Introduction
Previously termed the peripheral benzodiazepine
receptor, the translocator protein (TSPO) is a key element of the
mitochondrial permeability transition pore (MPTP), which is a
multiprotein complex located at the contact sites between the inner
and outer mitochondrial membranes of various different cell types,
including cells of the hematopoietic system. TSPO is physically
associated with the backbone of MPTP via its adenosine nucleotide
translocase and voltage-dependent anion channel (VDAC) (1). Consistent with its localization in the
MPTP, TSPO has been implicated in various mithochondrial functions,
such as the control of respiration, the modulation of inner
membrane ion channel activities, as well as the regulation of
apoptosis, cell proliferation and a number of other associated
processes (2). It has previously
been demonstrated that overexpression of TSPO inhibits apoptosis
induced by free radicals or ultraviolet light. Concordantly, TSPO
ligands have been demonstrated to induce caspase-3 (3) and -9 activation, the cytosolic release
of cytochrome c and cause an increase in the production of
reactive oxygen species (ROS), resulting in the activation of p38
mitogen activated protein kinase and the opening of the MPTP
(4). Furthermore, Carayon et
al (5) demonstrated that TSPO
expression correlated with H2O2 cytotoxicity
resistance in hematopoietic cell lines (5); following the transfection of Jurkat
cells with human TSPO complementary DNA, H2O2
resistance increased compared with wild-type cells. These results
indicate that TSPO may aid in preventing free radical-induced
cellular damage of mitochondria and may regulate cell apoptosis in
the hematopoietic system.
Previously, TSPO and its ligands have been
highlighted as potential targets for the development of novel
anticancer agents (6). TSPO is
highly expressed in various cancer cell types, including colon
(7), brain (8), breast (9), ovary (10–11)
and liver (12) cancer, and its
expression is particularly high in organs involved in
steroidogenesis (13). Although
additional studies are required to improve the understanding of the
biological functions of TSPO, clear evidence exists regarding its
involvement in steroid biosynthesis. Furthermore, TSPO is key in
cancer cell growth (14–17), with Maaser et al (18) demonstrating that specific TSPO
ligands are able to induce apoptosis and cell cycle arrest in
colorectal cancer cells.
Additionally, TSPO is regarded as a potential
prognostic factor in cancer. In particular, it has been reported
that lymphoma cell lines (19–20)
and myeloid/lymphoid cells obtained from leukemia patients
(5,20) express high levels of TSPO, and
ovarian, hepatic and colonic carcinomas, as well as glioma
(7–8,10–12)
have demonstrated increased TSPO densities compared with the
corresponding healthy tissue. In specific cases, TSPO expression
correlates with the grade of the tumor malignancy and patient
survival (21); for example,
relatively high levels of TSPO density were observed in more
rapidly proliferating breast cancer cells (22) and more aggressive breast cancer
phenotypes (23).
CLL is characterized by the accumulation of mature
malignant cluster of differentiation (CD) 5+
B-lymphocytes. Despite the use of numerous agents in the treatment
of CLL, patients may develop mechanisms of resistance (24). In order to stratify patients
according to their potential to respond to targeted therapy,
personalized targeted therapy has been proposed; however, this
requires the identification of novel (bio)markers.
In the present study, the role of TSPO as prognostic
factor in CLL patients was investigated by evaluating the response
to bendamustine and rituximab treatment according to TSPO
expression. In addition, thiobarbituric acid reactive substance
(TBARS) and nitric oxide (NO) levels, as well as caspase-3
activity, were analyzed in the lymphocytes of healthy donors
compared with CLL patients.
