Introduction
AITL is a rare and unique subtype of peripheral
T-cell lymphoma, accounting for 2–5% of all non-Hodgkin lymphomas
(1). POEMS syndrome is a rare
paraneoplastic syndrome secondary to a plasma cell dyscrasia. The
POEMS acronym, which was coined by Bardwich et al (2) in 1980, refers to the features of the
syndrome: Polyneuropathy (P), organomegaly (O), endocrinology (E),
monoclonal protein (M), skin changes (S), which combine with a
range of other clinical and pathological symptoms, including fever,
cachexia, edema, thrombocytosis and multicentric Castleman’s
disease. The current study presents a rare case of AITL with POEMS
syndrome and reviews the literature on AITL. Written informed
consent was obtained from the patient.
Case report
A 53-year-old male developed weakness of the lower
limbs, numbness and stabbing pains below the ankle and wrist
joints, accompanied by a low fever and night sweating. One month
later, the patient developed a red, full-body rash and noticed
gradually worsening instability when walking. A rapidly
proliferative painless lymph node on the left side of the neck was
also noted. As a consequence, the patient was admitted to the
Xiangya Second Hospital of Center South University (Changsha,
Hunan, China). A physical examination revealed lower limb paresis
(grade 4/5 according to the manual muscle testing) (3), a high fever, a red rash and swelling
of the left neck lymph nodes to a size of 2.0×1.5 cm2.
Blood analysis revealed the following results: White blood cell
count, 14.6×109/l (normal range,
3.0–10.0×109/l) [87.6% neutrophils (normal range,
50–70%) and 5.6% lymphocytes (normal range, 20–40%)]; hemoglobin,
88 g/l (normal range, 20–160 g/l); platelet count,
462×109/l (normal range, 100–300×109/l);
sodium, 133 mmol/l (normal range, 136–146 mmol/l); calcium, 1.96
mmol/l (normal range, 2.08–2.80 mmol/l); albumin, 22.8 g/l (normal
