Introduction
Breast cancer is the second most frequent type of
cancer in the world and is by far the most common malignant disease
in female individuals (1). Due to
advancements in methods for earlier diagnosis and adjuvant therapy,
the prognosis of patients with breast cancer has improved in recent
years, however, ~12% of breast cancer patients succumb to the
disease within the first five years (2). The most recent statistics for China
demonstrate that the mortality rate of breast cancer increased by
10% between 2012 and 2013, and increased by 30.5% between 2007 and
2013. Despite the application of the American Joint Committee on
Cancer tumor-node-metastasis system for staging and prognosis, ≤30%
of lymph node-negative patients ultimately develop recurrent
disease (3). This is possibly due
to occult metastatic cells, which are undetectable by currently
employed methodology, that have spread via the lymphatic or
hematogenous systems. Hence, it is clinically important that
disseminated tumor cells are detected using effective markers to
supplement the staging method, prediction of metastasis and
prognosis in breast cancer.
To identify circulating markers for the detection of
disseminated tumor cells in breast cancer patients, the current
study used in silico analysis of the National Cancer
Institute Cancer Genome Anatomy Project database (http://cgap.nci.nih.gov/cgap.html). NPY1R, a
novel peripheral blood marker, was determined to exhibit the
largest differential expression ratios. Neuropeptide Y (NPY) is the
most abundant neuropeptide in the mammalian brain and modulates
various mechanisms, such as appetite, anxiety, circadian rhythm,
memory and blood pressure (4). The
effect of NPY may be mediated by a number of NPY receptor subtypes,
termed Y1R-6R, of which upregulation of Y1R and Y2R has been
reported in numerous types of human carcinoma, including breast
cancer, adrenal tumors, renal cell carcinoma and ovarian cancer.
This activation of Y1R and Y2R by NPY resulted in tumor cell
proliferation, angiogenesis and metastasis (5).
In the present study, the correlation between NPY1R
expression and various clinicopathological features of breast
cancer patients was analyzed. Therefore, it was proposed that NPY1R
may serve as a useful marker to predict cancer metastasis and to
evaluate the prognosis of breast cancer patients.
Patients and methods
Patients and samples
The present study was conducted on 142 blood samples
provided by breast cancer patients, who were histopathologically
and clinically diagnosed with breast cancer at the Affiliated
Hospital of Chengde Medical College Cancer Center (Chengde, China)
between November 2008 and December 2011. The patient age range was
21–82 years, with a mean age of 52 years. In addition, 60 healthy
female volunteers (normal group) were enrolled (median age, 49
years; range 22–76 years). No patients received antihormonal
treatment, chemotherapy or radiotherapy prior to surgery, and all
data, including age, pathological type, tumor size, lymph node
metastasis, clinical stage according to the American Joint
Committee on Cancer (6), estrogen
receptor (ER) status, progesterone receptor (PgR) status, human
epidermal growth factor receptor 2 (HER2) score, according to the
American Society of Clinical Oncology/College of American
Pathologists guidelines (7), and
recurrence were obtained from the clinical and pathological
records. All participants provided written informed consent and
this study was approved by the ethics committee of Chengde Medical
College (Chengde, China).
Peripheral blood samples were acquired from
superficial veins on the opposite side to the breast cancer using
standard percutaneous venipuncture and collected into two citrate
sodium-containing tubes; the first 1 ml of blood was collected in
the first tube and the subsequent 5 ml was collected in the second
tube. The blood sample in the first tube, which may have been
contaminated with epithelial cells picked up by the needle when it
pierced the skin, was discarded; however, the blood in the second
tube was loaded onto a Ficoll-Hypaque layer (Gibco BRL, Carlsbad,
CA, USA). Following density gradient centrifugation, the peripheral
blood mononuclear cell pellet was collected, washed twice with
sterile phosphate buffer solution, snap frozen and stored at −80°C
until RNA extraction.
Identification of candidate marker
gene
The complementary DNA (cDNA) Digital Gene Expression
Displayer developed by the Cancer Genome Anatomy Project is an
expressed sequence tag database, which contains vast amounts
information generated from cancer cell lines. Thus, the cDNA
Digital Gene Expression Displayer was used to identify genes that
were differentially expressed in breast cancer cells and
leukocytes. The P-value filter was set at 0.01 and the
differentially expressed genes were ranked according to sequence
odds ratio (OR). The gene with the highest sequence OR was selected
as the candidate marker gene to undergo subsequent quantitative
polymerase chain reaction (qPCR) assays.