Materials and methods
Preclinical characteristics, treatment
strategy and peripheral blood mononuclear cell (PBMC)
isolation
The present study included 10 healthy blood bank
donors and 30 patients from the Hematology Unit of San Gennaro
Hospital (Naples, Italy). The patients enrolled in the present
study were diagnosed with CLL according to the following criteria:
Monotypic expansion of lymphoid cells (≥15×103/μl),
morphologically consistent with CLL (small lymphocytes), in the
blood for ≥60 days prior to treatment; >30% lymphocytes in the
bone marrow; and normal renal (creatinine, <2.0 mg/dl) and
hepatic (bilirubin, <2.0 mg/dl) function. No patients had
previously been treated for CLL and no patients had previously
received four cycles of bendamustine plus rituximab. The treatment
strategy administered in the present study was intravenous
rituximab (375 mg/m2) every 28 days, followed by
intravenous bendamustine (90 mg/m2) on the next day, for
two consecutive days. This treatment regimen was continued until
the occurrence of disease progression or unacceptable levels of
toxicity.
The response criteria used was that previously
defined by the National Cancer Institute Working Group (25). Complete remission (CR) was defined
as the absence of all palpable disease and the return of the blood
counts to within the following normal ranges: Neutrophils,
>1.5×103/ml; platelets, >100×103/ml;
hemoglobin, >11 g/dl; and bone marrow aspirate lymphocyte
percentage, <30%. Partial remission (PR) was defined as a
50% decrease in palpable disease accompanied by a 50% improvement
in all abnormal blood parameters. Patients were considered to be
responsive to the treatment if a PR of ≥6 months was observed and
resistant to the treatment if a PR of <6 months or no remission
(NR) were observed. PBMCs were isolated from blood samples obtained
prior to (T0), one week after (T1) and six months after (T6) the
commencement of treatment. The PBMCs were isolated using Ficoll
density gradient centrifugation, as previously described (26) and stored at -80°C for use in the
following experiments. In addition, sera was collected from the
patients at the abovementioned time periods and stored at −20°C for
use in the following experiments. This study was approved by the
ethics committee of San Gennaro Hospital and written informed
consent was obtained from all patients.
Analysis of TSPO protein expression
levels by flow cytometry
The PBMCs were fixed for 20 min in a 3% (w/v)
paraformaldehyde (PFA) solution and permeabilized for 10 min with
0.1% (w/v) Triton X-100 in phosphate-buffered saline (PBS) at room
temperature. To prevent non-specific interactions occurring between
antibodies, the cells were treated with 5% bovine albumin serum
(BSA) in PBS for 2 h and incubated with a specific mouse monoclonal
antibody raised against TSPO [cat. no. SAB1405525; dilution,
1:1,000 in blocking solution; 3% (w/w) BSA in 0.1% Tris-buffered
saline-Tween; Sigma-Aldrich, St. Louis, MO, USA] for 2 h at 37°C.
Following multiple washes, the cells were incubated with a
secondary IgG goat anti-mouse monoclonal antibody (cat. no.
A-11001; Alexa Fluor® 488; Life Technologies, Grand
Island, NY, USA) diluted 1:1,000 in blocking solution for 1 h at
room temperature. The samples were subsequently washed twice and,
for each sample, 10,000 cells were counted using a FACSCalibur™
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and
CellQuest™ software (BD Biosciences). As TSPOs are expressed on
mitochondria, the cellular mitochondrial content of TSPO was
determined as follows: The cells were fixed with 3% PFA, labeled
with 10−7 mol/l nonyl acridine orange for 15 min at room
temperature, washed twice in PBS and analyzed using flow cytometry.
Thus, the mitochondrial TSPO density was determined by calculating
the number of TSPO sites per unit of mitochondrial mass.
Nitrite assay
Nitric oxide (NO) is a molecular mediator of
numerous physiological processes, including vasodilation,
inflammation, thrombosis, immunity and neurotransmission. Thus, a
number of methods exist, which analyze NO levels in biological
systems. Under physiological conditions, NO is rapidly converted
into the stable end products nitrite (NO2-) and nitrate and
subsequently the hematic concentrations are frequently assessed as
an index of systemic NO production. Therefore, nitrite levels were
measured using the Griess reaction, as previously described by
Gomez-Monterrey et al (27).
