Introduction
Colorectal cancer remains the leading cause of
cancer-associated death in developed and developing countries
(1). Despite progress in surgery,
chemotherapy, biotherapy and radiotherapy, the mortality rate of
colorectal cancer remains high. In total, ~136,830 novel cases and
50,310 mortalities are estimated to occur due to colorectal cancer
in the United States in 2014 (2).
Lack of specificity is one of the main factors that limit the
efficacy of treatment, which leads to severe intolerable
side-effects (3). Therefore, the
development of effective and tumor-specific therapeutic approaches
is urgently required.
Previous studies have suggested that the unique
metabolism of cancer cells may become a novel target of
tumor-specific therapy (4–8). Cancer cells possess a different
bioenergetic metabolism to that of normal cells. Normal cells
mostly depend on mitochondrial oxidative phosphorylation to produce
energy, while cancer cells primarily depend on glycolysis, even in
the presence of oxygen. This altered metabolism is known as the
Warburg effect and gives rise to enhanced lactate production
(9–13). Targeting this type of metabolism may
offer a possibility for tumor-specific treatment.
D-erythrose is a tetrose carbohydrate that exists in
the human body in the form of D-erythrose 4-phosphate, which is an
intermediate in the pentose phosphate pathway (14). The metabolism of administered
D-erythrose in the human body remains unknown. Although the
transformation between D-erythrose and D-erythrose 4-phosphate
occurs in certain microorganisms (15), there are no studies investigating
this transformation in the human body. A previous study using
radioactive D-erythrose revealed that D-erythrose could be utilized
by rats, as the radioactivity eventually appeared in carbon dioxide
and glucose, indicating that the administered D-erythrose can be
completely oxidized to carbon dioxide (16). Based on the aforementioned findings,
at the 101st Annual Meeting of the American Association for Cancer
Research, Wang and Wei (17) put
forward the hypothesis that, in tumor tissues, the administered
D-erythrose is oxidized in the cytosol or mitochondria to carbon
dioxide, which is then converted to carbonic acid under the
catalysis of carbonic anhydrase. Since cancer cells possess
increased lactate production, this results in increased formation
of lactic acid and intracellular acidosis, which can ultimately
cause cell death once the acidosis exceeds a certain threshold. In
the previous study by Wang and Wei, D-erythrose was used as an
antitumor agent, and it was found that D-erythrose could inhibit
the viability of several cancer cell lines in vitro, and
suppress the growth of lung cancer in a subcutaneous tumor model of
LL-2 Lewis lung cells in vivo.
However, little is known about the antitumor
activity of D-erythrose in colorectal cancer in an animal model.
Therefore, in the present study, the antitumor effect of
D-erythrose in an abdominal metastatic model of colon carcinoma was
investigated. The present results revealed that intraperitoneal
administration of D-erythrose significantly suppressed the growth
of colon carcinoma, reduced the development of ascites and
increased tumor cell apoptosis.
Materials and methods
Cell culture
The mouse colon adenocarcinoma C-26 cell line
(American Type Culture Collection, Manassas, VA, USA) was cultured
in RPMI-1640 medium supplemented with 10% fetal bovine serum. The
cells were incubated at 37°C in a 5% CO2 humidified
incubator and passaged every three days using trypsin.
Establishment of the animal model and
therapy studies
The following procedures were approved by the
Institutional Animal Care and Use Committee of Sichuan University
(Chengdu, China). To evaluate the antitumor effect of D-erythrose
in colon cancer in vivo, an abdominal metastatic model of
colon carcinoma was established, as previously described (18). Male BALB/c mice (eight to nine weeks
old) were obtained from the Laboratory Animal Center of Sichuan
University and received IP injections of 0.2 ml C-26 cell
suspension, containing 2×105 cells. Three days after the
injection (day 0), the mice were randomly divided into two groups,
with eight mice per group, and received daily IP administration of
D-erythrose (500 mg/kg; Carbosynth, Compton, UK) or equal volume of
normal saline (NS), respectively, for the following 15 days. The
weight of the mice was recorded every three days. All mice were
sacrificed by cervical dislocation 24 h after the last
administration and the tumors were collected and weighed. The tumor
inhibition rate was calculated by the following formula: Tumor
inhibition rate (%) = (1 − mean tumor weight in D-erythrose-treated
group / mean tumor weight in NS group) × 100. The volume of ascites
was recorded at the same time.