range, 35–50 g/l); lactate dehydrogenase, 290 IU/l (normal range,
100–300 IU/l); rheumatoid factors, 23.7 IU/ml (normal range,
0.0–20.0 IU/l); erythrocyte sedimentation rate, 65 mm/h (normal
range, 0–15 mm/h); and C-reactive protein, 15 mg/dl (normal range,
0–10 mg/dl). Endocrine investigations revealed reduced levels of
neo-hombreol F (130 ng/dl; normal range, 250–836 ng/dl). The serum
immunoglobulin (Ig)G (31.5 g/l), IgA (8.55 g/l) and IgM (3.12 g/l)
levels were all high (normal ranges, 7.6–166 g/l, 0.71–3.35 g/l and
0.48–2.12 g/l, respectively), and immunoelectrophoresis revealed
monoclonal gammopathy (γ globulin, 51.5 g/l; normal range,
20.0–30.0 g/l). Bence-Jones protein was not detected in the urine.
The electromyography test showed a motor-dominant polyneuropathy
with demyelinating features in the lower limbs. An ultrasonic
cardiogram revealed that the interventricular septal and left
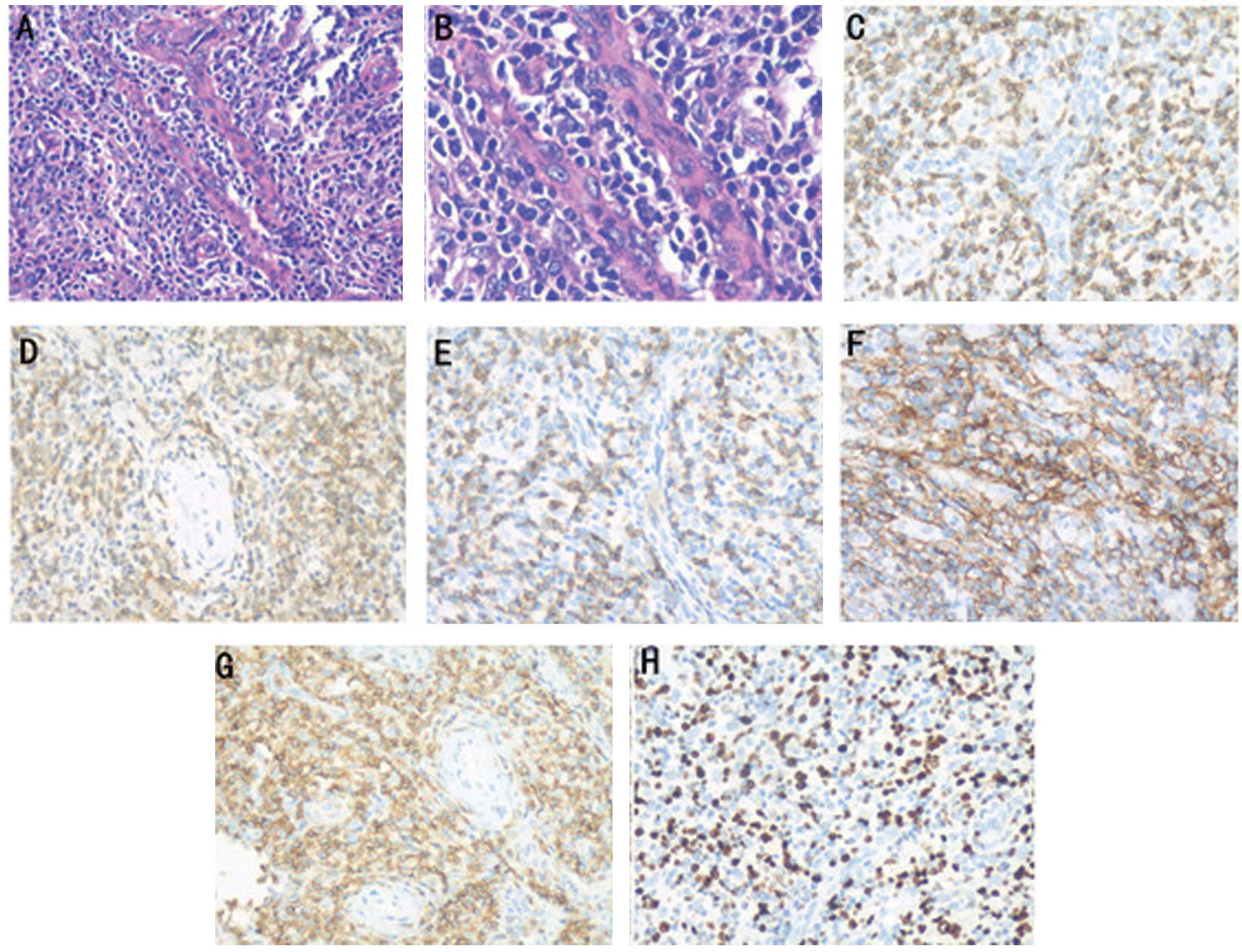
ventricular posterior walls were thickened. A biopsy of the left
neck lymph nodes revealed that the normal architecture was lost,
except for the presence of occasional depleted follicles with
concentrically arranged follicular dendritic cells, and that the
architecture was effaced by polymorphic infiltrate with marked
vascular proliferation (Fig. 1).
Immunohistochemistry revealed the following results: CD21 (++), CD3
(+), CD4 (+), CD45RO (++), CD8 (+), Ki-67 (++; 60%), CD79a (−),
anaplastic lymphoma kinase (−) and CD20 (−). The staining scores
were defined as follows: −, negative (<3% cells positively
stained); +, weakly positive (3–24% cells positively stained); ++,
moderately positive (25–49% cells positively stained); +++,
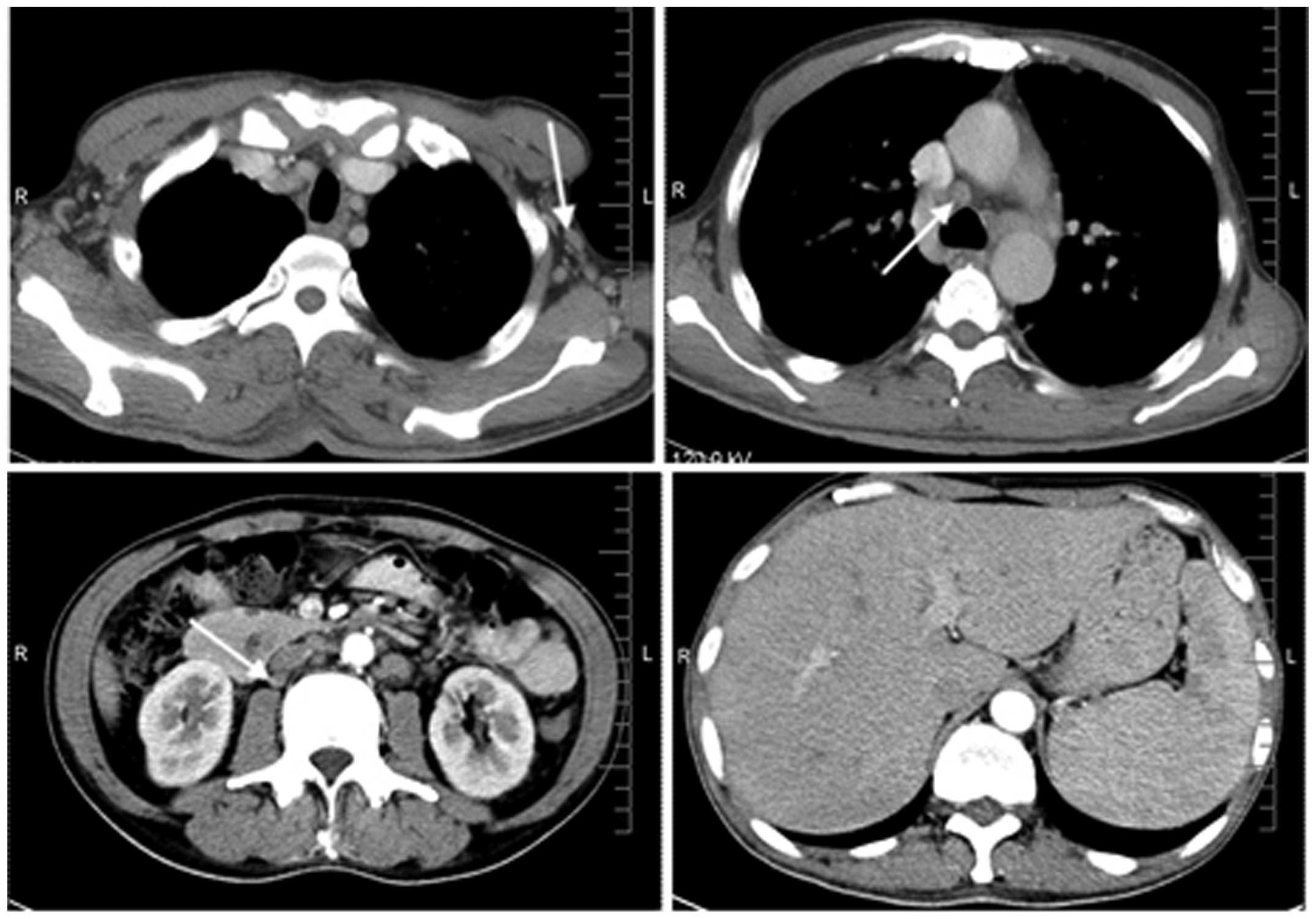
strongly positive (≥50% cells positively stained). Computed
tomography scans (Fig. 2) revealed
swelling of the axillary, mediastinal and retroperitoneal lymph
