Introduction
The swim bladder is important organ in Osteichthyes
that is used to maintain balance and contains ~10% polysaccharide.
Larimichthys crocea is used as drug in traditional Chinese
medicine (TCM) and it has been reported that L. crocea swim
bladder may remove free radicals and protect against cancer
(1). Polysaccharides are an
important material for producing drugs, and the polysaccharide
obtained from Phellinus linteus and Pleurotus
ostreatus have been shown to exhibit an anticancer effect in
colon cancer cells in vitro (2,3).
Apoptosis induction in cancer cells is characterized
by changes in cell morphology, which include cell shrinkage,
membrane blebbing, chromatin condensation and nuclear fragmentation
(4). Apoptosis presents a critical
defense mechanism against cancer, which leads to the death of
potentially harmful cells. Dysregulated apoptotic processes have
been implicated in numerous diseases; these lead to the inhibition
of cell death and the progression of diseases, including cancer
(5). Identifying the critical
events involved with carcinogenesis may present an opportunity to
prevent cancer development using TCM that triggers apoptosis,
particulary with the extraction of natural substances. However, TCM
may augment disease progression. In addition to the effects on
protein expression and function, an increasing number of studies
have reported that a large number of components of TCM may exert
effects on the human genome, via the direct or indirect modulation
of gene expression (6).
In the present study, the in vitro anticancer
effects of polysaccharide of L. crocea swim bladder (PLCSB)
were determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, 4,6-diamidino-2-phenylindole (DAPI) staining test, flow
cytometry analysis, mRNA and protein expression analysis, and the
molecular mechanisms underlying the anticancer effects of PLCSB
were also investigated.
Materials and methods
PLCSB preparation
Wild Yellow Sea L. crocea were purchased from
Shandong Linyi Dahai Aquaculture Company (Linyi, China). The swim
bladder of L. crocea (1 kg) was freeze-dried and the samples
were crushed. A total of 3 l petroleum ether was mixed with the
swim bladder of L. crocea and reflux extraction was
performed twice, for 1 h at 60°C to remove the protein. Next, the
residue was collected following filtration. A total of 3 l absolute
ethyl alcohol was then added and reflux extraction was performed
for 3 h, and the residue without protein was filtrated and
collected. Finally, 3 l water was added and the residue was
extracted at 60°C for 2 h and the filter liquid was collected
(7). The crude PLCSB was obtained
following evaporation.
Cancer cell preparation
HCT-116 human colon carcinoma cells and human HaCaT
keratinocyte cells were purchased from the American Type Culture
Collection (Manassas, VA, USA). The HCT-116 cells were cultured in
RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA) and HaCaT cells
were cultured in Dulbecco’s modified Eagle’s medium (Gibco-BRL)
supplemented with 10% fetal bovine serum (Gibco-BRL) and 1%
penicillin-streptomycin (Gibco-BRL) at 37°C in a humidified
atmosphere containing 5% CO2 (Forma 311 S/N29035
CO2 incubator; Forma Therapeutics, Inc., Watertown, MA,
USA). The medium was changed two-three times a week.
MTT assay
The anticancer effects of PLCSB were assessed by MTT
assay. HCT-116 cells were seeded in a 96-well plate
(2×104 cells/ml per well) in a volume of 180 μl. Next,
20 μl of 100, 200 or 400 μg/ml PLCSB were added. The cells were
then incubated with the PLCSB solutions for 48 h at 37°C in an
incubator (311 S/N29035; Forma Therapeutics, Inc.) in a humidified
atmosphere containing 5% CO2. An MTT solution (200 μl; 5
mg/ml; Amresco LLC, Solon, OH, USA) was added to each well and the
cells were cultured for an additional 4 h under the same
conditions. The supernatant was discarded and 150 μl dimethyl
sulfoxide (per well) was added and mixed for 30 min. Finally, the
absorbance of each well was measured by an ELISA plate reader
(Bio-Rad 680; Bio-Rad, Hercules, CA, USA) at a wavelength of 540 nm
(8).
DAPI staining
Untreated control cells and cells treated with
different concentrations of PLCSB were harvested, washed with
phosphate-buffered saline (PBS), and fixed with 3.7%
paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) in PBS for 10
min at room temperature. The fixed cells were then washed with PBS
and stained with a 1 mg/ml DAPI (Sigma-Aldrich) solution for 10 min
at room temperature (9). The cells
were washed twice with PBS and examined with a fluorescence
microscope (BX50; Olympus Corporation, Tokyo, Japan).
Flow cytometry analysis
For histological analysis, liver tissues were fixed
in 10% (v/v) buffered formalin for 24 h, dehydrated in ethanol and
embedded in paraffin. Next, 4-μm-thick sections were prepared and
stained with hematoxylin and eosin, and then observed under a
microscope (BX41; Olympus Corporation) (10).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from cancer cells using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. RNA was digested by
RNase-free DNase (Roche Diagnostics, Basel, Switzerland) for 15 min
at 37°C and purified using an RNeasy kit (Qiagen, Hilden, Germany)
according to the manufacturer’s instructions. cDNA was synthesized
from 2 μg of total RNA by incubation at 37°C for l h with avian
myeloblastosis virus reverse transcriptase (GE Healthcare Life
Sciences, Chalfont, UK) according to the manufacturer’s
instructions. The primer sequences used to amplify the genes were
as follows: Forward, 5′-AAGCTGAGCGAGTGTCTCCGGCG-3′ and reverse,
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ for Bax; forward,
5′-CTCGTCGCTACCGTCGTGACTTGG-3′ and reverse,
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ for B-cell lymphoma 2 (Bcl-2);
forward, 5′-CAGCTGCACCTGACG-3′ and reverse, 5′-GCTGGGTAGGTGCAT-3′
for Bcl-extra large (xL); forward, 5′-GCTCTGACTGTACCACCATCC-3′ and
reverse, 5′-CTCTCGGAACATCTCGAAGCG-3′ for p53; forward,
5′-CTCAGAGGAGGCGCCATG-3′ and reverse, 5′-GGGCGGATTAGGGCTTCC-3′ for
p21; forward, 5′-CTATGAGCTAGTCATGTGTTAGA-3′ and reverse,
5′-CCAATTCACAGACACTGACA-3′ for apoptotic protease activating factor
1 (Apaf-1); forward, 5′-CAAACTTTTTCAGAGGGGATCG-3′ and reverse,
5′-GCATACTGTTTCAGCATGGCA-3′ for caspase-3; forward,
5′-CTGCTGGGGATGGCCACTGTG-3′ and reverse,
5′-TCGCCTCGAGGACATCGCTCTC-3′ for caspase-8; forward,
5′-GGCCCTTCCTCGCTTCATCTC-3′ and reverse,
5′-GGTCCTTGGGCCTTCCTGGTAT-3′ for caspase-9; forward,
5′-GAAATGAAATCCAAAGCT-3′ and reverse, 5′-TAATTTAGAGGCAAAGTGGC-3′
for Fas; and forward, 5′-GGATTGGGCCTGGGGATGTTTCA-3′ and reverse,
5′-TTGTGGCTCAGGGGCAGGTTGTTG-3′ for Fas ligand (L). Glyceraldehyde
3-phosphate dehydrogenase was amplified as an internal control gene
using the following primers: Forward, 5′-CGGAGTCAACGGATTTGGTC-3′
and reverse, 5′-AGCCTTCTCCATGGTCGTGA-3′. Amplification was
performed in a thermal cycler (Mastercycler Nexus X1; Eppendorf,
Hamburg, Germany). The PCR products were separated in 1.0% agarose
gels and visualized with ethidium bromide staining (11).