Histological analysis
Tumors of each group were fixed in 10% formalin (pH
7.0) for ≥24 h, and then embedded in paraffin. The
paraffin-embedded tumors were then sliced into 3–5 μm sections.
Subsequent to slicing, a terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay was
performed to detect apoptotic cells in the tumor tissues using the
DeadEndTM Fluorometric TUNEL System, according to the
manufacturer’s instructions (Promega, Madison, WI, USA). The cell
nuclei stained with green fluorescence were identified as
TUNEL-positive nuclei. The apoptosis index was calculated by
analyzing the average percentage of TUNEL-positive cells in five
random fields from at least three different sections at a
magnification of ×400.
Toxicity assessment
To evaluate the possible side effects of
D-erythrose, health-associated indexes, including anorexia,
diarrhea, skin ulceration and toxic death, were observed every
three days. Furthermore, the main organs of the mice, including the
heart, liver, spleen, lung and kidney, were collected for
hematoxylin and eosin (H&E) staining.
Statistical analysis
The data were recorded as the mean ± standard error.
The two-tailed unpaired Student’s t-test was used for comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Antitumor effect of D-erythrose in an
abdominal metastatic model of colon carcinoma
The abdominal metastatic model of colon carcinoma
was used to evaluate the antitumor effect of D-erythrose in colon
cancer. The mice were administered daily with D-erythrose (500
mg/kg) or NS, respectively, for 15 days. All mice were sacrificed
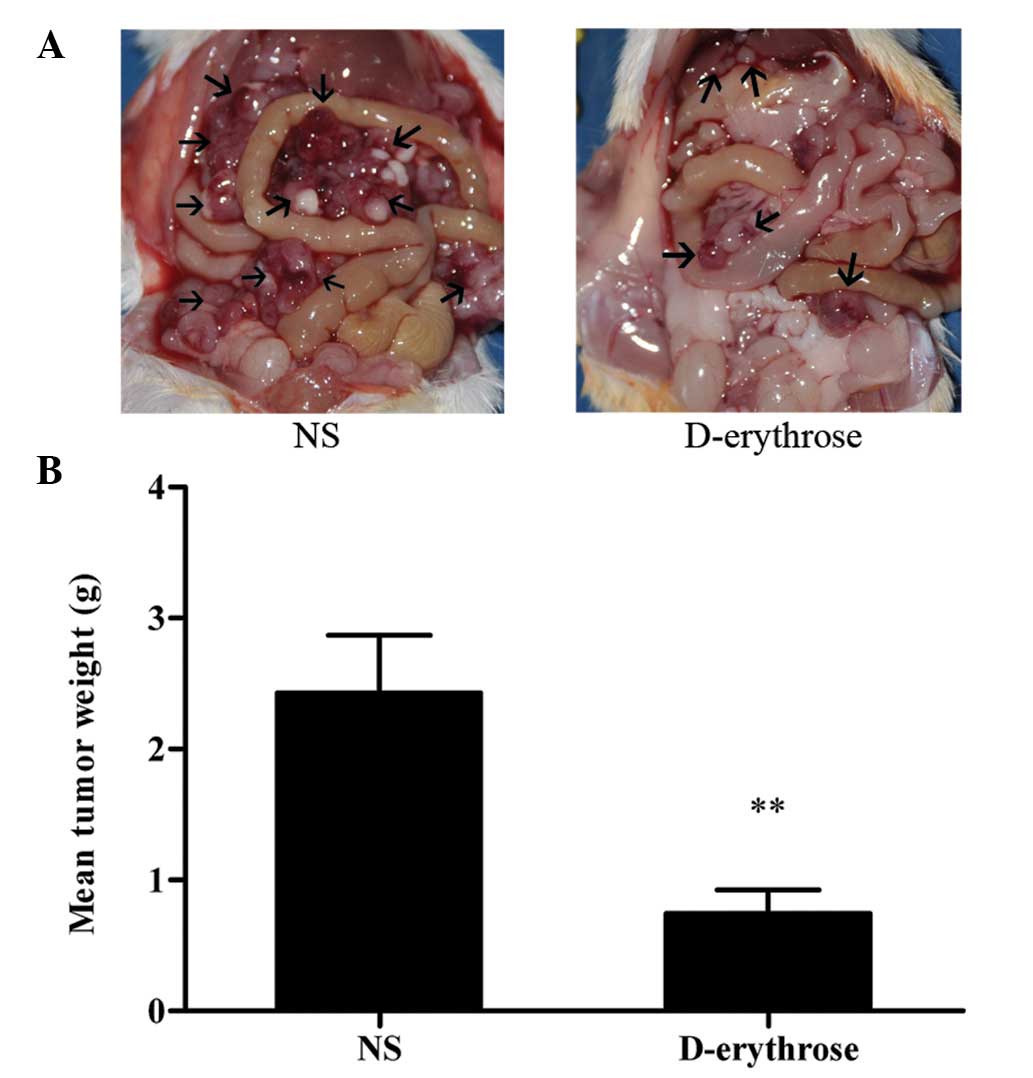
24 h after the last treatment. Fig.
1A shows representative images of intraperitoneal metastases of
C-26 colon carcinoma in the two groups. It is clear that mice
treated with D-erythrose bore fewer intraperitoneal metastases than
mice treated with NS. The metastases in each group were collected
and weighed. As shown in Fig. 1B,
the mean tumor weight was 0.75±0.17 in the D-erythrose-treated
group vs. 2.43±0.44 g in the NS group. IP administration of
D-erythrose (500 mg/kg) significantly suppressed tumor growth
compared with the control agent, with a tumor inhibition rate of
69.1% (P<0.01).
D-erythrose reduces the development of
ascites
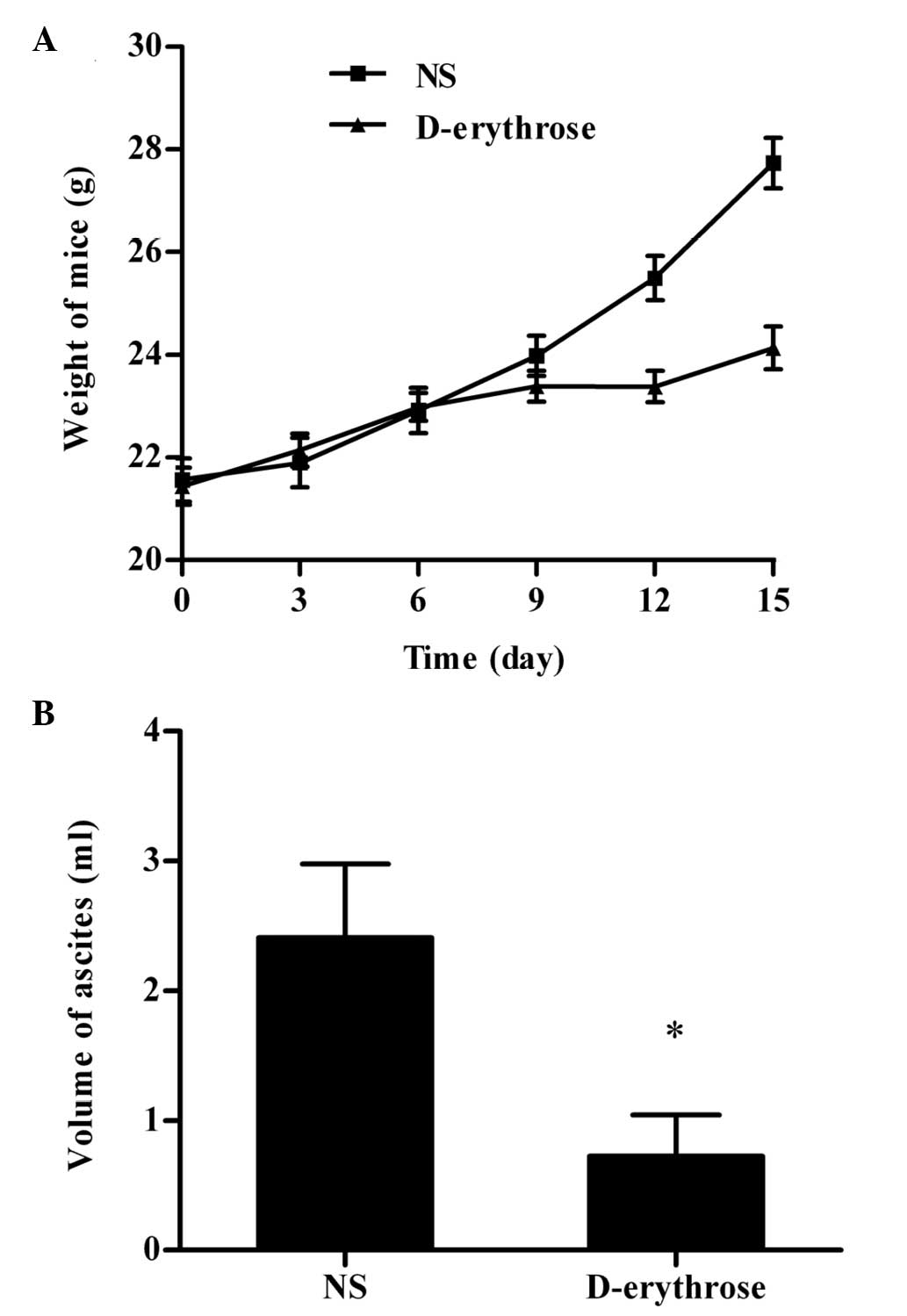
The mice were weighed every three days and the
weights of the mice increased rapidly over time due to the
accumulation of ascites. As shown in Fig. 2A, the ascites-associated weight gain
was evidently slower in the D-erythrose-treated group.
