Introduction
Colorectal carcinoma is one of the most prevalent
forms of cancer that exists within Western countries (1), and is the second and third most common
type of cancer in males and females, respectively. The majority of
patients with advanced colon cancer require cytotoxic chemotherapy
as a primary treatment (2).
Recently, 5-fluorouracil (5-FU) has been widely used to treat cases
of colon cancer. In addition, a number of attempts have been made
to improve the objective response rates to chemotherapy, including
the use of 5-FU in combination with other agents. However, the
optimal combination regimen has not yet been identified, and the
standard treatment modality remains debatable (3). Therefore, a requirement exists to
identify novel compounds and optimized combined therapies for the
treatment of colon cancer. A growing number of patients have
selected herbal medicinal compounds as complementary therapies, in
combination with conventional chemotherapeutic treatments (4). Due to the narrow therapeutic windows
of existing chemotherapeutic drugs, these synergistic or additive
interactions may improve the therapeutic results and decrease the
necessary doses of current chemotherapeutic agents.
The Chinese herbal formula, Guan Chang Fu Fang
(GCFF), contains ingredients from three medicinal plants,
Agrimonia pilosa Ledeb., Patrinia scabiosaefolia and
Solanum nigrum L. serve as adjuvants to assist the effects
of the primary ingredient, A. pilosa. In traditional Chinese
medicine, A. pilosa is a plant that possesses anti-cancer
(5), anti-oxidant (6), acetylcholinesterase inhibitory
(7) and anti-inflammatory (8) activities. Certain studies have
identified that A. pilosa contains the phenolic compounds
catechin, agrimonin and quercetin (9). However, ethanol extracts of A.
pilosa have not yet been examined. P. scabiosaefolia,
another component of GCFF, has also been used in Chinese medicinal
formulas for the treatment of carbuncles, stasis, intestinal
abscess and dysmenorrhea (10).
Furthermore, P. scabiosaefolia is also an important
component of formulated traditional Chinese medicine prescriptions
to treat gastrointestinal and breast cancer (11). In addition, a number of in
vitro studies have revealed that Solanum nigrum L. has
antitumor effects against various types of cancer, including
leukemia and stomach, colon and endometrial cancers (12). Further studies indicated that an
aqueous extract of Solanum nigrum L. was able to enhance the
cytotoxicity of 5-FU, docetaxel, cisplatin and doxorubicin in
colorectal cells (13). Due to the
variety of adjuvant components, each herbal formula has a different
name. The term Guan Chang Fu Fang, meaning ‘enema of compound’ in
Chinese, was derived from the fact that the compound is clinically
used for enemas. Our preliminary experiments confirmed that the
ethanol extract of GCFF was more effective than the aqueous extract
(14), which led to the use of the
ethanol extract within the present study. In vitro and in
vivo studies have revealed that each component of the GCFF
compound has a significant cytotoxic effect upon numerous types of
cancer, particularly cancers of the digestive system (12). Despite this, the role of GCFF in the
treatment of cancer has not yet been addressed by modern science.
Therefore, the present preclinical study aimed to investigate
whether the combination of GCFF and 5-FU could produce a
significant synergistic interaction, which could treat colon
cancer. Furthermore, the expression of chemotherapeutic agent
resistance-related genes in colon cancer cells following treatment
with GCFF and 5-FU, either alone or in combination, was
investigated.
Materials and methods
Preparation of the GCFF extract
The medicinal plants used for the preparation of the
GCFF extract were provided by Bozhou Yonggang Medicinal Herbs
Factory Co., Ltd., (Bozhou, China). The preparation included
obtaining the ethanol extracts from the crude plant ingredients of
A. pilosa, P. scabiosaefolia and Solanum
nigrum L., at a ratio of 5:1:1. The plants were homogenized
with a Waring blender (Shanghai Specimen Model Factory, Shanghai,
China), and then soaked at a 10:l dilution in double-distilled
water for 24 h. The mixture was then heated to 100°C for 2 h, after
which an 8-fold volume of distilled water was added, followed by
further heating for 1.5 h. Next, the residue from the two combined
extracts was extracted twice with 80% ethanol. Firstly, the
plant-extract residue was extracted in a 10-fold volume of ethanol
for 2 h, and then an 8-fold volume of 80% ethanol was added. The
mixture was heated for a further 1.5 h, prior to the merging of the
two extracts, and then heated to 70°C to evaporate the ethanol.
Next, the ethanol extract was concentrated, and the decoction was
filtrated. For GCFF, the raw ethanol extract was mixed at a
concentration of 1.4 g herb/ml, and then filtered through a 0.2-mm
filter (Microgen, Laguna Hills, CA, USA) prior to use. The quality
control of the GCFF preparation, including definition of the
correct plants, production origin, implantation, harvesting and
processing, was conducted according to the guidelines defined by
Nanjing Herb Pharmaceutics, Ltd. The species, plant parts and
origins used within the GCFF formula are revealed in Table I. The total weight of the boiled
herbs was 210 g.
 | Table IGuan Chang Fu Fang components. |
Table I
Guan Chang Fu Fang components.
| Family | Latin binomial | Plant part | Origin |
|---|
| Rosaceae | Agrimonia
pilosa Ledeb. | Everything above
ground | Hubei, China |
| Valerianaceae | Patrinia
scabiosaefolia | Root | Sichuan, China |
| Solanaceae | Solanum nigrum
L. | Everything above
ground | Anhui, China |
Cell lines and cell culture
The poorly-differentiated human colon adenocarcinoma
LoVo cell line was provided by the Center Laboratory of the Jiangsu
Province Chinese Hospital (Nanjing, China). The LoVo cell lines
were propagated in RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA),
which was supplemented with 10% bovine serum, 100 U/ml penicillin
and 100 μg/ml streptomycinat 37°C in a water-saturated atmosphere
with 5% CO2.
Drugs
5-FU was supplied by the Jiangsu Hengrui Medicine
Company (Jiangsu, China). The Cell Titer 96 AQueous One Solution
Cell Proliferation Assay kit was purchased from Promega (Madison,
WI, USA) and the Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Detection kit was purchased from Invitrogen (Carlsbad,
CA, USA).