TBARS levels
The PBMC samples were incubated with 0.5 ml of 20%
acetic acid (pH 3.5) and 0.5 ml of 0.78% aqueous thiobarbituric
acid solution. The mixture was heated to 95°C for 45 min and
centrifuged at 1,600 × g for 5 min. To quantify the amount of TBARS
in the supernatant fractions, spectrophotometry was performed at an
absorbance of 532 nm (27) and data
were expressed as TBARS/serum protein in μM/μg. Data are presented
as the average of triplicate measurements from duplicate
experiments.
Caspase-3 activity
The BD ApoAlert™ Caspase-3 Fluorogenic assay kit (BD
Biosciences Clontech, Palo Alto, CA, USA) and a fluorescent
microplate reader (Applied Biosystems Life Technologies, Foster
City, CA, USA) were used to determine the caspase-3 activity
levels, as previously described (28).
Statistical analysis
Statistical analyses were conducted by performing an
analysis of variance with Neumann-Keul’s multiple comparison test
or the Kolmogorov-Smirnov test, as appropriate. Analyses of the
differences between the CR and NR patients were performed using the
Mann-Whitney U test for non-parametric independent and continuous
variables and all data are expressed as the mean ± standard
deviation. *P<0.01 indicates a statistically
significant difference between the control and CLL patients.
Results
Patient characteristics and clinical
response to therapy
A total of 30 patients were enrolled from the
Hematology Unit of San Gennaro Hospital. The pretreatment clinical
characteristics of the 30 patients are indicated in Table I. Among the 30 enrolled patients,
67% were male and the median age was 73 years, with 48% patients
aged ~65 years and 52% patients aged ≥75 years. Compared with the
healthy patients, the CLL patients exhibited a significantly higher
median white blood cell count (75,000/mm3; P=0.004), a
marginally lower median hemoglobin level (11.5 g/dl; P=0.05) and a
marginally higher median platelet count (180,000/mm3;
P=0.05).
 | Table IPretreatment clinical characteristics
(n=30). |
Table I
Pretreatment clinical characteristics
(n=30).
| Variable | Value |
|---|
| Median age,
years | 73 |
| Age distribution,
% |
| 65 years | 48 |
| 75–80 years | 50 |
| ≥80 years | 2 |
| Gender
distribution, % |
| Male | 67 |
| Female | 33 |
| Median white blood
cell count,/mm3 | 78,000 |
| Median hemoglobin
level, g/dl | 11.5 |
| Median platelet
count,/mm3 | 180,000 |
The CLL patients received four cycles of treatment
with bendamustine plus rituximab. Patients were considered to be
responsive to the treatment if a PR of ≥6 months was observed and
resistant to the treatment if a PR of <6 months or NR were
observed. Following six months of treatment with bendamustine plus
rituximab, 12/30 (40%) patients achieved CR, eight (26%) patients
achieved PR, six (20%) patients achieved no remission (NR) and four
(13%) patients succumbed to the disease (Table II).
 | Table IIClinical response to therapy
(n=30). |
Table II
Clinical response to therapy
(n=30).
| Clinical
response | Patients, n
(%) |
|---|
| Complete
remission | 12 (40) |
| Partial
remission | 8 (26) |
| No remission | 6 (20) |
| Mortality | 4 (13) |
Modulation of TSPO expression
To evaluate the use of TSPO as a therapeutic target
and prognostic marker, the response of CLL patients to bendamustine
and rituximab treatment was determined according to TSPO
expression. As TSPO is located on the mitochondria, the cellular
mitochondrial content was determined by performing flow cytometry
analysis; furthermore, TSPO density at the mitochondrial level was
calculated by the number of TSPO sites per unit of mitochondrial
mass.
TSPO expression was evaluated at T0 and T6. Compared
with the lymphocytes of the healthy participants, the leukemic
cells of the 30 CLL patients exhibited an increased level of TSPO,
normalized for mitochondrial expression (Fig. 1A).