Western blot analysis
Total cell lysates were obtained using extraction
buffer as previously described (12). Protein was extracted using cell
lysis solution (WB-0061, Beijing Dingguo Changsheng Biotechnology
Co. Ltd., Beijing, China) and protein concentrations were
determined using a protein assay kit (Bio-Rad). For western blot
analysis, aliquots of lysate containing 30–50 μg of protein were
separated by electrophoresis on 12% sodium dodecyl
sulfate-polyacrylamide gels and then electrotransferred onto a
nitrocellulose membrane (Schleicher & Schuell BioScience, Inc.,
Keene, NH, USA). After blocking with skimmed milk, the membranes
were probed with specific primary antibodies for 1 h and then
incubated with the appropriate horseradish peroxidase-conjugated
polyclonal secondary antibodies (goat anti-human; ab6958; dilution,
1:1,000; Abcam, Cambridge, UK). Primary antibodies included mouse
anti-human Bax monoclonal antibody (sc-65532; dilution, 1:200),
mouse anti-human Bcl-2 monoclonal antibody (sc-509; dilution,
1:200), mouse anti-human Bcl-xL monoclonal antibody (sc-136207;
dilution, 1:100), mouse anti-human p53 monoclonal antibody
(sc-55476; dilution, 1:200), mouse anti-human p21 monoclonal
antibody (sc-56335; dilution, 1:200), goat anti-human Apaf-1
polyclonal antibody (sc-33870; dilution, 1:200), mouse anti-human
caspase-3 polyclonal antibody (sc-56052; dilution, 1:200), mouse
anti-human caspase-8 monoclonal antibody (sc-81657; dilution,
1:200), mouse anti-human caspase-9 monoclonal antibody (sc-56073;
dilution, 1:200) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), rabbit anti-human Fas polyclonal antibody (ab82419; dilution,
1:1,000), rabbit anti-human FasL polyclonal antibody (ab15285;
dilution, 1:200) and rabbit anti-human β-actin polyclonal antibody
(ab16039; dilution, 1:200) (Abcam). Antibody binding was visualized
by enhanced chemiluminescence according to the manufacturer’s
instructions (GE Healthcare). Chemiluminescence was visualized
using a LAS3000 luminescent image analyzer (Fujifilm Corporation,
Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between individual groups were assessed by one-way
analysis of variance and Duncan’s multiple range test. P<0.05
was considered to indicate a statistically significant difference.
SAS version 9.1 software (SAS Institute Inc., Cary, NC, USA) was
used for statistical analyses.
Results
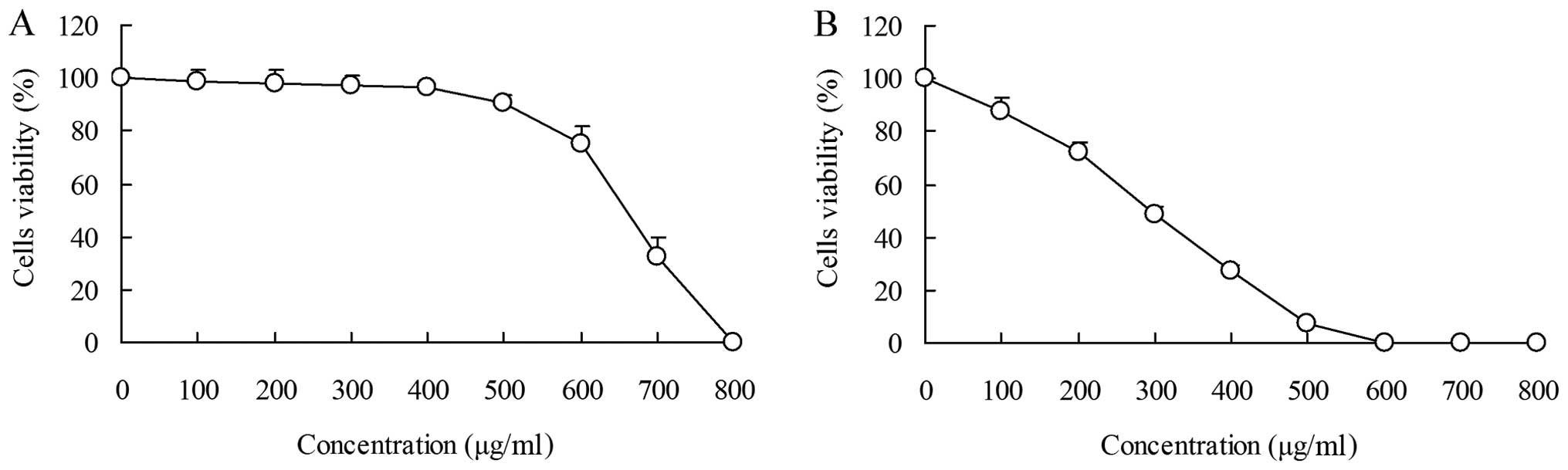
Inhibitory effects of PLCSB on cancer
cell growth
The inhibitory effects of PLCSB on HCT-116 cell
growth were analyzed by MTT assay. HaCaT keratinocyte cells were
evaluated and the growth inhibitory rates were not associated with
the concentration of PLCSB. When the cells were treated with 0–400
μg/ml PLCSB, no significant difference in the growth inhibitory
rates were identified, and the rates were all <10%. When the
cells were treated with >400 μg/ml PLCSB, the growth inhibitory
rate increased and, following treatment with 800 μg/ml PLCSB, the
rate was 100% (Fig. 1A). At
concentrations ranging between 0 and 400 μg/ml, cell viability was
decreased by the PLCSB in a concentration-dependent manner. At the
concentration of 600 μg/ml, the survival rate of the cells treated
with PLCSB reached 0% (Fig. 1B). No
inhibitory or toxic effects on the growth of normal human cells
were observed at concentrations of 0–400 μg/ml PLCSB; however, an
inhibitory effect on growth was exhibited in HCT-116 cancer cells.