Cytotoxicity assay and analysis of
combination effects
The LoVo tumor cells were grown until the log-phase
had been reached, and then were seeded at a density of
8×103 cells per well into 96-well plates. The RPMI-1640
medium in each well was replaced with fresh medium, or with medium
containing various drug concentrations (0.21, 0.43, 0.87, 1.75, 3.5
and 7 mg/ml GCFF, and 0.02, 0.04, 0.16, 0.64, 2.5 and 10 μg/ml
5-Fu, as a single drug or in combination), for 48 h. The cells were
incubated for an additional 4 h with MTT, prior to absorbance
analysis at 490 nm using a microplate reader (elx800; Bio-Tek
Instruments, Inc., Winooski, VT, USA). The cell growth inhibition
rate was calculated using the following formula: Inhibition rate =
1 − ODexperiment/ODcontrol, where OD is the
optical density. The dose-response curves were obtained for GCFF:
0.21, 0.43, 0.88, 1.75, 3.5 and 7 mg/ml and 5-FU: 0.02, 0.04, 0.16,
0.64, 2.5 and 10 μg.ml, alone, and for multiple dilutions of
fixed-ratio combinations of the two drugs (0.02:0.21, 0.04:0.43,
0.16:0.88, 0.64:1.75, 2.5:3.5 and 10 μg/ml:7 mg/ml, 5-FU:GCFF,
respectively). The median-effect analysis was performed using the
combination index (CI) method, according to Chou and Talalay
(15). The CI is defined by the
following equation: CI = (D)1/(Dx)1 +
(D)2/(Dx)2 +
α(D)1(D)2/(Dx)1(Dx)2.
(Dx)1 and (Dx)2 are the concentrations of
D1 (GCFF) and D2 (5-FU) alone, which give x%
inhibition, whereas (D)1 and (D)2, as the
numerators, are the concentrations of GCFF and 5-FU that produce an
identical effect level when in combination. For example, α=0 when
GCFF and 5-FU are mutually exclusive (with similar modes of
action), whereas α=1 when GCFF and 5-FU are mutually non-exclusive
(with independent modes of action). A CI level of >1 indicates
antagonism, whereas a CI level of <1 indicates synergy and a CI
level equal to 1 indicates additivity. The CI ratio represented in
the present study was the mean value derived from at least three
independent experiments.
Apoptosis assay
The LoVo cells were briefly plated on a 60-mm Petri
dish and allowed to grow to reach 75–80% confluence. The cells were
then exposed to GCFF and 5-FU, either alone or in combination, for
48 h. Following incubation, the tumor cells were compared with the
untreated control cells. Next, the cells were collected and
resuspended in 500 μl binding buffer, to which 5 μl each of Annexin
V-FITC and propidium iodide (PI) was added. The analyses were
performed on a flow cytometer (FACScalibur; BD Biosciences,
Franklin Lakes, NJ, USA).
Cell cycle analysis
In total, 1×105 cells were seeded into
6-well plates and incubated overnight. The cells were then treated
with with 0.43 mg/ml GCFF and 0.04 μg/ml 5-FU, as a single drug or
in combination), for 48 h. Next, the cells were harvested, washed
with cold phosphate-buffered saline (PBS) and then fixed for 12 h
with 70% ethanol in PBS at 4°C. Following incubation, the cells
were resuspended in PBS with 100 μg/ml RNase and 50 μg/ml PI, and
incubated at 37°C for 30 min. The cell cycle distribution of
nuclear DNA was determined by flow cytometry using an FC500
cytometer (Beckman Coulter Inc., Pasadena, CA, USA).
Fluorescence microscopy
In total, 1×106 LoVo cells were seeded
into 6-well plates, incubated overnight and then treated with
either 0.43 mg/ml GCFF, 0.04 μg/ml 5-FU or a combination of GCFF
and 5-FU for 48 h. The cells were then washed twice with PBS, fixed
overnight with cold methanol and acetic acid at a ratio of 3:1, and
then stained with 1 μg/ml Hoechst 33342 (Life Technologies,
Carlsbad, CA, USA) for 30 min in the dark. The stained cells were
observed using a fluorescence microscope (magnification, ×400;
IX51; Olympus Corporation, Tokyo, Japan).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was isolated using TRIzol reagent
(Life Technologies), and reverse-transcribed into cDNA using a RT
reagent kit (Takara Bio, Inc., Shiga, Japan). The PCR reactions
were performed using the ABI 7500 fast real-time PCR system (Life
Technologies) and 1X ABsolute QPCR Mix (Thermo Fisher Scientific,
Waltham, MA, USA). The sequences of the primers (GenScript USA
Inc., Piscataway, NJ, USA) were as follows: Orotate phosphoribosyl
transferase (OPRT) forward, 5′-CGAGTAAGCATGAAA CCAGA-3′ and
reverse, 5′-CTACTCAAATACGCTTCC CCA-3; thymidylate synthase (TS)
forward, 5′-ACCTGAATC ACAATCGAGCCA-3′ and reverse,
5′-TTGGATGCGGATTGT ACCCT-3′; dihydropyrimidine dehydrogenase (DPD)
forward, 5′-TGTTCGGACAGAGCAAGATG-3′ and reverse, 5′-CTF
CAATCCGGCCATITCTA-3′; and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) forward, 5′-CCATGGAGA AGGCTGGGG-3′ and reverse,
5′-CAAAGTTGTCATGGA TGACC-3′. The PCR conditions were 50°C for 2 min
and 95°C for 15 min, followed by 45 cycles at 95°C for 15 sec and
60°C for 1 min. The relative gene expression quantifications were
calculated according to the comparative CT method, using
glyceraldehyde 3-phosphate dehydrogenase as an endogenous control
and commercial human total RNA (Clontech Laboratories Inc.,
Mountain View, CA, USA) as a calibrator. The final results were
determined according to the 2−ΔΔCT method (16).