The volume of ascites was recorded subsequent to the
mice being sacrificed (Fig. 2B). In
the NS group, hemorrhagic ascites developed in seven of the eight
mice. By contrast, hemorrhagic ascites only developed in four of
the eight mice in the D-erythrose-treated group. In addition, the
mean volume of ascites in the D-erythrose-treated group was
significantly smaller compared with that of the NS group
(P<0.05).
D-erythrose increases apoptosis in tumor
tissues
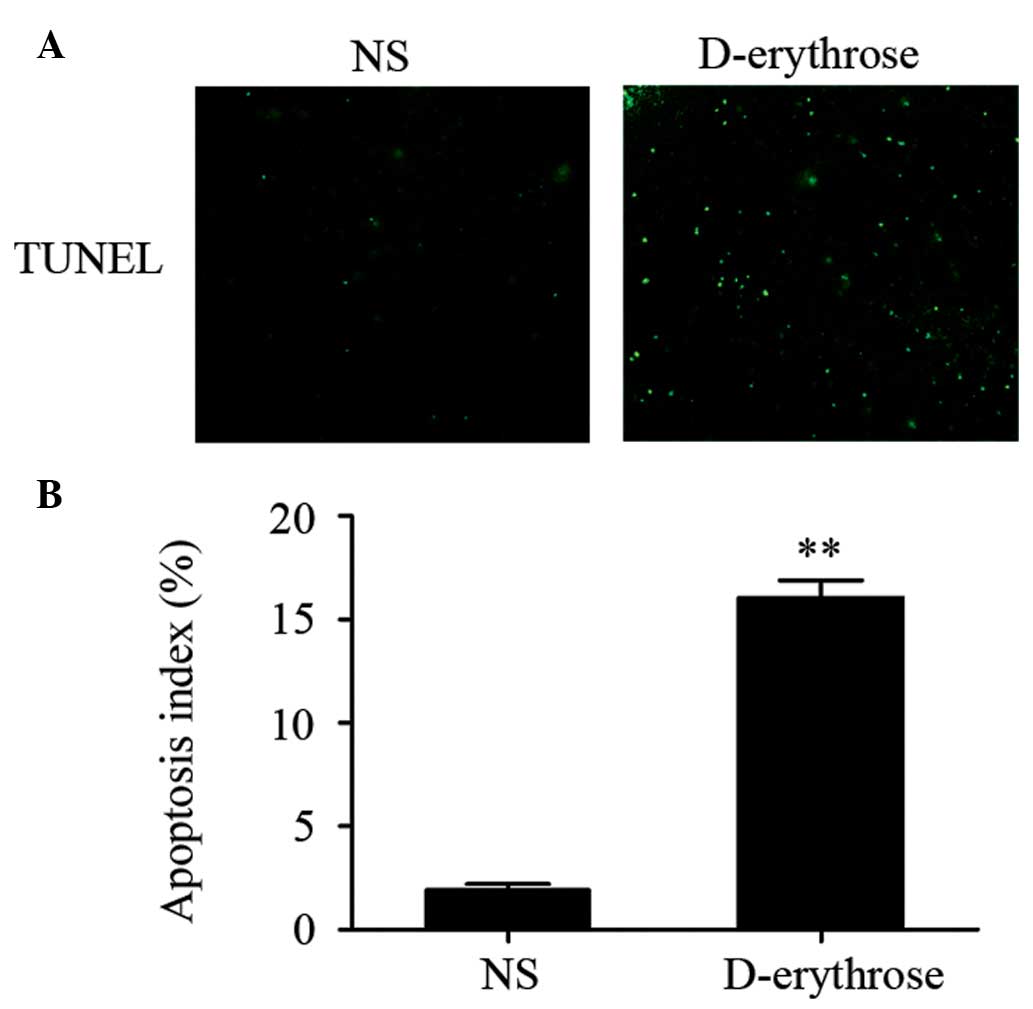
A TUNEL assay was used to detect apoptotic tumor
cells in each group. The cell nuclei stained with green
fluorescence were considered to be TUNEL-positive nuclei. As shown
in Fig. 3, the percentage of
TUNEL-positive cells (apoptotic cells) was significantly increased
in the D-erythrose treated group compared with that in the NS group
(P<0.01). Apoptotic cells were rare in the NS group.
Toxicity observation
No evident abnormalities, including anorexia,
diarrhea, skin ulceration and toxic death, were noted in the two
groups. Additionally, H&E staining of the heart, liver, spleen,
lung and kidney did not exhibit any clear pathological alteration
in the D-erythrose treated group compared with the NS group.
Discussion
The antitumor effect of D-erythrose in an abdominal
metastatic model of colon carcinoma was observed in the present
study. The results revealed that IP administration of D-erythrose
significantly suppressed the growth of intraperitoneal tumors
compared with the control agent, as revealed by mean tumor weight
analysis (Fig. 1). The present
results are similar to those obtained by Wang and Wei, who
subcutaneously injected D-erythrose beside a tumor in a
subcutaneous tumor model of LL-2 Lewis lung cells (17). Furthermore, in the present study,
malignant ascites were markedly reduced in the D-erythrose-treated
group (Fig. 2), indicating that
D-erythrose may also inhibit tumor invasion. The present study
provides proof of the principle that D-erythrose possesses
antitumor activity against colon cancer, and provides a focus for
future investigations.
The inhibition of apoptosis is considered essential
for tumor growth, and therefore induction of tumor cell apoptosis
has been demonstrated to be a promising strategy for tumor therapy
(19,20). In the present study, TUNEL staining
of the tumor tissue sections demonstrated that IP administration of
D-erythrose resulted in increased apoptosis of colon cancer cells
compared with the cells treated with the control agent (Fig. 3), indicating that D-erythrose may
exert its antitumor effect by induction of apoptosis.