RNA preparation and cDNA synthesis
Total RNA was extracted from the blood samples using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions, treated with DNase I
(Promega Corporation, Madison, WI, USA) and quantified using
ultraviolet spectrophotometry (UV2000; LabTech, Beijing, China).
Furthermore, cDNA was synthesized from 2 μg total RNA using the
Advantage™ Reverse Transcriptase-for-PCR kit (Clontech
Laboratories, Inc., Mountainview, CA, USA). The integrity of RNA
samples and the accuracy of the cDNA synthesis were verified by
performing amplification of GAPDH in a standard PCR reaction.
Nested qPCR assay
A highly sensitive nested qPCR is required to detect
just a few circulating cancer cells. The first round of nested qPCR
was performed using 1 μl cDNA (dilution, 1:20) with a PCR mixture
(Beijing Tian Wei Yaida Technology Co., Ltd., Beijing, China)
containing 0.2 μmol/l outer primers for NYP1R (forward,
5′-TATACCACTCTTCTCTTGGTGCTG-3′ and reverse,
5′-CTGGAAGTTTTTGTTCAGGAACCCA-3′), 0.2 mM deoxynucleotide
triphosphate, 50 mM Tris-HCl, 10 mM KCl, 5 mM
(NH4)2SO4, 2 mM MgCl2
and 0.75 U Taq polymerase, to a total volume of 25 μl. The PCR
conditions were as follows: 35 cycles at 94°C for 20 sec, 62°C for
20 sec and 72°C for 40 sec, followed by a final extension at 72°C
for 10 min.
For the second round of nested qPCR amplification,
the reaction mixture contained 2 μl of the first round PCR product,
0.25 μmol/l inner primers for NYP1R (forward,
5′-ATCTGCCCTTGGCCATGAT-3′ and reverse, 5′-AGGCCAGGTTTCCAGAGACA-3′)
and SYBR® Green PCR master mix (Applied Biosystems,
Warrington, UK), to a total volume of 20 μl. The qPCR assays were
performed using an ABI PRISM® 7000 sequence detection
system (Applied Biosystems Life Technologies, Foster City, CA, USA)
under the following conditions: 94°C for 4 min followed by 40
cycles at 94°C for 15 sec, 58°C for 30 sec and 72°C for 35 sec. All
reactions were performed in triplicate and GAPDH mRNA (forward,
5′-ACCACAGTCCATGCCATC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′;
Shanghai Shenggong Co., Ltd., Shanghai, China), was used as the
internal control. The relative quantity of mRNA, normalized against
the GAPDH mRNA, was expressed as in terms of cycle threshold (Ct)
using the following equations: ΔCtNPY1R =
CtNPY1R − CtGAPDH; ΔΔCt = ΔCttumor
− mean of ΔCtnormal If the fluorescence signal was
undetected after 40 cycles, the Ct value was defined as the maximum
cycle number of 40 for analysis convenience. Furthermore, the
differential expression ratio of the candidate marker gene (Q) was
calculated using the following equation: Q = 2−ΔΔCt. To
determine the marker positivity in the present study, receiver
operating characteristic (ROC) curves were plotted according to the
−ΔCt value in the breast cancer and normal control groups.
Follow-up
A follow-up study was conducted on 131 breast cancer
patients by a telephone interview between November 2008 and
December 2011, with additional verification of clinical records.
Chest X-rays and mammographies were examined biannually, and liver
ultrasound and bone scans were examined annually. A total of 11
patients were lost to follow-up.
Statistical analysis
Statistical analyses were performed using the
Student’s t-test. Survival distributions were estimated using
Kaplan-Meier analysis and the log-rank test was performed to assess
the statistical significance of differences between the
NPY1R-positive and -negative groups. Furthermore, the
Mantel-Haenszel method was used to calculate χ2 in the
stratified correlation analysis between HER2 expression and patient
survival rate. Statistical analyses were conducted using SPSS
version 17.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05
indicated a statistically significant difference.
