
course of patients with non-small cell lung cancer and epidermal
growth factor receptor exon 19 and exon 21 mutations treated with
gefitinib or erlotinib. Clin Cancer Res. 12:839–844. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumors: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
25
|
Trotti A, Byhardt R, Stetz J, et al:
Common toxicity criteria: version 2.0. an improved reference for
grading the acute effects of cancer treatment: impact on
radiotherapy. Int J Radiat Oncol Biol Phys. 47:13–47. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cella D, Herbst RS, Lynch TJ, et al:
Clinically meaningful improvement in symptoms and quality of life
for patients with non-small-cell lung cancer receiving gefitinib in
a randomized controlled trial. J Clin Oncol. 23:2946–2954. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yokouchi H, Yamazaki K, Kinoshita I, et
al: Clinical benefit of readministration of gefitinib for initial
gefitinib-responders with non-small cell lung cancer. BMC Cancer.
7:512007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mok TS, Wu YL, Thongprasert S, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park K and Goto K: A review of the
benefit-risk profile of gefitinib in Asian patients with advanced
non-small-cell lung cancer. Curr Med Res Opin. 22:561–573. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inoue A, Suzuki T, Fukuhara T, et al:
Prospective phase II study of gefitinib for chemotherapy-naive
patients with advanced non-small-cell lung cancer with epidermal
growth factor receptor gene mutations. J Clin Oncol. 24:3340–3346.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hotta K, Matsuo K, Ueoka H, et al:
Continued gefitinib treatment after disease stabilisation prolongs
survival of Japanese patients with non-small-cell lung cancer:
Okayama Lung Cancer Study Group experience. Ann Oncol.
16:1817–1823. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang JJ, Chen HJ, Yan HH, et al: Clinical
modes of EGFR tyrosine kinase inhibitor failure and subsequent
management in advanced non-small cell lung cancer. Lung Cancer.
79:33–39. 2013. View Article : Google Scholar
|
|
33
|
Omuro AM, Kris MG, Miller VA, et al: High
incidence of disease recurrence in the brain and leptomeninges in
patients with nonsmall cell lung carcinoma after response to
gefitinib. Cancer. 103:2344–2348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fukuhara T, Saijo Y, Sakakibara T, et al:
Successful treatment of carcinomatous meningitis with gefitinib in
a patient with lung adenocarcinoma harboring a mutated EGF receptor
gene. Tohoku J Exp Med. 214:359–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jackman DM, Holmes AJ, Lindeman N, et al:
Response and resistance in a non-small-cell lung cancer patient
with an epidermal growth factor receptor mutation and
leptomeningeal metastases treated with high-dose gefitinib. J Clin
Oncol. 24:4517–4520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma S, Xu Y, Deng Q and Yu X: Treatment of
brain metastasis from non-small cell lung cancer with whole brain
radiotherapy and Gefitinib in a Chinese population. Lung Cancer.
65:198–203. 2009. View Article : Google Scholar
|
|
37
|
Ma S, Xu Y, et al: Prophylactic Cranial
Irradiation (PCI) in Erlotinib/Gefitinib-Responders With Non-small
Cell Lung Cancer (NSCLC) (RT1001). ClinicalTrialsgov Identifier:
NCT01158170. 2010.
|