These results indicated that PLCSB only exerts an effect on cancer
cells. Consequently, concentrations of 100, 200 and 400 μg/ml PLCSB
were selected for subsequent experiments.
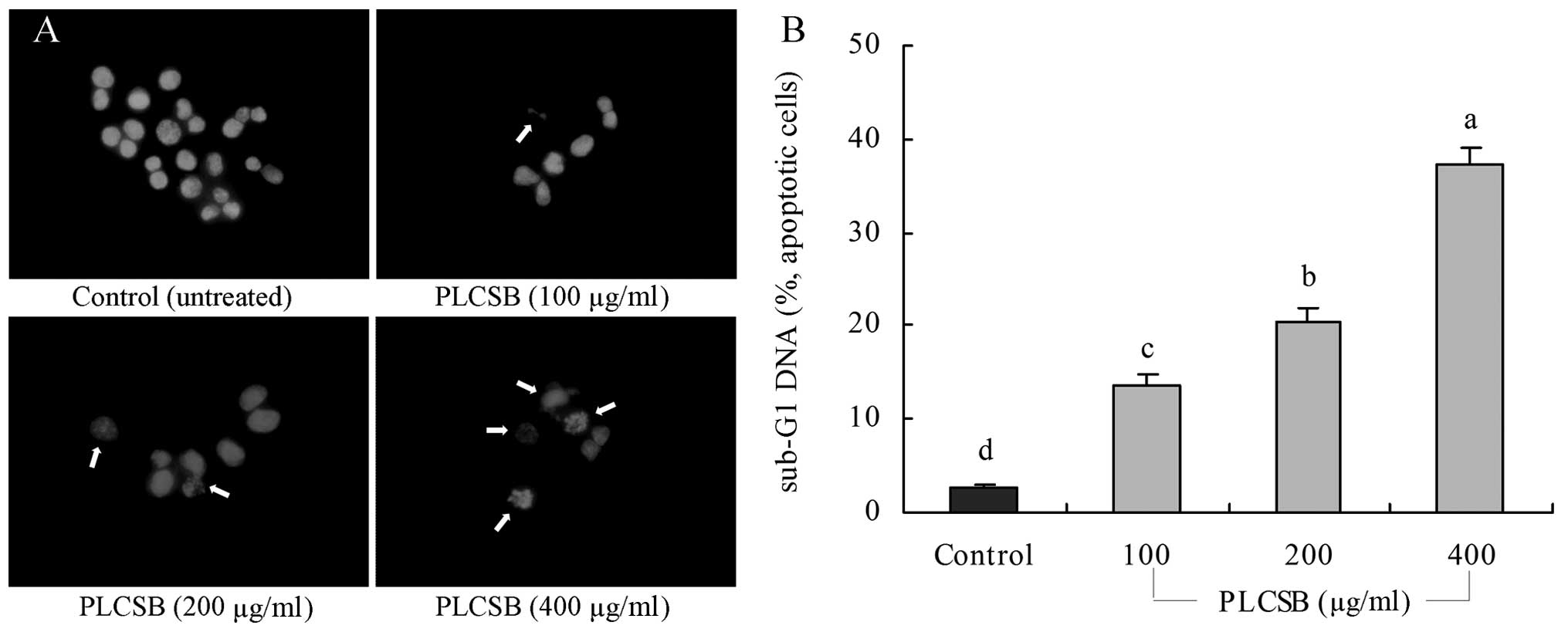
Induction of apoptosis by PLCSB
To determine a possible mechanism underlying the
growth inhibitory activity of PLCSB in HCT-116 cancer cells, the
induction of apoptosis was analyzed. The extent of chromatin
condensation was determined by fluorescence microscopy of the cells
stained with the DNA-binding fluorescent dye DAPI and flow
cytometric analysis. While the untreated HCT-116 cells exhibited
nuclei with homogeneous chromatin distribution, treatment with the
PLCSB induced chromatin condensation and nuclear fragmentation,
which indicated the presence of apoptotic cells (Fig. 2A). Chromatin condensation and the
formation of apoptotic bodies, which are two hallmarks of
apoptosis, were observed in cells cultured with 200 and 400 μg/ml
PLCSB. By contrast, the level of chromatin condensation was low in
the cells treated with 100 μg/ml PLCSB. Flow cytometric analyses
revealed that treatment with PLCSB promoted apoptosis of the
HCT-116 cells, compared with the untreated control cancer cells.
This conclusion is based on the significant accumulation of cells
with a sub-G1 DNA content (Fig.
2B). The induction of apoptosis was almost negligible (2.7%) in
untreated control cancer cells; however, cancer cells treated with
400 μg/ml PLCSB exhibited a higher level of apoptosis (37.2%) than
cells treated with 100 μg/ml (13.7%) and 200 μg/ml (20.5%)
PLCSB.