nodes, and hepatosplenomegaly. Magnetic resonance imaging of the
head was normal. Abnormal cells were confirmed by bone marrow
punctures. These findings were consistent with a diagnosis of stage
IV AITL, according to the World Health Organization classification
(4). The patient was also diagnosed
with POEMS syndrome, as determined by the criteria defined by
Dispenzieri et al (5).
Following two courses of gemcitabine (1,600 mg, days 1 and 8),
oxaliplatin (150 mg, day 1), DXM (10 mg, days 1–5) and
L-asparaginasum (1,000 IU, days 1–5) therapy, in a 21-day cycle,
the lymphadenopathy was reduced, and the skin changes and limb
neurological symptoms improved markedly.
Discussion
AITL is classified as a high-grade malignancy, with
a range of clinical symptoms and signs, including fever, weight
loss, anemia, hepatosplenomegaly, thrombocytopenia, lymphadenopathy
and polyclonal hypergammaglobulinemia (6). AITL mainly affects the elderly
population (median age, 59–65 years), with a slight predominance in
males. Geographically, the disease occurs more often in Europe
compared with North America or Asia (7).
Anthracycline-based chemotherapy regimens are
currently used as the first-line treatment for AITL, however, the
results are mostly short term and associated with early relapse
(8,9). For young patients or those suffering
from relapse, studies have shown that high-dose chemotherapy
combined with autologous stem cell transplantation has a higher
response rate and a lower recurrence rate (10,11).
Recent studies have used several chemotherapeutic agents, including
pralatrexate (12), bendamustine
(13), bortezomib (13) and immunomodulators such as
thalidomide (14) and cyclosporin-A
(15), for the treatment of AITL,
both as single agents and in combination. Certain targeted drugs,
such as alemtuzumab (16,17) and bevacizumab (18), also prevail in the treatment of
AITL.