Results
Identification of the marker gene NPY1R
for detecting circulating breast cancer cells
The in silico Digital Gene Expression
Displayer program search of the National Cancer Institute Cancer
Genome Anatomy Project database identified 30,460 sequences in four
breast cancer cDNA libraries and 21,036 sequences in five leukocyte
cDNA libraries with a P-value filter set at 0.01. Of these, 23
overexpressed genes with a sequence OR of >16 were yielded from
the breast cancer and the leukocyte cDNA libraries. NPY1R exhibited
the largest differential expression ratios; therefore, nested qPCR
of the peripheral blood samples using NPY1R primers was performed.
Positive NPY1R gene expression was identified in 5/10 breast cancer
patient samples; however, 0/10 normal controls appeared to express
NPY1R.
Expression of NPY1R in the peripheral
blood of breast cancer patients
Nested qPCR was performed to determine the
expression level of NPY1R in the peripheral blood of 142 clinical
samples obtained from breast cancer patients. The −ΔCt value, which
represents the relative quantity of NYP1R mRNA, was significantly
higher in the cancerous samples compared with the corresponding
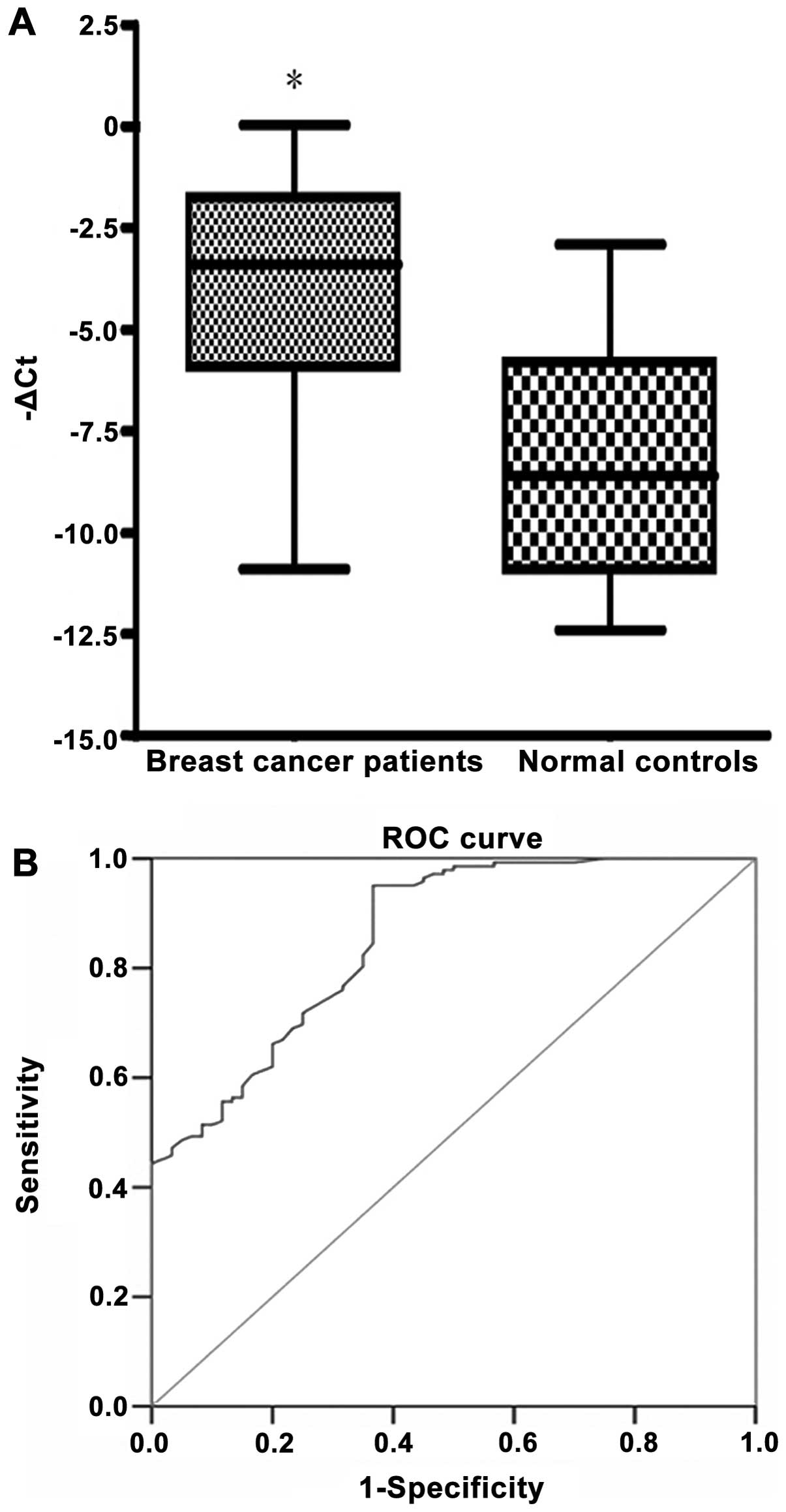
normal control samples (−3.93±2.5 vs. −8.21±2.9; P<0.01; Q,
55.54±27.3; Fig. 1A). The threshold
of −ΔCt was set at a conservative value of −2.75; this threshold
corresponds to 100% specificity (i.e., no normal control samples
were positive) as determined by ROC curve analysis (Fig. 1B). Using this clinical threshold for
marker positivity, it was observed that the positive detection rate
of circulating cancer cells in 142 breast cancer patients was 44.4%
(63/142) for the NPY1R gene.