Toxicity is an important factor that influences the
ultimate therapeutic effect of antitumor drugs (21). In the present study, no gross
abnormalities, including anorexia, diarrhea, skin ulceration and
toxic death, were noticed during the in vivo treatment
period. In addition, H&E staining of the major organs (heart,
liver, spleen, lung and kidney) did not exhibit any evident
pathological alteration following treatment with D-erythrose. These
results indicate that the administration of D-erythrose appears to
be safe and without detectable systemic toxic effects, at least at
the dose used (500 mg/kg). Furthermore, according to the public
report by the National Industrial Chemicals Notification and
Assessment Scheme, erythrulose, an isomer of erythrose, exhibits
low acute oral toxicity in rats (acute oral mean lethal dose, >2
g/kg), and the no-observed-effect-level was 1 g/kg/day in a 28-day
repeat dose oral toxicity study (22). The aforementioned results reveal
that the administration of D-erythrose (500 mg/kg) exhibits a low
toxicity and possesses potential clinical applications.
The antitumor mechanism of D-erythrose may be
associated with the unique bioenergetic metabolism of cancer cells.
Differing from normal cells, cancer cells mostly depend on
glycolysis rather than mitochondrial oxidative phosphorylation to
produce energy, even in the presence of ample oxygen. This
phenomenon was first reported by the Nobel Prize winner Warburg 90
years ago (9), and has repeatedly
been observed in various types of cancer cells. The increased
dependency upon glycolysis is a hallmark of cancer cell metabolism,
and gives rise to enhanced lactate production (11,12).
D-erythrose, a tetrose carbohydrate, can be used as cellular fuel,
and its final products are carbon dioxide and water (16). According to the hypothesis of Wang
and Wei, in cancer tissues, D-erythrose is oxidized to carbon
dioxide, which is then converted to carbonic acid catalyzed by
carbonic anhydrase (17). Due to
the increased lactate production in cancer cells, it ultimately
results in an increased formation of lactic acid. The lactic
acid-induced acidosis, once above a certain threshold, can finally
lead to cancer cell death. However, the exact mechanism requires
further investigation.
One of the key factors that restricts therapeutic
advances is the lack of tumor-specific therapy. Traditional
chemotherapy drugs against colorectal cancer, including
5-fluorouracil, irinotecan and oxaliplatin, are widely used in
clinical practice. However, they exhibit little or no specificity,
leading to various side effects that necessitate a dose reduction
or even termination of the therapy (23–27),
and any extremely serious side effect impairs the quality of life
of the individual. Therefore, it is necessary to develop additional
specific therapeutic strategies. For this purpose, the unique
bioenergetic metabolism of cancer cells has been increasingly
investigated (5–7). Based on the high levels of glycolysis
and lactate production in cancer cells, D-erythrose significantly
inhibited the growth of colon cancer in vivo in the present
study, without any observed effect on normal tissues, indicating
that D-erythrose may become a potentially effective and specific
antitumor agent against colon cancer. In addition, D-erythrose may
be more effective in combination with other antitumor drugs, which
provides an attractive therapeutic strategy for further
investigation.
In conclusion, the present data suggest that
D-erythrose can markedly suppress the growth of colon carcinoma,
inhibit tumor cell invasion and increase tumor cell apoptosis,
without any observed toxic effects. The present study may provide
an effective and specific therapeutic strategy for colon cancer
treatment.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. NSFC81071861), the
National 973 Program of China (no. 2010CB529905 and 2011CB910703),
the National Science and Technology Major Project (no.
2009zx09503-020), and the Specialized Research Fund for the
Doctoral Program of Higher Education of China (no. 20120181110029).
The authors would like to thank Mrs. Youcheng Wei for her valuable
comments on the original manuscript.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ojima I, Zuniga ES, Berger WT and Seitz
JD: Tumor-targeting drug delivery of new-generation taxoids. Future
Med Chem. 4:33–50. 2012. View Article : Google Scholar :
|
|
4
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schulz TJ, Thierbach R, Voigt A, et al:
Induction of oxidative metabolism by mitochondrial frataxin
inhibits cancer growth: Otto Warburg revisited. J Biol Chem.