Western blot analysis
In total, 1×106 LoVo cells were seeded
into 6-well plates, and incubated overnight. The cells were treated
according to the aforementioned instructions. Next, the cells were
washed twice with ice-cold PBS. The total proteins were solubilized
and extracted using a lysis buffer, which consisted of 20 mM HEPES
(pH 7.9), 20% glycerol, 200 mM KCl, 0.5 mM EDTA, 0.5% NP40, 0.5 mM
DTT and 1% protease inhibitor cocktail. The protein concentrations
were determined using a bicinchoninic acid protein assay. The
samples were separated using SDS-PAGE, and then transferred to
polyvinylidene fluoride membranes by electroblotting, prior to
antibody probing with rabbit anti-human B-cell lymphoma-2
(Bcl-2)-associated X protein (Bax) polyclonal antibody (1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
anti-human Bcl-2 polyclonal antibody (1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and rabbit anti-human
Bcl-2 19-kDa interacting protein 3 (Bnip3) polyclonal antibody
(1:1,000; Santa Cruz). The membranes were then incubated with goat
anti-rabbit IgG-horseradish peroxidase secondary antibodies
(dilution, 1:10,000; Cell Signaling Technology, Inc.). The blots
were developed with an enhanced chemiluminescence kit, and each
western blot assay was repeated three times.
Statistical analysis
The values are expressed as the mean ± standard
deviation. The statistical comparisons were performed using
Student’s t-test. P<0.05 was used to indicate a statistically
significant difference.
Results
Cytotoxicities of GCFF and 5-FU against
LoVo cells
The cytotoxic activities of GCFF and 5-FU were
investigated individually. As expected, GCFF and 5-FU individually
inhibited the proliferation of the LoVo cells in a dose-dependent
manner. Table II reveals the half
maximal inhibitory concentration (IC50) doses for the
LoVo cells upon exposure to GCFF or 5-FU. The response of the LoVo
cells to the different drugs were significantly different
(P=0.003).
 | Table IIIC50 doses of GCFF and
5-FU. |
Table II
IC50 doses of GCFF and
5-FU.
| IC50 (mean
± SD) |
|---|
|
|
|---|
| Cell line | GCFF, mg/ml | 5-FU, μg/ml |
|---|
| LoVo | 1.62±0.09 | 2.91±0.46 |
Median-effect analysis of combined GCFF
and 5-FU in vitro
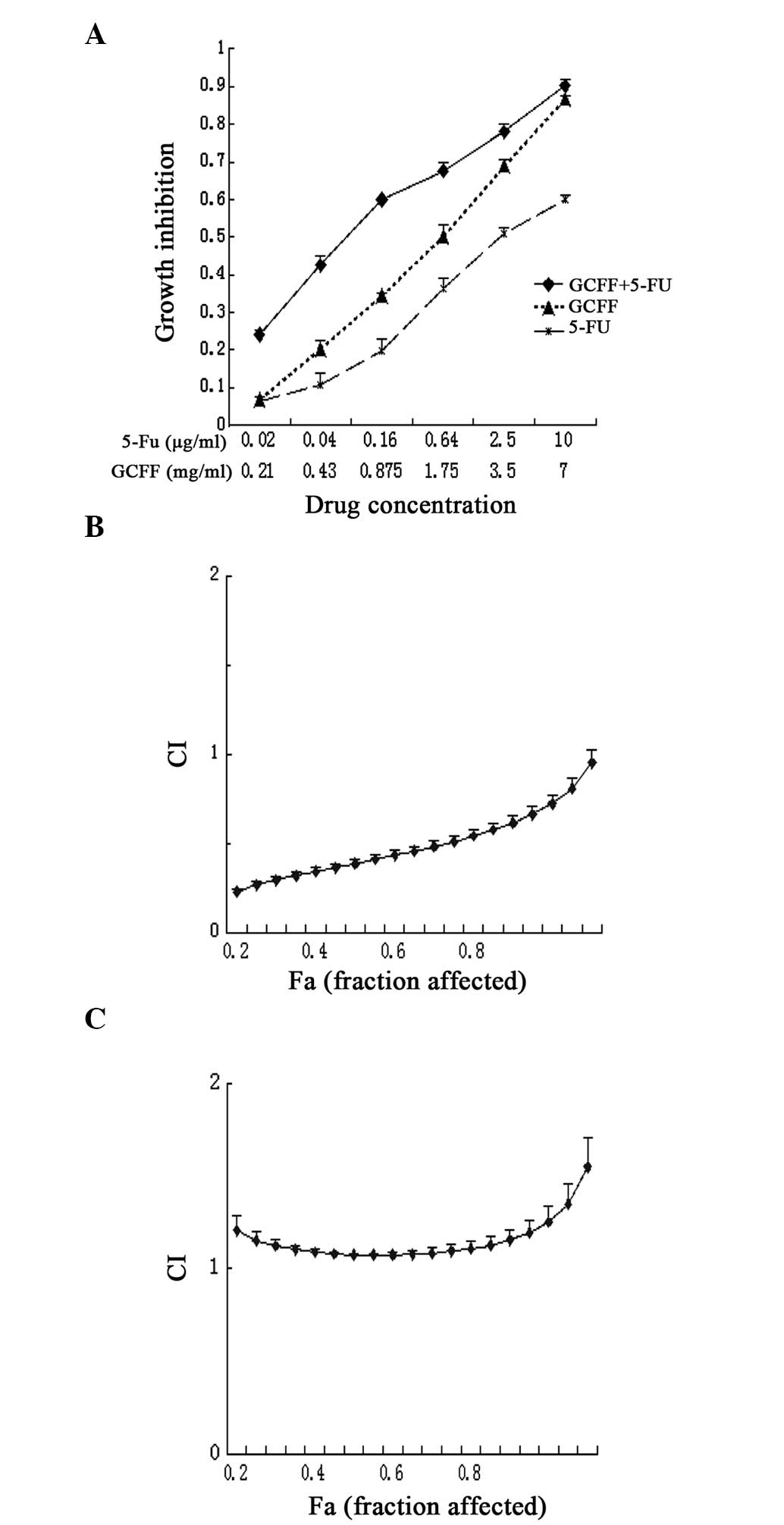
Fig. 1A presents the
dose-response curves for the LoVo cells that were exposed to GCFF
and 5-FU, alone or in combination. The combination of GCFF and 5-FU
demonstrated significant proliferative inhibition of the LoVo cells
at the majority of doses (0.21–3.5 mg/ml GCFF) (P=0.008). Fig. 1B reveals the cytotoxic effect upon
the cells simultaneously treated with GCFF and 5-FU. As the CI
values were below a relatively broad range of killed cell
fractions, this suggested that GCFF exhibited a synergistic effect
upon the cytotoxicity of 5-FU over a broad dose-inhibition range.