Relative expression of NPY1R and patient
characteristics
The association between clinicopathological
variables and NPY1R transcript expression in the peripheral blood
of breast cancer patients was analyzed. High expression levels of
NPY1R correlated with the progression of clinical stages
(P<0.001). Furthermore, statistical analysis was performed to
determine that the HER2 score was significantly higher in the high
NPY1R expression group compared with the low NPY1R expression group
(P=0.001), the relative NPY1R expression level was significantly
higher in ER-positive compared with ER-negative patients (P=0.001),
and the relative NPY1R expression level was significantly higher in
PgR positive compared with the PgR-negative patients (P=0.037). Of
note, high NPY1R expression levels were detected in the lymph node
metastasis group, which highlights the value of NPY1R as a
predictive peripheral blood marker of lymph node metastasis in
breast cancer. However, no statistically significant association
was identified between marker detection and tumor size, pathology
type or patient age (P>0.05; Table
I).
 | Table IAssociation between the expression
level of the NPY1R gene in the peripheral blood of breast cancer
patients (n=142) and patient clinicopathological features. |
Table I
Association between the expression
level of the NPY1R gene in the peripheral blood of breast cancer
patients (n=142) and patient clinicopathological features.
| Clinicalpathological
feature | Patients, n | Relative NPY1R
expression, −ΔCt (mean ± standard deviation) | P-value |