281:977–981. 2006. View Article : Google Scholar
|
|
6
|
Zhao Y, Liu H, Riker AI, et al: Emerging
metabolic targets in cancer therapy. Front Biosci (Landmark Ed).
16:1844–1860. 2011. View
Article : Google Scholar
|
|
7
|
Nagasawa H: Pathophysiological response to
hypoxia - from the molecular mechanisms of malady to drug
discovery: drug discovery for targeting the tumor microenvironment.
J Pharmacol Sci. 115:446–452. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan DA, Sutphin PD, Nguyen P, et al:
Targeting GLUT1 and the Warburg effect in renal cell carcinoma by
chemical synthetic lethality. Sci Transl Med.
3:94ra702011.PubMed/NCBI
|
|
9
|
Warburg O, Posener K and Negelein E: On
metabolism of tumors. Biochem Z. 152:319–344. 1924.(In German).
|
|
10
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ristow M: Oxidative metabolism in cancer
growth. Curr Opin Clin Nutr Metab Care. 9:339–345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: cancer’s Achilles’ heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horecker BL: The pentose phosphate
pathway. J Biol Chem. 277:47965–47971. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hiatt HH and Horecker BL: D-erythrose
metabolism in a strain of Alcaligenes faecalis. J Bacteriol.
71:649–654. 1956.PubMed/NCBI
|
|
16
|
Batt RD, Dickens F and Williamson DH:
Tetrose metabolism 2. The utilization of tetroses and tetritols by
rat tissues. Biochem J. 77:281–294. 1960.PubMed/NCBI
|
|
17
|
Wang X and Wei Y: Erythrose kill cancer
cell in vitro and inhibit tumor growth in vivo. Cancer Res.
70(Suppl 1): 45482010. View Article : Google Scholar
|
|
18
|
Gou M, Men K, Zhang J, et al: Efficient
inhibition of C-26 colon carcinoma by VSVMP gene delivered by
biodegradable cationic nanogel derived from polyethyleneimine. ACS
Nano. 4:5573–5584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jensen M, Engert A, Weissinger F, et al:
Phase I study of a novel pro-apoptotic drug R-etodolac in patients
with B-cell chronic lymphocytic leukemia. Invest New Drugs.
26:139–149. 2008. View Article : Google Scholar
|
|
21
|
Jansman FG, Sleijfer DT, de Graaf JC,
Coenen JL and Brouwers JR: Management of chemotherapy-induced
adverse effects in the treatment of colorectal cancer. Drug Saf.
24:353–367. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Industrial Chemicals Notification
and Assessment Scheme (NICNAS). Full Public Report: Erythrulose
(Erythrulose Pentapharm). http://www.nicnas.gov.au/__data/assets/pdf_file/0015/10338/SN19FR.pdf.
Accessed February 11, 2008
|
|
23
|
Macdonald JS: Toxicity of 5-fluorouracil.
Oncology (Williston Park). 13(7 Suppl 3): 33–34. 1999.
|
|
24
|
Alimonti A, Gelibter A, Pavese I, et al:
New approaches to prevent intestinal toxicity of irinotecan-based
regimens. Cancer Treat Rev. 30:555–562. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grothey A: Clinical management of
oxaliplatin-associated neurotoxicity. Clin Colorectal Cancer.
5(Suppl 1): S38–S46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hotta T, Takifuji K, Arii K, et al:
Toxicity during l-LV/5FU adjuvant chemotherapy as a modified RPMI
regimen for patients with colorectal cancer. Oncol Rep. 14:433–439.
2005.PubMed/NCBI
|
|
27
|
Grenon NN and Chan J: Managing toxicities
associated with colorectal cancer chemotherapy and targeted
therapy: a new guide for nurses. Clin J Oncol Nurs. 13:285–296.
2009. View Article : Google Scholar : PubMed/NCBI
|