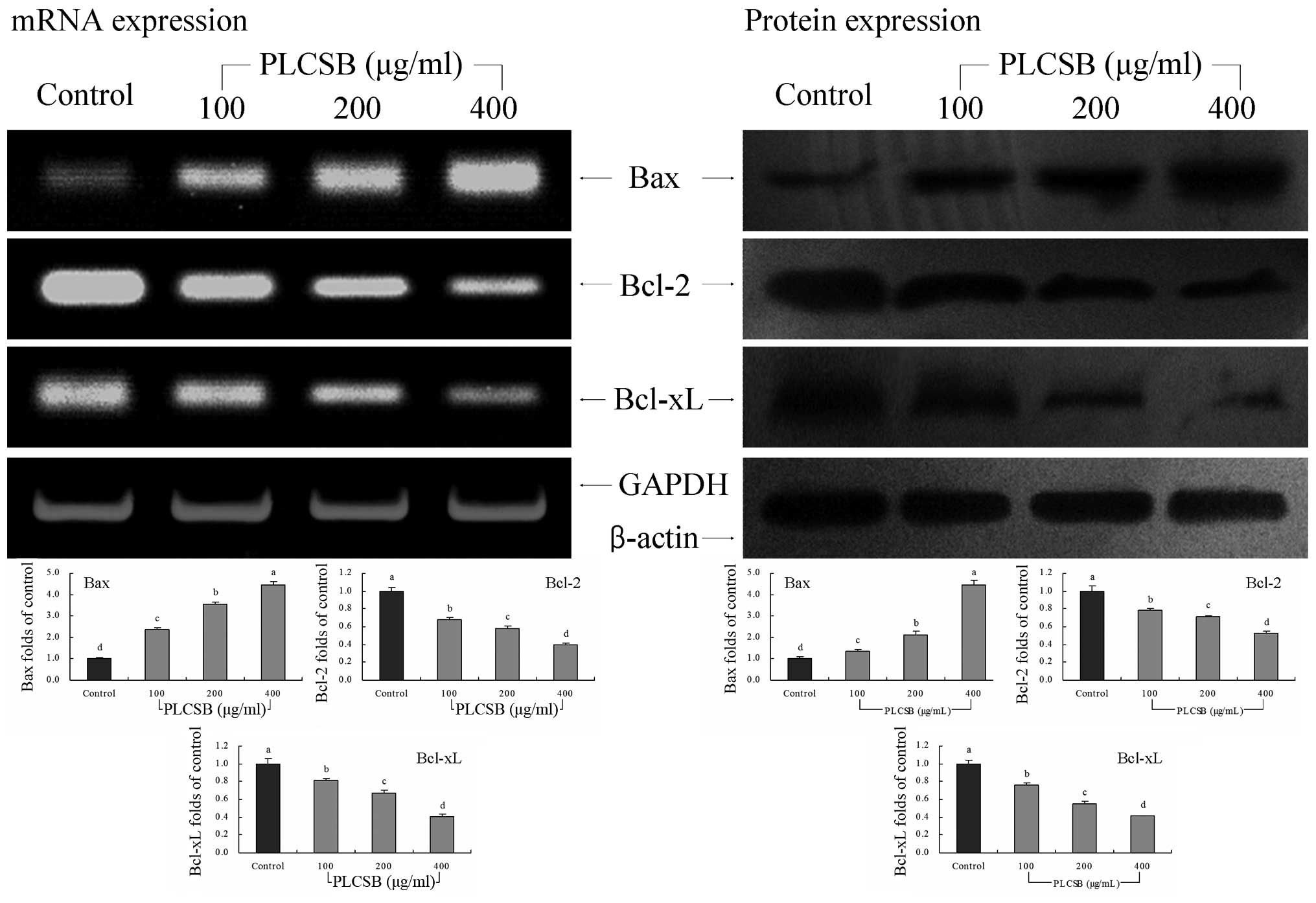
Bcl-2 family gene expression
To investigate the mechanisms underlying the
inhibition of cancer cell growth by PLCSB, the expression of Bax,
Bcl-2 and Bcl-xL in HCT-116 human colon cancer cells was analyzed
by RT-PCR and western blot analysis following incubation with 100,
200 or 400 μg/ml PLCSB for 48 h. The expression of pro-apoptotic
Bax and anti-apoptotic Bcl-2 and Bcl-xL exhibited significant
changes (P<0.05) following treatment with PLCSB (Fig. 3). These results indicate that
treatment with PLCSB leads to apoptosis induction in HCT-116 cells
via a Bax-, Bcl-2- and Bcl-xL-dependent pathway.
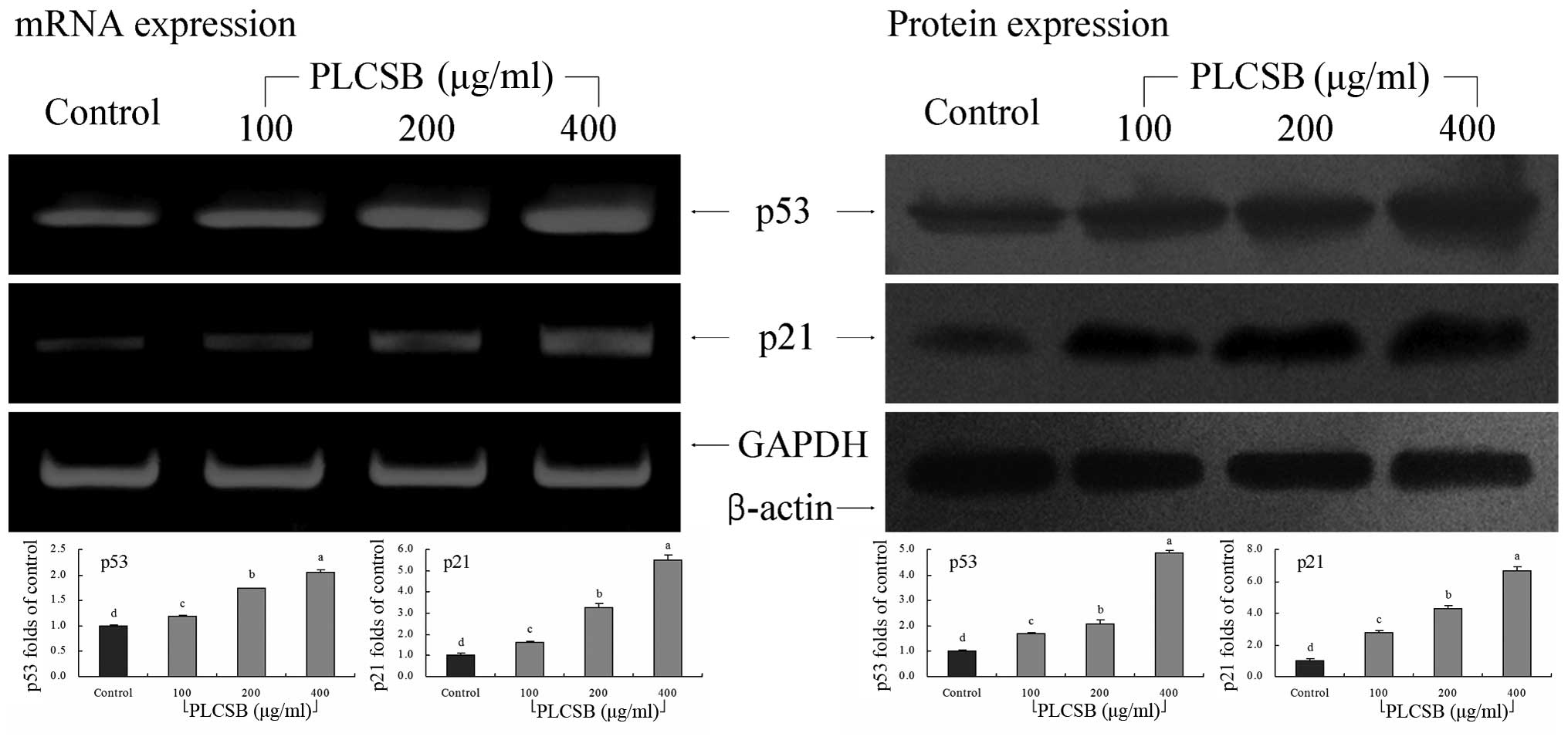
p53 and p21 gene expression
As shown in Fig. 4,
PLCSB significantly increased the level of p53 and p21 mRNA
expression (P<0.05). These changes in p53 and p21 expression as