In addition, the present study analyzed the effect of sequential
drug delivery upon the LoVo cells; GCFF or 5-FU were administered
alone for 24 h, prior to administration of the second drug. The
treatment schedule in which GCFF was administered prior to 5-FU
demonstrated a synergistic growth inhibitory effect, similar to
that observed in the simultaneous treatment regimen. However,
significant antagonistic effects were identified when the cells
were treated in the reverse order (P=0.01; Fig. 1C). These results indicated that the
simultaneous treatment and administration of GCFF prior to 5-FU was
more effective compared with the reverse order.
Apoptotic effects mediated by GCFF and
5-FU
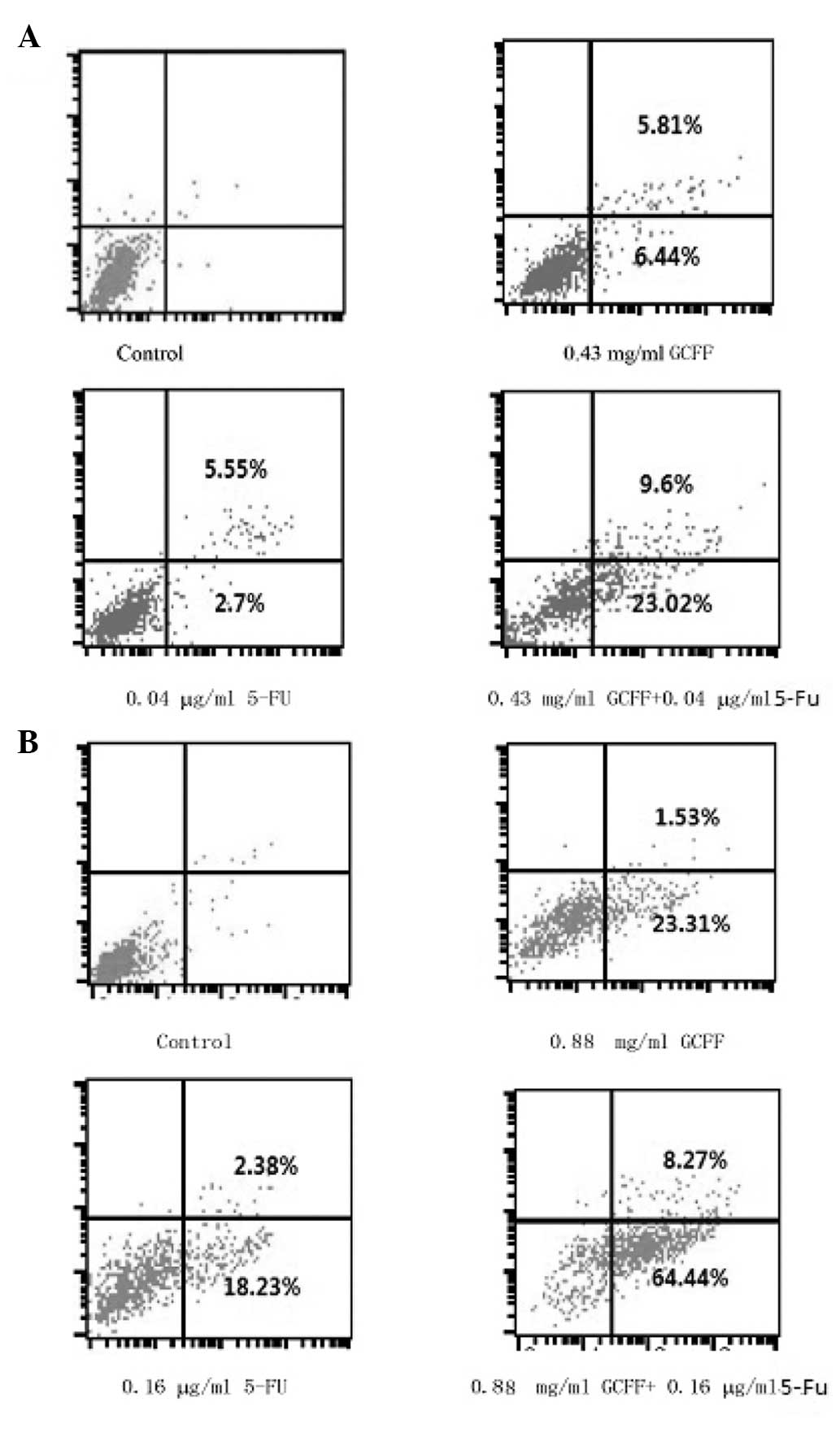
The Annexin V-FITC and PI double-staining was
performed in order to distinguish between the apoptotic cells and
the other cell populations. The LoVo cell lines were treated with
GCFF and 5-FU, alone and in combination. The percentage of
apoptotic cells present following treatment with 5-FU was
significantly increased by the co-administration of GCFF (P=0.003).
This indicated that the simultaneous treatment of GCFF and 5-FU
induced apoptosis in a synergistic manner (Fig. 2). In particular, the percentages of
LoVo cells that had undergone early apoptosis, induced by
single-agent treatment with either GCFF and 5-FU, were 23.31 and
18.23%, respectively, whereas the percentage of apoptotic cells
following the combined drug regimen increased to 64.44%.