The overall prognosis of AITL is poor and is
associated with a five-year survival rate of 30–36% and a median
survival time of less than three years. Poor prognosis factors of
AITL comprise an age of >60 years, a performance status of
>2, more than one extranodal site, the presence of B symptoms
and a platelet count of <150×109/l (19).
Lymphoma with POEMS or POEMS-like syndrome is
extremely rare in clinical practice, and to the best of our
knowledge, only three cases have previously been reported in the
literature, all of which were B-cell lymphoma (20–22).
With regard to the unique manifestation of the present case, it
must be determined whether a correlation exists between AITL and
POEMS syndrome. We hypothesize that AITL is derived from a
follicular helper T-cell subset of a germinal center; this T-cell
subset promotes positive selection, proliferation and
differentiation of germinal center antigen-specific B cells. A
number of studies have shown that chemokine (C-X-C motif) ligand 13
(23) and CD20 (24) are highly expressed in AITL. Iqbal
et al (25) also reported
that the AITL classifier is largely reflective of the
non-neoplastic cells in the microenvironment, with a significant
contribution by B cells. Therefore, we postulate that T cells
overactivate B cells and B cells react to T cells in the
pathogenesis of AITL. This hypothesis also provides a rationale to
explain the symptoms linked to B-lymphocyte activation, such as the
presence of plasmacytic infiltrate in tumor biopsies and the
development of hypergammaglobulinemia, as well as the
manifestations of immunological dysfunction. When considering
B-cell hyperstimulation stigmata, like POEMS syndrome, and the
putative feeder role of B cells for neoplastic T cells, we further
hypothesize that the disruption of putative B cell-T cell
interactions by rituximab could improve the clinical outcome. The
use of anti-CD20 therapy, such as of rituximab (26), in AITL has also been reported, but
remains under debate.
In summary, this study is the first to report a case
of AITL with POEMS syndrome, which may be associated with B cell-T
cell interactions. The findings in this case suggest that the
aberrant clones of B cells can also be caused by AITL. Therefore,
clinicians should be aware of the possibility of POEMS syndrome in
AITL patients, particularly when associated with polyneuropathy or
endocrine alterations. Additionally, further investigation may be
warranted into the use of a rituximab combination treatment to
improve the clinical outcome.
References
|
1
|
Rüdiger T, Weisenburger DD, Anderson JR,
et al: Peripheral T-cell lymphoma (excluding anaplastic large cell
lymphoma): Results from the Non-Hodgkin’s Lymphoma Classification
Project. Ann Oncol. 13:140–149. 2002. View Article : Google Scholar
|
|
2
|
Bardwick PA, Zvaifler NJ, Gill GN, et al:
Plasma cell dyscrasia with polyneuropathy, organomegaly,
endocrinopathy, M protein, and skin changes: the POEMS syndrome.
Report on two cases and a review of the literature. Medicine
(Baltimore). 59:311–322. 1980. View Article : Google Scholar
|
|
3
|
Conable KM and Rosner AL: A narrative
review of manual muscle testing and implications for muscle testing
research. J Chiropr Med. 10:157–165. 2011.PubMed/NCBI
|
|
4
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J: World Health Organization
Classification of Tumors. Pathology and Genetics, Tumors of
Haematopoietic and Lymphoid Tissues. IARC Press; Washington, DC:
2008
|
|
5
|
Dispenzieri A, Kyle RA, Lacy MQ, et al:
POEMS syndrome: definitions and long-term outcome. Blood.
101:2496–2506. 2003. View Article : Google Scholar
|
|
6
|
Vokurka S, Koza V, Vozobulová V, et al:
Angioimmunoblastic T-cell lymphoma as a very poor-prognosis
malignancy - a single centre experience. Klin Onkol. 25:206–211.
2012.(In Czech).
|
|
7
|
Vose J, Armitage J, Weisenburger D, et al:
International T-Cell Lymphoma Project: International peripheral
T-cell and natural killer/T-cell lymphoma study: pathology findings
and clinical outcomes. J Clin Oncol. 26:4124–4130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mourad N, Mounier N, Brière J, et al:
Groupe d’Etude des Lymphomes de l’Adulte: Clinical, biologic, and
pathologic features in 157 patients with angioimmunoblastic T-cell
lymphoma treated within Groupe d’ Etude des-Lymphoms de l’Adulte
(GELA) trials. Blood. 111:4463–4470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abouyabis AN, Shenoy PJ, Sinha R, et al: A
systematie review and meta-analysis of front-line
anthracycline-based chemotherapy regimens for peripheral T-cell
lymphoma. ISRN Hematol. 2011:6239242011. View Article : Google Scholar
|
|
10
|
Schetelig J, Fetscher S, Reichle A, et al:
Long-term disease-free survival in patients with angioimmunoblastic
T-cell lymphoma after high-dose chemotherapy and autologous stem
cell transplantation. Haematologica. 88:1272–1278. 2003.PubMed/NCBI
|
|
11
|
Kyriakou C, Canals C, Sibon D, et al:
High-dose therapy and autologous stem-cell transplantation in
Waldenstrom macroglobulinemia: the Lymphoma Working Party of the
European Group for Blood and Marrow Transplantation. J Clin Oncol.