|---|
| Age, years |
| <50 | 56 | −2.51±0.23 | |
| ≥50 | 86 | −2.23±1.07 | 0.350 |
| Pathology |
| Invasive ductal
carcinoma | 98 | −2.25±0.85 | |
| Simple cancer | 7 | −2.42±1.27 | |
| Eczematous
cancer | 5 | −2.48±1.34 | |
| Medullary
carcinoma | 19 | −2.56±1.22 | |
| Invasive lobular
carcinoma | 13 | −2.51±1.08 | 0.952 |
| Tumor size, cm |
| ≤2 | 75 | −2.69±1.41 | |
| >2 | 67 | −2.12±0.48 | 0.055 |
| Clinical stage |
| I – II | 89 | −3.11±1.62 | |
| III – IV | 53 | −1.72±0.91 | <0.001 |
| Lymph node
metastasis |
| Yes | 84 | −2.04±1.39 | |
| No | 58 | −3.17±1.49 | 0.001 |
| ER |
| + | 82 | −1.96±1.28 | |
| − | 60 | −2.88±1.12 | 0.001 |
| PgR |
| + | 63 | −2.17±1.27 | |
| − | 79 | −2.80±1.34 | 0.037 |
| HER2 |
| + | 68 | −1.86±0.87 | |
| − | 74 | −2.96±1.07 | 0.001 |
Association between NPY1R expression and
disease progression
To investigate the association between the detection
of circulating tumor cells and the clinical outcome of breast
cancer patients, a follow-up study was performed for 38 months in
131 patients following surgical removal of the tumor mass. The
survival rate was 61.1% (80/131), of which 46 patients displayed no
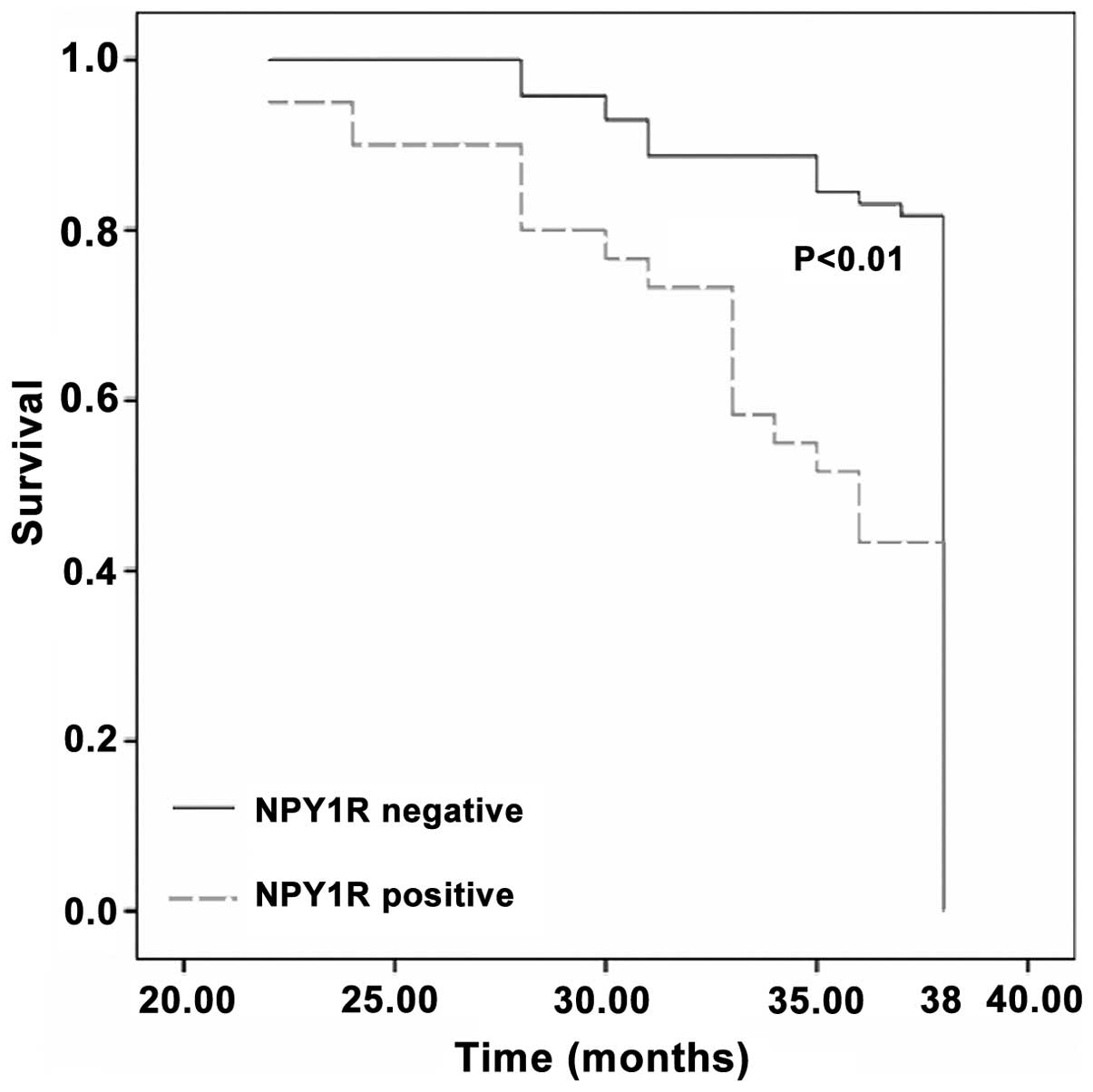
recurrence. The breast cancer patients with NPY1R-positive
circulating cancer cells exhibited shorter tumor-specific survival
compared with individuals with absent NPY1R expression (P<0.01;
Fig. 2). In addition, the 38-month
actuarial overall survival rates were 43.3% (26/60) and 76.1%
(54/71) in NPY1R-positive and -negative patients, respectively.