GCFF induces S-phase cell cycle arrest in
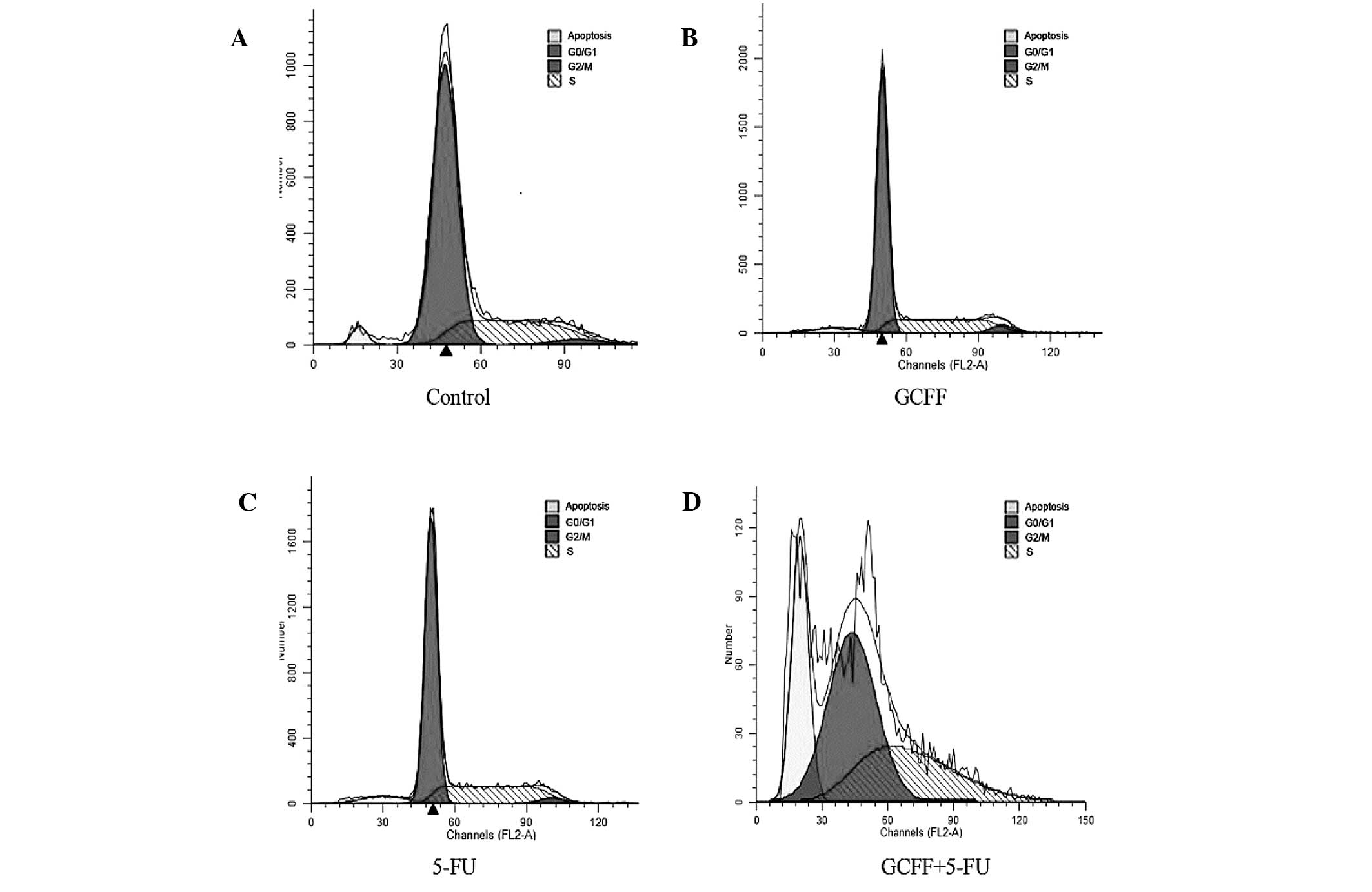
LoVo cells
Due to the significant effect of the GCFF and 5-FU
co-administration upon the apoptotic rate of the LoVo cells, the
present study also examined the potential effects of the combined
GCFF and 5-FU regimen upon the cell cycle distribution of LoVo
cells at doses below IC30. As revealed in Fig. 3 and Table III, the LoVo cells treated with
0.43 mg/ml GCFF and 0.04 μg/ml 5-FU demonstrated a larger number of
cells in the S-phase (38.06±1.90%) compared with the cells treated
with GCFF alone (27.99±0.38%). Furthermore, at lower doses,
treatment with GCFF increased the number of cells in the S-phase of
the cell cycle. Therefore, the results of the present study
suggested that following a 48-h treatment with a low-dose
combination of the two drugs, the cells were arrested in the
S-phase of the cell cycle.
 | Table IIIPercentage of cells in each phase of
the cell cycle, and total percentage of apoptotic cells, following
48 h of incubation. |
Table III
Percentage of cells in each phase of
the cell cycle, and total percentage of apoptotic cells, following
48 h of incubation.
| Drug
(concentration) | Cell cycle phase (%
of cells) | % of apoptotic
cells |
|---|
| Control |
G0/G1 (71.45) | |
| S (25.76) | |
| G2/M
(2.79) | |
| GCFF (0.43
mg/ml) |
G0/G1 (67.81) | 5.08 |
| S (27.99) | |
| G2
(4.25) | |
| 5-FU (0.04
μg/ml) |
G0/G1 (67.33) | 5.47 |
| S (30.08) | |
| G2/M
(2.59) | |
| GCFF (0.43 mg/ml) +
5-FU (0.04 μg/ml) |
G0/G1 (60.98) | |
| S (38.06) | 24.92 |
| G2/M
(0.97) | |
Percentage of apoptotic cells induced by
combination therapy is significantly higher compared with
monotherapy
Following a 48-h incubation with either 0.43 mg/ml
GCFF, 0.04 μg/ml 5-FU or a combination of the two, the cells were
examined by fluorescence microscopy. The chromatin condensation,
nuclear fragmentation and apoptotic bodies were clearly identified
in the treated cells (Fig. 4A).
Compared with the monotherapy-treated cells, the percentage of
apoptotic cells increased significantly following treatment with
the combined therapy (P=0.005).