28:2227–2232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O’Connor OA, Pro B, Pinter-Brown L, et al:
Pralatrexate in patients with relapsed or refractory peripheral
T-cell lymphoma: result from the pivotal PROPEL study. J Clin
Oncol. 29:1182–1189. 2011. View Article : Google Scholar
|
|
13
|
Kim SJ, Yoon DH, Kang HJ, Kim JS, et al:
Bortezomib in combination with CHOP as first-line treatment for
patients with stage III/IV peripheral T-cell lymphomas: a
multicentre, single-arm, phase 2 trial. Eur J Cancer. 48:3223–3231.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fabbri A, Cencini E, Pietrini A, Gozzetti
A, et al: Impressive activity of lenalidomide monotherapy in
refractory angioimmunoblastic T-cell lymphoma: report of a case
with long-term follow-up. Hematol Oncol. 31:213–217. 2013.
View Article : Google Scholar
|
|
15
|
Mori M, Inoue D, Arima H, et al:
Therapeutic efficacy of cyclosporin A for refractory
angiommunoblastic T cell lymphoma. Rinsho Ketsueki. 51:332–338.
2010.(In Japanese). PubMed/NCBI
|
|
16
|
Kuan JW, Chang KM, Lau NS, et al: The
outcome of hyperCVAD combined with alemtuzumab for the treatment of
aggressive T-cell and NK-cell neoplasms. Indian J Hematol Blood
Transfus. 27:136–145. 2011. View Article : Google Scholar :
|
|
17
|
Gallamini A, Zaja F, Patti C, Billio A, et
al: Alemtuzumab (Campath-1H) and CHOP chemotherapy as first-line
treatment of peripheral T-cell lymphoma: results of a GITIL (Gruppo
Italiano Terapie Innovative nei Linfomi) prospective multicenter
trial. Blood. 110:2316–2323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aguiar Bujanda D: Complete response of
relapsed angioimmunoblastic T-cell lymphoma following therapy with
bevacizumab. Ann Oncol. 19:396–397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mosalpuria K, Bociek RG and Vose JM:
Angioimmunoblastic T-cell lymphoma management. Semin Hematol.
51:52–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Viala K, Béhin A, Maisonobe T, et al:
Neuropathy in lymphoma: a relationship between the pattern of
neuropathy, type of lymphoma and prognosis? J Neurol Neurosurg
Psychiatry. 79:778–782. 2008. View Article : Google Scholar
|
|
21
|
Nakayama S, Yokote T, Kobayashi K, et al:
Primary cutaneous diffuse large B-cell lymphoma, leg type, with
features simulating POEMS syndrome. Eur J Haematol. 84:79–83. 2010.
View Article : Google Scholar
|
|
22
|
Sasaki T, Onishi S, Onishi R, Takemura R,
et al: POEMS syndrome complicated by follicular lymphoma. Rinsho
Ketsueki. 50:1621–1625. 2009.PubMed/NCBI
|
|
23
|
Dupuis J, Boye K, Martin N, et al:
Expression of CXCL13 by neoplastic cells in angioimmunoblastic
T-cell lymphoma (AITL): a new diagnostic marker providing evidence
that AITL derives from follicular helper T-cells. Am J Surg Pathol.
30:490–494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tachibana T, Tomita N, Furuya M, et al:
Aberrant CD20 expression in angioimmunoblastic T-cell lymphoma.
Intern Med. 50:495–499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iqbal J, Weisenburger DD, Greiner TC, et
al: Molecular signatures to improve diagnosis in peripheral T-cell
lymphoma and prognostication in angioimmunoblastic T-cell lymphoma.
Blood. 115:1026–1036. 2010. View Article : Google Scholar :
|
|
26
|
Delfau-Larue MH, de Leval L, Joly B, et
al: Targeting intratumoral B cells with rituximab in addition to
CHOP in angioimmunoblastic T-cell lymphoma. A clinicobiological
study of the GELA. Haematologica. 97:1594–1602. 2012. View Article : Google Scholar : PubMed/NCBI
|