a result of PLCSB treatment may lead to the induction of apoptosis
in HCT-116 cells. These results showed that PLCSB exhibits
significant anticancer activity via the induction of apoptosis.
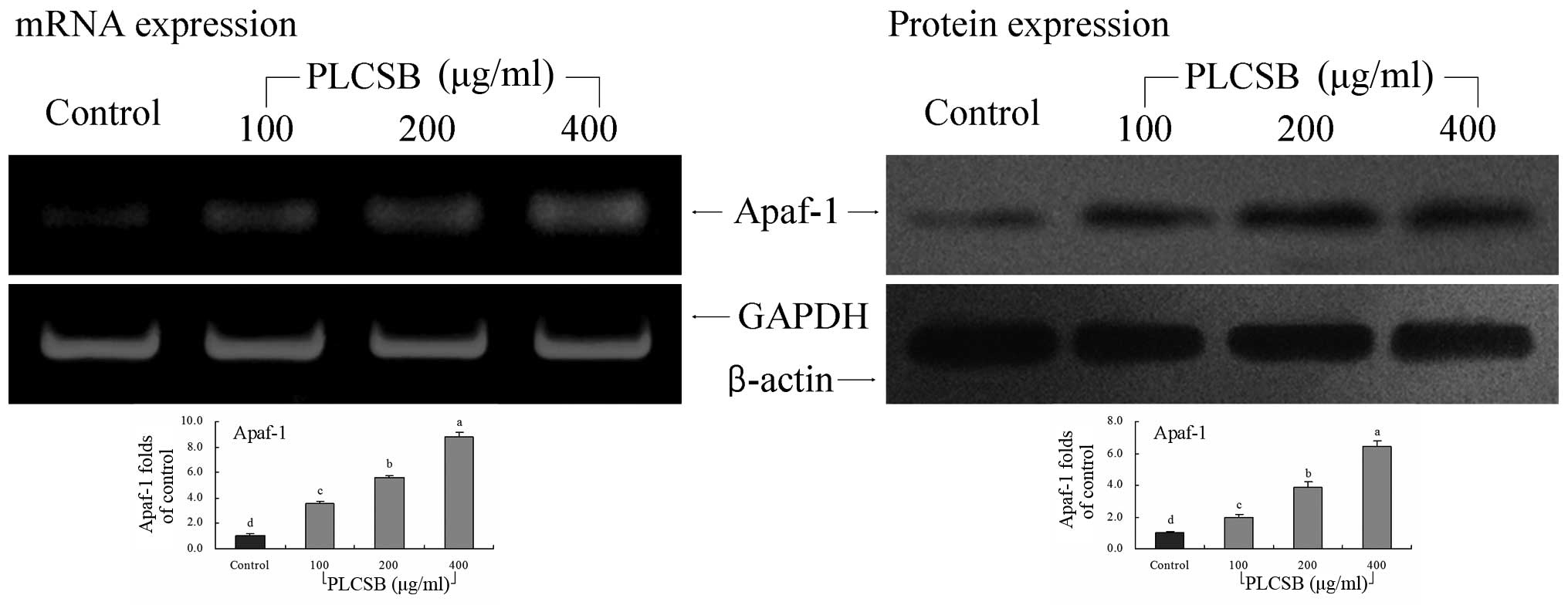
Apaf-1 gene expression
The mRNA and protein expression of Apaf-1 was
increased by treatment with PLCSB, with the greatest Apaf-1
expression observed in the 400-μg/ml PLCSB-treated cells (8.85- and
6.45-fold that of the mRNA and protein expression in the untreated
cancer cells, respectively) (Fig.
5). The Apaf-1 mRNA and protein expression in 200-μg/ml
PLCSB-treated cells was 5.57- and 3.90-fold that of the control
cells. The 100-μg/ml PLCSB-treated cells also showed higher Apaf-1
mRNA and protein expression than the control cells (3.59- and
1.94-fold, respectively), but this was lower than that of cells
treated with 200 and 400 μg/ml PLCSB.