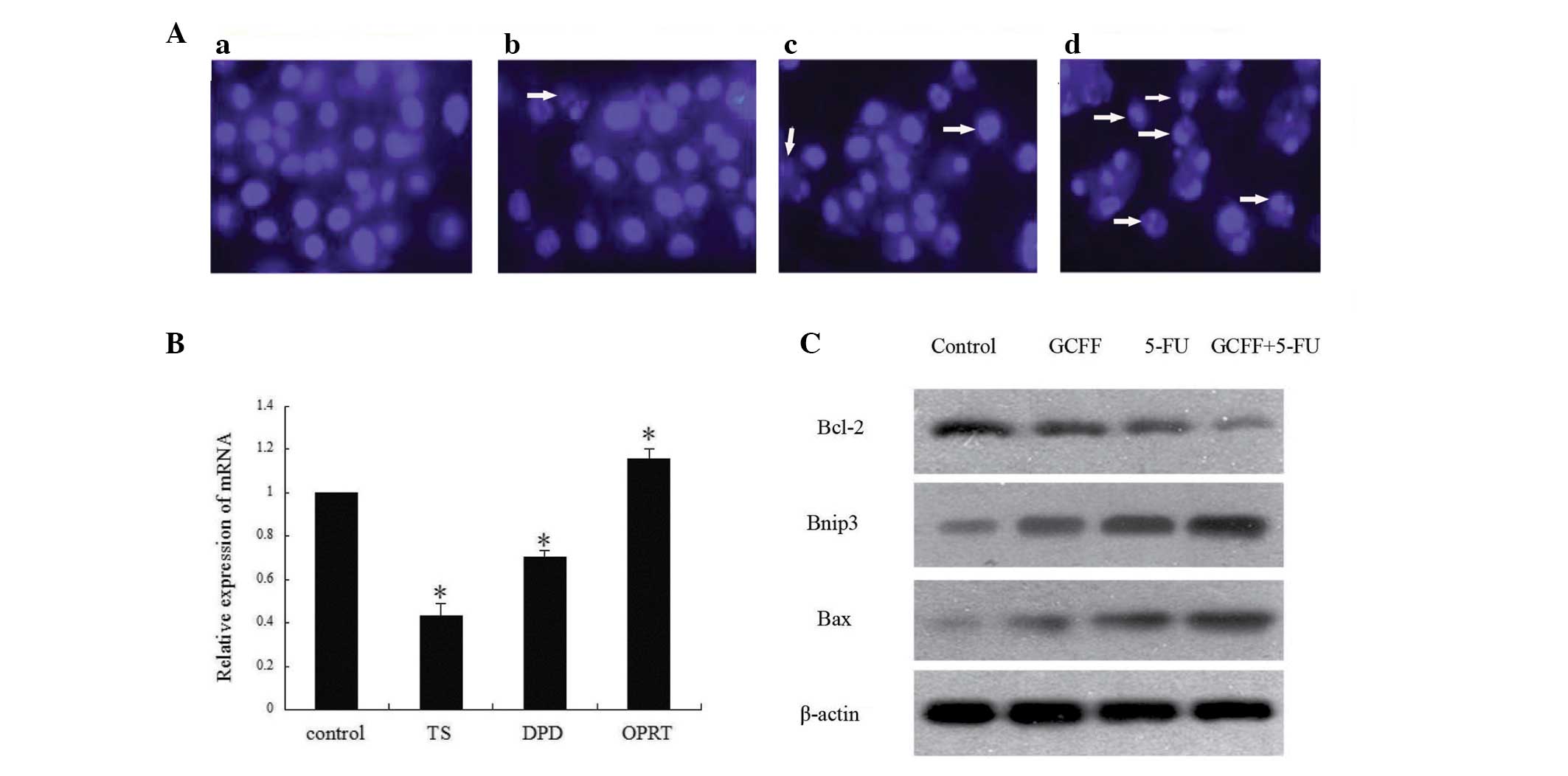 | Figure 4(A) Hoechst 33342 staining revealing
the presence of apoptotic cells. The human colon adenocarcinoma
LoVo cells were (a) left untreated or treated for 48 h with either
(b) 0.43 mg/ml Guan Chang Fu Fang (GCFF), (c) 0.04 μg/ml
5-fluoroucil (5-FU) or (d) GCFF + 5-FU. Compared with the control
group, the GCFF- and 5-FU-treated cells exhibited chromatin
condensation, nuclear fragmentation and apoptotic bodies (white
arrows). (B) GCFF suppressed the mRNA expression of certain
chemotherapeutic agent resistance-related genes (TS, DPD and OPRT)
in the LoVo cells. The changes in the relative gene expression
levels following a 48-h treatment with the half maximal inhibitory
concentration of GCFF are revealed (*P<0.05 vs. the
control). (C) Western blot analysis revealing the combined effects
of GCFF and 5-FU upon the expression of members of the B-cell
lymphona-2 (Bcl-2) family of proteins. LoVo cells were treated with
0.43 mg/ml GCFF, 0.04 μg/ml 5-FU or GCFF + 5-FU for 48 h. β-actin
served as the control. Bnip3, Bcl-2 19-kDa interacting protein 3;
Bax, Bcl-2-associated X protein; TS, thymidylate synthase; DPD,
dihydropyrimidine dehydrogenase; OPRT, orotate phosphoribosyl
transferase. |
GCFF affects the mRNA expression of
chemotherapeutic agent resistance-related genes
To explain the mechanisms that underlie the
synergistic association between GCFF and 5-FU, the present study
hypothesized that GCFF may affect the expression of certain
chemotherapeutic agent resistance-related genes, namely OPRT, TS
and DPD, which could effect the sensitivity of the LoVo cells to
5-FU. The cells were incubated for 48 h with GCFF or 5-FU alone, at
their respective IC40 values, or in combination. As
revealed in Fig. 4B, the expression
levels of TS and DPD were significantly downregulated following
treatment with GCFF alone. By contrast, the expression levels of
OPRT were significantly upregulated by GCFF. However,
administration of 5-FU alone did not downregulate the expression of
the drug resistance-associated genes.