Association between HER2 expression and
patient survival rate in NPY1R-positive and -negative groups
Stratified correlation analysis was performed
between HER2 expression and patient survival rate in the
NPY1R-positive and -negative groups: NPY1R-positive group,
χ2=4.85 and OR=3.27 [95% confidence interval (CI),
1.14–9.38]; and NPY1R-negative group, χ2=14.73
and OR=11.08 (95% CI, 3.25–37.77) (Table II). These data indicate that
mortality rate is associated with HER2 expression in NPY1R-positive
and -negative groups. Furthermore, use of the Mantel-Haenszel
method (χ2=11.48; P<0.01) and adjusted
Mantel-Haenszel method [OR, 4.29 (95% CI, 1.85–9.95)] indicate that
HER2 expression is associated with patient survival rate and,
therefore, is one of the important risk factors of mortality rate
in breast cancer.
 | Table IIStratified correlation analysis
between HER2 expression and patient survival rate in NPY1R-positive
and -negative groups, as determined by follow-up (n=131). |
Table II
Stratified correlation analysis
between HER2 expression and patient survival rate in NPY1R-positive
and -negative groups, as determined by follow-up (n=131).
| HER2+ | HER2− |
|---|
|
|
|
|---|
| Patients, n |
NPY1R+ |
NPY1R− | Total |
NPY1R+ |
NPY1R− | Total |
|---|
| Mortalities | 24 | 14 | 38 | 10 | 3 | 13 |
| Survivors | 11 | 16 | 27 | 15 | 38 | 53 |
| Total | 35 | 30 | 65 | 25 | 41 | 66 |
Discussion
The detection of circulating cancer cells holds
promise as a powerful tool for cancer diagnosis and disease
monitoring (8). However,
conventional diagnostic methods, such as imaging and serum marker
detection assays, are unable to detect circulating tumor cells as
they exist in such small numbers. To overcome this problem, the
present study used nested qPCR; a sensitive method that is capable
of detecting one breast cancer cell in 107 cells
(9). Although various studies have
demonstrated that qPCR-based tumor cell detection assays yield
higher sensitivity compared with conventional methods, qPCR-based
assays for breast cancer have been limited by the availability of
molecular markers. Therefore, the present study employed in
silico analysis to identify marker genes for the detection of
circulating tumor cells. The National Cancer Institute Cancer
Genome Anatomy Project database and the Digital Gene Expression
Displayer program were useful tools for identifying genes that were
expressed in the two pools of samples; this also applies to
subsequent experiments, providing that a sufficient number of
expressed sequence tag libraries for the tissue of interest are
archived in the database (10).
Differentially expressed genes identified using the abovementioned
method may be developed into marker genes for diagnostic or
prognostic application by performing experimental verification
procedures, such as qPCR. In the present study, nested qPCF was
used to identify NPY1R as a novel marker of circulating cancer
cells in breast cancer patients; favorable markers are
characterized by a high level of expression in breast cancer
tissues but no or low expression in the peripheral blood cells of
healthy patients (11).
To date, NPY is the most abundant neuropeptide
reported in the mammalian brain. In the periphery, NPY is co-stored
and co-released with norepinephrine in the sympathetic nerve
endings (12). NPY exerts potent
biological effects on numerous target areas in the brain and in the
periphery. NPY is important in the regulation of the cardiovascular
system, lung function, feeding behavior, anxiogenesis, and the
release of hypothalamic and pituitary hormones. Kiyokawa et
al (13) studied the
interaction between estrogen, NPY and its receptors, and identified
the concerted action of estrogen and progesterone on increased NPY
level, as well as the associated increase in luteinizing hormone
release (13).
Recent studies have indicated that NPY and its
receptors are associated with human cancer. Körner et al
(14) reported that the Ewing’s
sarcoma family of tumors and synovial sarcomas expressed the NPY
receptor subtype Y1 at a high incidence rate (84 and 40%,
respectively) and density (mean, 5,314 and 7,497
disintegrations/min/mg tissue). Furthermore, a different study
identified that numerous types of sarcoma expressed Y1 on
intratumoral blood vessels (15).
Furthermore, data from a study conducted by Ruscica et al
(16) indicated that NPY may
directly regulate prostate cancer cell growth via its receptor.