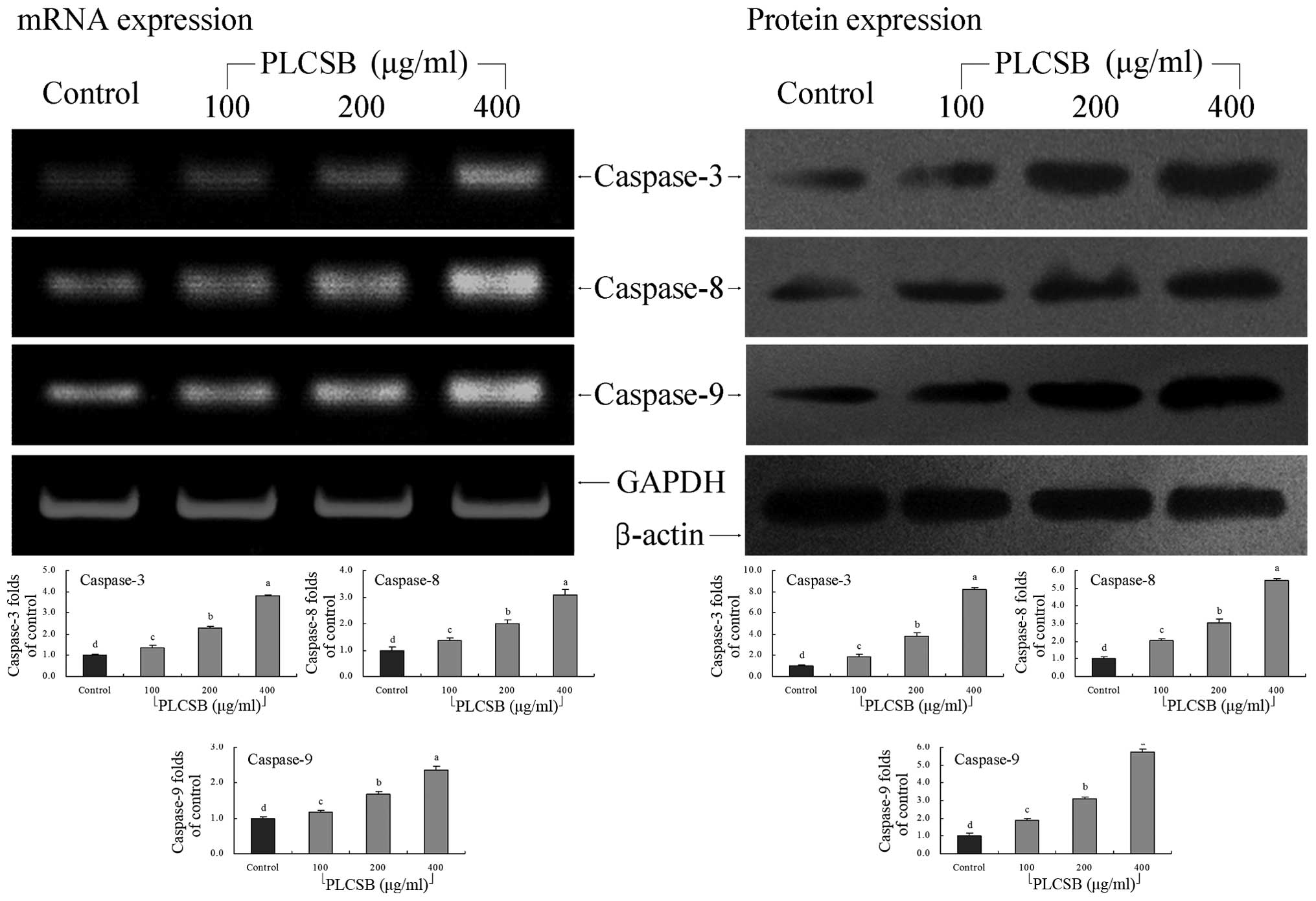
Caspase gene expression
The mRNA expression levels of caspase-3, -8 and -9
were extremely low in untreated control HCT-116 cells; however, the
levels significantly increased following treatment with 400 μg/ml
PLCSB (P<0.05). Following PLCSB treatment, the mRNA expression
of caspase-3, -8 and -9 were gradually increased in a
dose-dependent manner (Fig. 6).
Furthermore, the induction of apoptosis by PLCSB was associated
with the upregulation of caspase-3, -8 and -9 mRNA and protein
expression.
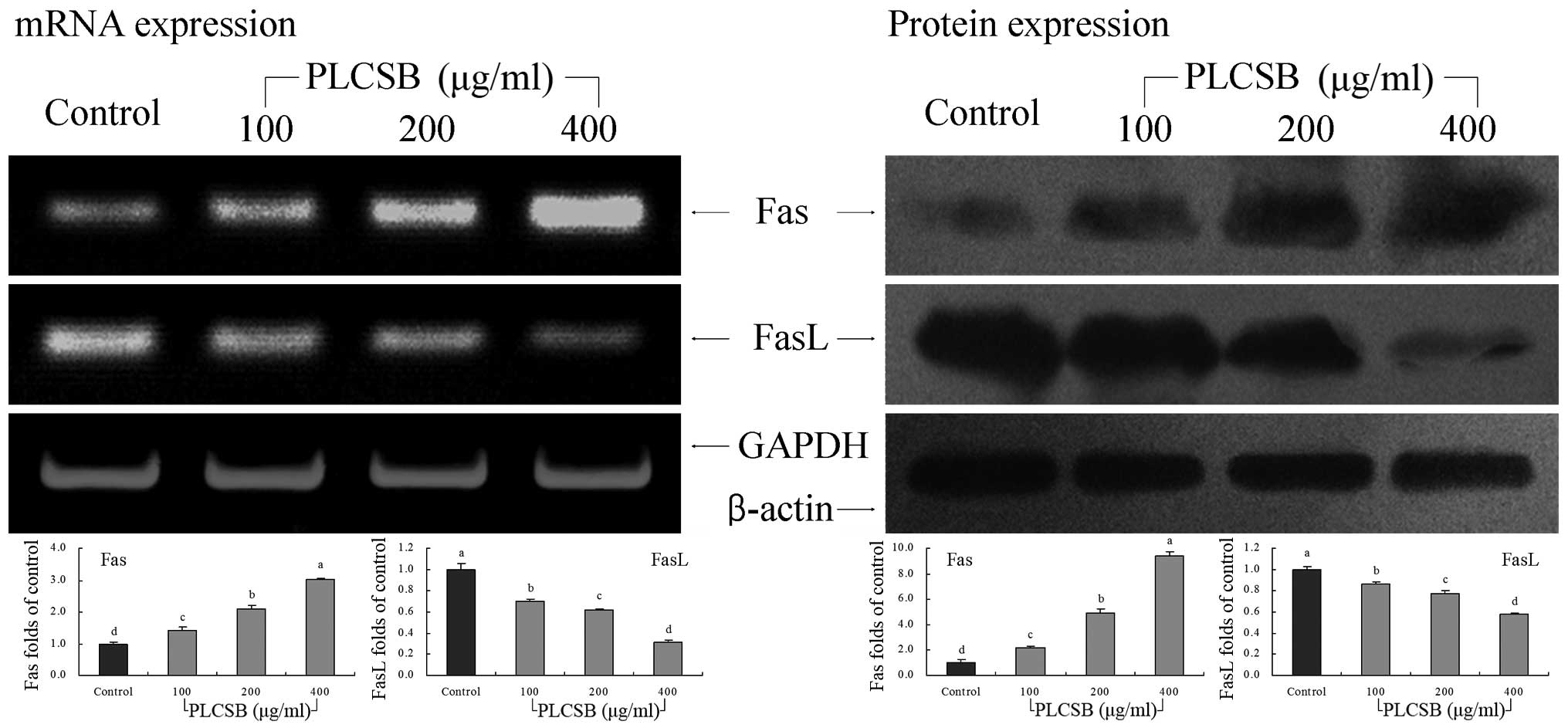
Fas and FasL gene expression
This study further determined whether the
apoptosis-inducing actions of PLCSB were associated with inhibition
of Fas and FasL gene expression. As shown in Fig. 7, PLCSB demonstrated induction
activity of apoptosis in HCT-116 cells, as indicated by increased
mRNA and protein expression of Fas along with decreased FasL
expression when compared with untreated cancer cells
(P<0.05).
Discussion
The swim bladder has been historically used as a
folk medicine. Recently, swim bladder has been shown to alleviate
various inflammatory conditions, and it may also augment the
function of platelets, capillary vessels and clotting factors
(11). Polysaccharides are the main
component of swim bladder; however, few studies have investigated
the polysaccharides of the swim bladder, and these studies showed
that polysaccharides of the swim bladder posses anti-inflammatory
effects (12,13). To the best of our knowledge, the
present study was the first to investigate the anticancer effect of
apoptosis induction by PLCSB in vitro. The results
demonstrated that the PLCSB exhibited a marked apoptosis-inducing
effect against the HCT-116 colon cancer cells.
Apoptosis induction in cancer cells is a potentially
promising approach for cancer therapy (14). In the present study, PLCSB decreased
the growth of HCT-116 cells via the induction of apoptosis.
Apoptotic cells with degraded DNA exhibit hypodiploid DNA content
and are presented as sub-G1 peaks on DNA histograms, which are used
to count the percentage of apoptotic cells (15). The formation of apoptotic bodies was
observed, in addition to increased sub-G1 DNA (apoptotic cells)
accumulation in cells treated with PLCSB.
Apoptosis is a critical cellular event and, thus,
eludicating its mechanisms of action may present potential for
improvements in tumor diagnosis and therapy (16). In normal cells, the anti-apoptotic
protein Bcl-2 is expressed on the outer mitochondrial membrane
surface (17). The apoptosis
regulator BAX promotes apoptosis by binding to and antagonizing the
Bcl-2 protein (18). Bcl-xL is a
member of the Bcl-2 protein family and it is hypothesized that the
relative amount of pro- and anti-survival Bcl-2 family of proteins