Combined effect of GCFF and 5-FU on the
expression of the Bcl-2 family of proteins
In order to investigate the molecular mechanisms
that underlie the combined anticancer effects of GCFF and 5-FU, the
effects of GCFF and 5-FU, alone or in combination, upon the
expression of the Bax, Bcl-2 and Bnip3 were investigated. As shown
in Fig. 4C, the western blot
analysis demonstrated that treatment with either GCFF or 5-FU alone
reduced Bcl-2 expression, and increased Bax and Bnip3 expression.
Furthermore, the combined GCFF and 5-FU treatment significantly
reduced Bcl-2 expression, and increased Bax and Bnip3
expression.
Discussion
The use of active combination chemotherapy has the
potential to reduce drug toxicity and dosages, and address the
issue of drug resistance. The aim of the present study was to
investigate whether the cytotoxic effect of 5-FU could be enhanced
by the Chinese herbal medicinal compound, GCFF; this was determined
using media-effect analysis, flow cytometry and fluorescence
microscopy. The results of the present study indicated that the
simultaneous administration of GCFF and 5-FU inhibited cell growth
and induced apoptosis in a synergistic manner within the LoVo cell
line. It was concluded that GCFF contributed to 5-FU-induced
apoptosis and growth inhibition. Next, low doses of GCFF and 5-FU
were revealed to induce S-phase cell cycle arrest within the LoVo
cells via the Bcl-2 family of proteins. Finally, the expression
levels of certain chemotherapeutic agent resistance-related genes
were identified to be downregulated by GCFF alone and in
combination with 5-FU. Overall, the results indicated that GCFF may
be a potential candidate for a combined therapy approach alongside
5-FU. The underlying therapeutic mechanisms of this combined
therapy may be attributable to a downregulation of certain
chemotherapeutic agent resistance-related genes and a synergistic
effect upon the rate of cellular apoptosis. Complementary and
alternative medicines have been increasingly accepted by patients
with cancer in China (6), and a
number of patients have taken herbal medicines prior to or during
chemotherapy. One previous study revealed that certain herbal
medicinal formulae improve the clinical outcomes of chemotherapy
(17). However, the potential
underlying mechanisms have not yet been identified. Another prior
study demonstrated the potential of a number of plant-derived
compounds to sensitize tumor cells to chemotherapeutic agents and
restore the sensitivity of certain drug-resistant cells (16). However, further elucidation was
required as to whether the herbal medicinal formulae could
sensitize tumor cells to chemotherapeutic agents. In the present
study, a media-effect analysis was performed in order to observe
the interaction between GCFF and 5-FU in the LoVo cell lines. In
the future, similar studies could be performed upon other cell
lines to further investigate the therapeutic potential of
combination regimens against different types of tumor cells.
In recent years, 5-FU has been widely administered
for the treatment of colon cancer (18). 5-FU is able to induce DNA damage,
either directly or indirectly (19), and initiate apoptosis via the
p53-dependent pathway (20). A
number of experimental studies have revealed that the
overexpression of chemotherapy agent resistance-related genes is
associated with drug resistance. The DNA synthase enzyme, TS, is
targeted by 5-FU and possesses an important role in the efficacy of
5-FU. High levels of TS expression have been demonstrated to
contribute to the occurrence of 5-FU resistance and poor clinical
outcomes (21). Certain in
vivo and in vitro studies have revealed that OPRT is one
of the key enzymes involved in 5-FU metabolism, and that
phosphorylation of its active metabolite is necessary to inhibit
cellular DNA synthesis and induce RNA dysfunction (22). In addition, OPRT mRNA expression has
been demonstrated to predict 5-FU sensitivity; specifically,
patients with high OPRT expression are more sensitive to 5-FU
treatment, and are therefore more likely to benefit from it
(23). DPD is an enzyme that
catalyzes the major catabolic step during pyrimidine metabolism
(24). A previous study identified
that low levels of DPD were associated with low TS expression, and
were also correlated with responses to 5-FU-based chemotherapy
(25). Further studies have
revealed that, in addition to being an important determinant of
5-FU pharmacokinetics and clinical toxicity, DPD activity is also a
significant factor involved in the determination of 5-FU
availability for the production of active metabolites within tumors
(26). This finding highlights the
potential of DPD activity to predict the response of tumors to 5-FU
therapy. In the present study, TS and DPD mRNA were revealed to be
significantly downregulated by GCFF, alone or in combination with
5-FU. By contrast, treatment with 5-FU alone did not downregulate
the expression of the resistance-associated genes. This finding may
explain why GCFF was able to sensitize the cells to 5-FU, and why
the synergistic effect of the simultaneous treatment regimen and
the administration of GCFF prior to 5-FU, differed from the effect
observed upon the administration of 5-FU prior to GCFF.
The Bcl-2 family of proteins are key regulators of
the apoptotic pathway (19). The
results of the present study revealed that treatment of the LoVo
cells with GCFF, in combination with 5-FU, significantly decreased
the expression of Bcl-2, but increased the expression of Bax and
Bnip3. This finding indicated that GCFF and 5-FU induce apoptosis
by regulating the expression of the Bcl-2 family of proteins. The
mitochondrial protein Bnip3, formerly known as NIP3, is a member of
the Bcl-2 family that induces apoptosis via the mitochondrial
permeability transition pore in the absence of a functional BH3
domain. In normal tissues, endogenous Bnip3 is loosely associated
with the mitochondrial membranes, but during the induction of
apoptosis, it is fully integrated within the outer mitochondrial
membrane, with the N-terminus remaining in the cytoplasm and the
C-terminus in the membrane (27).
The Bcl-2 proteins, in particular Bnip3, mediate the balance
between pro- and anti-apoptotic actions. This balance has a
significant role in tumor evolution processes. Further analysis,
through the examination of gene expression profiles of these
different pathways, is required.