This regulation appeared to be associated with the kinetics of
mitogen-activated protein kinase activation (i.e., long-lasting
versus transient) and to the clone-specific involvement of other
intracellular signals. The findings indicated that NPY-associated
mechanisms may be relevant in the progression of prostate cancer at
androgen-dependent and -independent stages of the disease. In
addition, a different study proposed a role of NPY in adrenal
cortical tumors as well as a Y1R-mediated physiological role in the
adrenal gland associated with strong NPY innervation of the cortex
(17).
The Y1R was the first NPY receptor subtype to be
cloned and characterized. A previous study determined that healthy
breast tissue expresses the Y2R subtype, whereas 85% of human
breast carcinoma tissue expresses the Y1R subtype (18). The high incidence of the Y1R subtype
expression in human breast carcinoma indicates that Y1R may be
important in the pathophysiology of breast malignancy; however, the
factors responsible for the high incidence of Y1R expression remain
unclear. Amlal et al (19)
hypothesized that the upregulated expression of Y1R was induced by
the activation of the estrogen signaling pathway. The effect of
estrogen on Y1R mRNA expression and the estrogen signaling pathway
were detected in vitro. The effect on the estrogen signaling
pathway was to induce cell proliferation in breast cancer. It was
suggested that the expression of the Y1R gene was increased in
response to estrogen treatment by using the MCF-7 cell line, an
estrogen receptor-positive human breast cancer cell line, which has
been demonstrated to express high-affinity NPY receptors. In
addition, a potential mechanism of NPY-inhibited
forskolin-stimulated adenosine 3′5′-cyclic monophosphate
accumulation and mobilized intracellular Ca2+ was
proposed in MCF-7 cells. Furthermore, the previous study treated
rats with estrogen and examined the upregulation of Y1R mRNA in the
hypothalamus by competitive reverse transcription-PCR. These
results indicate that estrogen exerts an important effect on the
upregulation of the Y1R, which in turn promotes estrogen-induced
proliferation in breast cancer cells. An interaction between
estrogen, NPY and its receptors has been proposed to explain the
concerted action of estrogen and progesterone on increased NPY
expression levels and an associated increase in luteinizing hormone
release (20). In the present
report, it was identified that expression of the marker gene NPY1R
in peripheral blood correlated with ER and PgR expression; the
expression level of NPY1R was significantly higher in the ER- and
PgR-positive groups compared with the negative group. The results
indicate that NPY1R may be involved in the activation of the
estrogen and progesterone signaling pathway in breast
carcinoma.
In the present study, the correlation between HER2
expression and patient outcome was investigated by performing
stratified analysis. The results indicated that HER2 expression was
associated with patient survival rate and, thus, was one of the
important risk factors of mortality in breast cancer. HER2 is
overexpressed in 20–30% of breast cancer patients; therefore,
recent studies have focused on investigating the role of HER2 as a
prognostic indicator to predict poor clinical outcome in breast
cancer patients (21). The
application of radioimmunohistochemical methods demonstrated that
85% of 296 breast tumor samples overexpressed HER2. Of these, 23%
expressed HER2 at 45–480 times greater than the normal level; this
high overexpression was associated with a poor clinical outcome,
therefore the serum HER2 expression level may be useful to predict
a poor clinical outcome in patients with HER2-positive breast
cancer. Furthermore, univariate analysis demonstrated that tumor
grade, necrosis, lymphovascular invasion and hormone receptor
negativity were significantly associated with HER-2/neu
overexpression. The upregulation of NPY1R appears to be specific to
ER+ breast cancer patients and thus, a combination of
multiple markers, including NPY1R, may be required to improve the
sensitivity and specificity for the detection of circulating breast
cancer cells. Future studies, which investigate the cellular
mechanisms underlying the role of NPY1R in breast cancer are
required, as this may lead to the development of drugs to target
NPY1R for breast cancer treatment.
Acknowledgements
The present study was supported by grants from the
Hebei Provincial Population and Family Planning Commission (grant
no. 2009-B21) and the Hebei Provincial Bureau of Health (grant no.
20090579).