determines whether a cell undergoes apoptosis (19). The Bax, Bcl-2 and Bcl-xL genes are
predominantly expressed during apoptosis and, thus, the effects of
these genes on apoptotic activity were determined.
p53, which is a tumor supressor, upregulates
expression of the Bax protein, which has been found to be involved
in p53-mediated apoptosis (20).
p53 is a transcription factor, which regulates the Bax downstream
target gene when activated in response to stress (21). The expression of the p21 gene is
tightly controlled by the tumor suppressor protein p53, through
which this protein mediates the p53-dependent cell cycle G1 phase
arrest in response to a variety of stress stimuli (22).
The Apaf-1 gene encodes a cytoplasmic protein, which
presents one of the most important factors in the apoptosis
regulatory network. The Apaf-1 protein binds and cleaves caspase-9
preproprotein, releasing the mature, activated caspase-9, which
stimulates a subsequent caspase cascade that causes the cancer cell
to undergo apoptosis (23).
Caspase-8 initiates disassembly in response to signals from
extracellular apoptosis-inducing ligands (24). Caspases present a proteolytic
network within the cell in which upstream initiator caspases are
activated early in the apoptotic process (caspase-9), leading to
the activation of downstream caspases (caspase-3). Caspase-3
amplifies caspase-9 and caspase-9 initiation signals to induce
nuclear disassembly (25).
Fas is a death domain-containing member of the tumor
necrosis factor receptor superfamily, which is associated with
apoptosis of cancer cells. The FasL-Fas system has been
investigated with respect to its death-inducing function. The Fas
receptor exerts an apoptotic signal by binding to FasL, which is
expressed on the surface of other cells (26). FasL signals via trimerization of
FasR, which spans the membrane of the target cell. This
trimerization usually leads to apoptosis (27).
In the death receptor pathway, a signaling cascade
leads to the activation (using an adaptor, Fas-associated protein
with death domain) of a caspase cascade involving caspase-8 and -3.
BH3-only proteins are firstly upregulated or activated by cytotoxic
injury during the mitochondrial pathway, and subsequently activate
and oligomerize Bax, which leads the oligomerized Bcl-2 family
members, Bax/Bak, to induce the release of cytochrome c from
the mitochondria into the cytosol. Consequently, cytochrome
c and Apaf-1 form a complex, the apoptosome, which activates
caspase-9 and subsequently caspase-3 (28). In agreement with the results of the
present study, the induction of apoptosis in drug or functional
material-treated cancer cells has been reported to increase Bax,
p53, p21, Apaf-1, caspase-3,-8,-9 and Fas gene expression, and
decrease Bcl-2, Bcl-xL and FasL gene expression (9,29–32).