Abbreviations:
|
GCFF
|
Guan Chang Fu Fang
|
|
5-FU
|
5-fluorouracil
|
|
OPRT
|
orotate phosphoribosyl transferase
|
|
TS
|
thymidylate synthase
|
|
DPD
|
dihydropyrimidine dehydrogenase
gene
|
|
CI
|
combination index
|
|
PI
|
propidium iodide
|
References
|
1
|
Ferlay J, Autier P, Boniol M, et al:
Estimates of the cancer incidence and mortality in Europe in 2006.
Ann Oncol. 18:581–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steele G Jr and Ravikumar TS: Resection of
hepatic metastases from colorectal cancer. Biologic perspective.
Ann Surg. 210:127–138. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 10(suppl 3): 49–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui Y, Shu XO, Gao Y, et al: Use of
complementary and alternative medicine by Chinese women with breast
cancer. Breast Cancer Res Treat. 85:263–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyamoto K, Kishi N and Koshiura R:
Antitumor effect of agrimonin, a tannin of Agrimonia pilosa Ledeb.,
on transplantable rodent tumors. Jpn J Pharmacol. 43:187–195. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He C, Ji X, Pan Y, et al: Antioxidant
activity of alcoholic extract of Agrimonia pilosa Ledeb. Med Chem
Res. 19:448–461. 2010. View Article : Google Scholar
|
|
7
|
Jung M and Park M: Acetylcholinesterase
inhibition by flavonoids from Agrimonia pilosa. Molecules.
12:2130–2139. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung CH, Kim JH, Park S, et al: Inhibitory
effect of Agrimonia pilosa Ledeb. on inflammation by suppression of
iNOS and ROS production. Immunol Invest. 39:159–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu X, Qi X, Wang W and Chen G: Separation
and determination of flavonoids in Agrimonia pilosa Ledeb. by
capillary electrophoresis with electrochemical detection. J Sep
Sci. 28:647–652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang HM and But PPH: Pharmacology and
Applications of Chinese Materia Medica. World Scientific;
Singapore; Philadelphia, PA, USA: 1986, View Article : Google Scholar
|
|
11
|
Chiu LC, Ho TS, Wong EY and Ooi VE: Ethyl
acetate extract of Patrinia scabiosaefolia downregulates
anti-apoptotic Bcl-2/Bcl-X(L) expression, and induces apoptosis in
human breast carcinoma MCF-7 cells independent of caspase-9
activation. J Ethnopharmacol. 105:263–268. 2006. View Article : Google Scholar
|
|
12
|
An L, Tang JT, Liu XM and Gao NN: Review
about mechanisms of anti-cancer of Solanum nigrum. Zhongguo Zhong
Yao Za Zhi. 31:1225–1226. 12602006.(In Chinese).
|
|
13
|
Tai CJ, Wang CK, Tai CJ, et al: Aqueous
extract of Solanum nigrum leaves induces autophagy and enhances
cytotoxicity of cisplatin, doxorubicin, docetaxel, and
5-fluorouracil in human colorectal carcinoma cells. Evid Based
Complement Alternat Med. 2013:5147192013.PubMed/NCBI
|
|
14
|
Yu C, Xu M, Ju W, et al: Active ingredient
of enema in treatment of colorectal cancer. Journal of Liaoning
University of TCM. 16:50–54. 2014.
|
|
15
|
Chou TC and Talalay P: Quantitative
analysis of doseeffect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−DeltaDelta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Li J, Sun GZ, Lin HS, et al: The herb
medicine formula ‘Yang Wei Kang Liu’ improves the survival of late
stage gastric cancer patients and induces the apoptosis of human
gastric cancer cell line through Fas/Fas ligand and Bax/Bcl-2
pathways. Int Immunopharmacol. 8:1196–1206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wagner AD, Grothe W, Haerting J, et al:
Chemotherapy in advanced gastric cancer: a systematic review and
meta-analysis based on aggregate data. J Clin Oncol. 24:2903–2909.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matuo R, Sousa FG, Escargueil AE, et al:
5-Fluorouracil and its active metabolite FdUMP cause DNA damage in
human SW620 colon adenocarcinoma cell line. J Appl Toxicol.
29:308–316. 2009. View
Article : Google Scholar
|
|
20
|
Matsuhashi N, Saio M, Matsuo A, et al:
Apoptosis induced by 5-fluorouracil, cisplatin and paclitaxel are
associated with p53 gene status in gastric cancer cell lines. Int J
Oncol. 26:1563–1567. 2005.PubMed/NCBI
|
|
21
|
Kuramochi H, Tanaka K, Oh D, et al:
Thymidylate synthase polymorphisms and mRNA expression are
independent chemotherapy predictive markers in esophageal
adenocarcinoma patients. Int J Oncol. 32:201–208. 2008.
|
|
22
|
Su F, Overholtzer M, Besser D and Levine
AJ: WISP-1 attenuates p53-mediated apoptosis in response to DNA
damage through activation of the Akt kinase. Genes Dev. 16:46–57.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian C, Zhou ZG, Meng WJ, et al:
Overexpression of connective tissue growth factor WISP-1 in Chinese
primary rectal cancer patients. World J Gastroenterol.
13:3878–3882. 2007.PubMed/NCBI
|
|
24
|
Lu Z, Zhang R and Diasio RB: Purification
and characterization of dihydropyrimidine dehydrogenase from human
liver. J Biol Chem. 267:17102–17109. 1992.PubMed/NCBI
|
|
25
|
Diasio RB and Lu Z: Dihydropyrimidine and
dehydrogenase activity and fluorouracil chemotherapy. J Clin Oncol.
12:2239–2242. 1994.PubMed/NCBI
|
|
26
|
Nita ME, Tominaga O, Nagawa H, et al:
Dihydropyrimidine dehydrogenase but not thymidylate synthase
expression is associated with resistance to 5-fluorouracil in
colorectal cancer. Hepatogastroenterology. 45:2117–2122. 1998.
|
|
27
|
Vande Velde C, Cizeau J, Dubik D, et al:
BNIP3 and genetic control of necrosis-like cell death through the
mitochondrial permeability transition pore. Mol Cell Biol.
20:5454–5468. 2000. View Article : Google Scholar : PubMed/NCBI
|