References
|
1
|
Suh MA, Atashili J, Fuh EA and Eta VA:
Breast self-examination and breast cancer awareness in women in
developing countries: a survey of women in Buea, Cameroon. BMC Res
Notes. 5:6272012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yilmaz YE, Lawless JF, Andrulis IL and
Bull SB: Insights from mixture cure modeling of molecular markers
for prognosis in breast cancer. J Clin Oncol. 31:2047–2054. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park Y, Chang M, Lee S, et al:
Heterogeneity of triple negative breast cancer (TNBC): TNBC might
be divided into two or more subgroups by clinicopathologic
findings. Cancer Res. 69:(Abstract 6032; Suppl 24). 2009.
View Article : Google Scholar
|
|
4
|
Kohno D and Yada T: Arcuate NPY neurons
sense and integrate peripheral metabolic signals to control
feeding. Neuropeptides. 46:315–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Memminger M, Keller M, Lopuch M, et al:
The neuropeptide y y(1) receptor: a diagnostic marker? Expression
in mcf-7 breast cancer cells is down-regulated by antiestrogens in
vitro and in xenografts. PLoS One. 7:e510322012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wolff AC, Hammond ME, Schwartz JN, et al:
American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar
|
|
8
|
Rahbari NN, Bork U, Motschall E, et al:
Molecular detection of tumor cells in regional lymph nodes is
associated with disease recurrence and poor survival in
node-negative colorectal cancer: a systematic review and
meta-analysis. J Clin Oncol. 30:60–70. 2012. View Article : Google Scholar
|
|
9
|
Liu L, Liao GQ, He P, Zhu H, et al:
Detection of circulating cancer cells in lung cancer patients with
a panel of marker genes. Biochem Biophys Res Commun. 372:756–760.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kavak E, Ünlü M, Nistér M and Koman A:
Meta-analysis of cancer gene expression signatures reveals new
cancer genes, SAGE tags and tumor associated regions of
co-regulation. Nucleic Acids Res. 38:7008–7021. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blackhall F, Peters S, Kerr KM, et al:
Biomarkers. Ann Onc. 23:ix73–ix94. 2012. View Article : Google Scholar
|
|
12
|
Higuchi H: Molecular analysis of central
feeding regulation by neuropeptide Y (NPY) neurons with NPY
receptor small interfering RNAs (siRNAs). Neurochem Int.
61:936–941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiyokawa M, Matsuzaki T, Iwasa T, et al:
Neuropeptide Y mediates orexin A-mediated suppression of pulsatile
gonadotropin-releasing hormone secretion in ovariectomized rats. J
Med Invest. 58:11–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Körner M, Waser B and Reubi JC: High
expression of neuropeptide Y1 receptors in ewing sarcoma tumors.
Clin Cancer Res. 14:5043–5049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu C, Tilan JU, Everhart L, et al:
Dipeptidyl peptidases as survival factors in Ewing sarcoma family
of tumors: implications for tumor biology and therapy. J Biol Chem.
286:27494–27505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruscica M, Dozio E, Boghossian S, et al:
Activation of the Y1 receptor by neuropeptide Y regulates the
growth of prostate cancer cells. Endocrinology. 147:1466–1473.
2006. View Article : Google Scholar
|
|
17
|
Körner M, Waser B and Reubi JC: High
expression of neuropeptide y receptors in tumors of the human
adrenal gland and extra-adrenal paraganglia. Clin Cancer Res.
10:8426–8433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reubi JC, Gugger M, Waser B and Schaer JC:
Y(1)-mediated effect of neuropeptide Y in cancer: breast carcinomas
as targets. Cancer Res. 61:4636–4641. 2001.PubMed/NCBI
|
|
19
|
Amlal H, Faroqui S, Balasubramaniam A and
Sheriff S: Estrogen up-regulates neuropeptide Y Y1 receptor
expression in a human breast cancer cell line. Cancer Res.
66:3706–3714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sheriff S, Ali M, Yahya A, et al:
Neuropeptide Y Y5 receptor promotes cell growth through
extracellular signal-regulated kinase signaling and cyclic AMP
inhibition in a human breast cancer cell line. Mol Cancer Res.
8:604–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lennon S, Barton C, Banken L, et al:
Utility of serum HER2 extracellular domain assessment in clinical
decision making: pooled analysis of four trials of trastuzumab in
metastatic breast cancer. J Clin Oncol. 27:1685–1693. 2009.
View Article : Google Scholar : PubMed/NCBI
|