In the present study, PLCSB induced a high level of
apoptotic activity in HCT-116 colon cancer cells, in vitro.
PLCSB, particularly at high concentrations, induced apoptosis,
which was demonstrated by DAPI staining, flow cytometry analysis,
and changes in the mRNA and protein expression of apoptosis-related
genes. PLCSB may be used as health product or medicine for cancer
prevention and treatment in the future.
Acknowledgements
This study was supported by Fundamental Research
Funds for the Central Universities, China (grant no.
XDJK2013B010).
References
|
1
|
Li C and Yao CL: Molecular and expression
characterizations of interleukin-8 gene in large yellow croaker
(Larimichthys crocea). Fish Shellfish Immunol. 34:799–809. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lavi I, Friesem D, Geresh S, Hadar Y and
Schwartz B: An aqueous polysaccharide extract from the edible
mushroom Pleurotus ostreatus induces anti-proliferative and
pro-apoptotic effects on HT-29 colon cancer cells. Cancer Lett.
244:61–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li G, Kim DH, Kim TD, et al: Protein-bound
polysaccharide from Phellinus linteus induces G2/M phase
arrest and apoptosis in SW480 human colon cancer cells. Cancer
Lett. 216:175–181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 1999. View Article : Google Scholar
|
|
5
|
Zhao X, Song JL, Wang Q, et al:
Comparisons of Shuidouchi, Natto, and Cheonggukjang in their
physicochemical properties, and antimutagenic and anticancer
effects. Food Sci Biotechnol. 22:1077–1084. 2013. View Article : Google Scholar
|
|
6
|
Efferth T, Li PC, Konkimalla VS and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan C and Yan X: Study on extraction of
Lycium barbarum polysaccharides by different methods and their
antioxidant effects in vitro. Food Sci. 29:179–182. 2008.
|
|
8
|
Zhao X, Deng XX, Park KY, Qiu L and Pang
L: Purple bamboo salt has anticancer activity in TCA8113 cells in
vitro and preventive effects on buccal mucosa cancer in mice in
vivo. Exp Ther Med. 5:549–554. 2013.PubMed/NCBI
|
|
9
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anticancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19. 2013.
View Article : Google Scholar
|
|
10
|
Zhao X, Ju JH, Kim HM and Park KY:
Antimutagenic activity and in vitro anticancer effects of bamboo
salt on HepG2 human hepatoma cells. J Environ Pathol Toxicol Oncol.
32:9–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao H, Tian XL and Liu X: Study on
molecular identification and pharmacology of hemostasis action for
isinglass. J Chinese Inst Food Sci Technol. 9:170–176. 2009.
|
|
12
|
Chen S, Zhu K, Wang R and Zhao X:
Preventive effect of polysaccharides from the large yellow croaker
swim bladder on HCl/ethanol induced gastric injury in mice. Exp
Ther Med. 8:316–322. 2014.PubMed/NCBI
|
|
13
|
Jiang X, Zhao X, Luo H and Zhu K:
Therapeutic effect of polysaccharide of large yellow croaker swim
bladder on lupus nephritis of mice. Nutrients. 6:1223–1235. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Telford WG, King LE and Fraker PJ:
Comparative evaluation of several DNA binding dyes in the detection
of apoptosis associated chromatin degradation by flow cytometry.
Cytometry. 13:137–143. 1992. View Article : Google Scholar
|
|
16
|
Milanezi F, Leitão D, Ricardo S, Augusto I
and Schmitt F: Evaluation of HER2 in breast cancer: reality and
expectations. Expert Opin Med Diagn. 3:607–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chao DT and Korsmeyer SJ: Bcl-2 family:
regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heiser D, Labi V, Erlacher M and Villunger
A: The Bcl-2 protein family and its role in the development of
neoplastic disease. Exp Gerontol. 39:1125–1135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyashita T, Krajewski S, Krajewska M, et
al: Tumor suppressor p53 is a regulator of bcl-2 and bax gene
expression in vitro and in vivo. Oncogene. 9:1799–1805.
1994.PubMed/NCBI
|
|
21
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodriguez R and Meuth M: Chk1 and p21
cooperate to prevent apoptosis during DNA replication fork stress.
Mol Biol Cell. 17:402–412. 2006. View Article : Google Scholar :
|
|
23
|
Pop C, Timmer J, Sperandio S and Salvesen
GS: The apoptosome activates caspase-9 by dimerization. Mol Cell.
22:269–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cryns V and Yuan JY: Proteases to die for.
Genes Dev. 12:1551–7150. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wajant H, Pfizenmaier K and Scheurich P:
Non-apoptotic Fas signaling. Cytokine Growth Factor Rev. 14:53–66.
2003. View Article : Google Scholar
|
|
27
|
Desagher S and Martinou JC: Mitochondria
as the central control point of apoptosis. Trends Cell Biol.
10:369–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Imao T and Nagata S: Apaf-1- and
Caspase-8-independent apoptosis. Cell Death Differ. 20:343–352.
2013. View Article : Google Scholar :
|
|
29
|
Zhu K, Li GJ, Sun P, et al: In vitro and
in vivo anti-cancer activities of Kuding tea (Ilex kudingcha C.J.
Tseng) against oral cancer. Exp Ther Med. 7:709–715.
2014.PubMed/NCBI
|
|
30
|
Kim YA, Rhee SH, Park KY and Choi YH:
Antiproliferative effect of resveratrol in human prostate carcinoma
cells. J Med Food. 6:273–280. 2003. View Article : Google Scholar
|
|
31
|
Choi S, Lew KL, Xiao H, et al: D,
L-Sulforaphane-induced cell death in human prostate cancer cells is
regulated by inhibitor of apoptosis family proteins and Apaf-1.
Carcinogenesis. 28:151–162. 2007. View Article : Google Scholar
|
|
32
|
Bush JA, Cheung KJ Jr and Li G: Curcumin
induces apoptosis in human melanoma cells through a Fas
receptor/caspase-8 pathway independent of p53. Exp Cell Res.
271:305–314. 2001. View Article : Google Scholar : PubMed/NCBI
|