Six months after the treatment commenced, a decrease
in the TSPO/mitochondria ratio occurred, resembling that of the
healthy controls in 24/30 CLL patients (Fig. 1A). CR patients exhibited low TSPO
levels at T0, which were marginally reduced following six months of
treatment compared with the NR group (Fig. 1B). Notably, the six patients who
were resistant to treatment (NR patients) displayed significantly
(P<0.01) higher TSPO levels compared with CR patients at T0;
furthermore, the NR TSPO levels were significantly (P<0.01)
reduced following six months of therapy (Fig. 1B). Thus, the results indicate an
inverse trend in the TSPO levels between the two groups of
patients.
Modulation of serum levels of NO, TBARS
and caspase-3 activity
The present study evaluated TBARS and NO levels, two
markers of oxidative stress, in the lymphocytes of 30 CLL patients
at T0, T1 and T6.
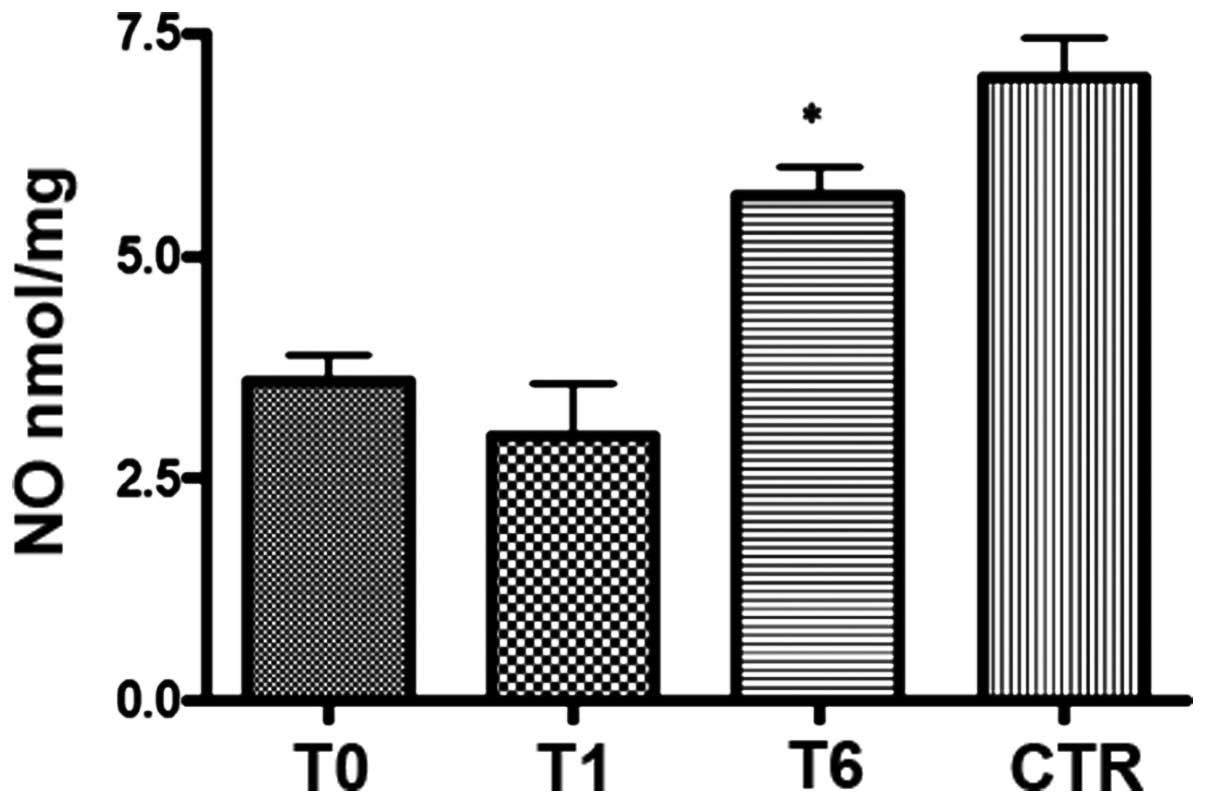
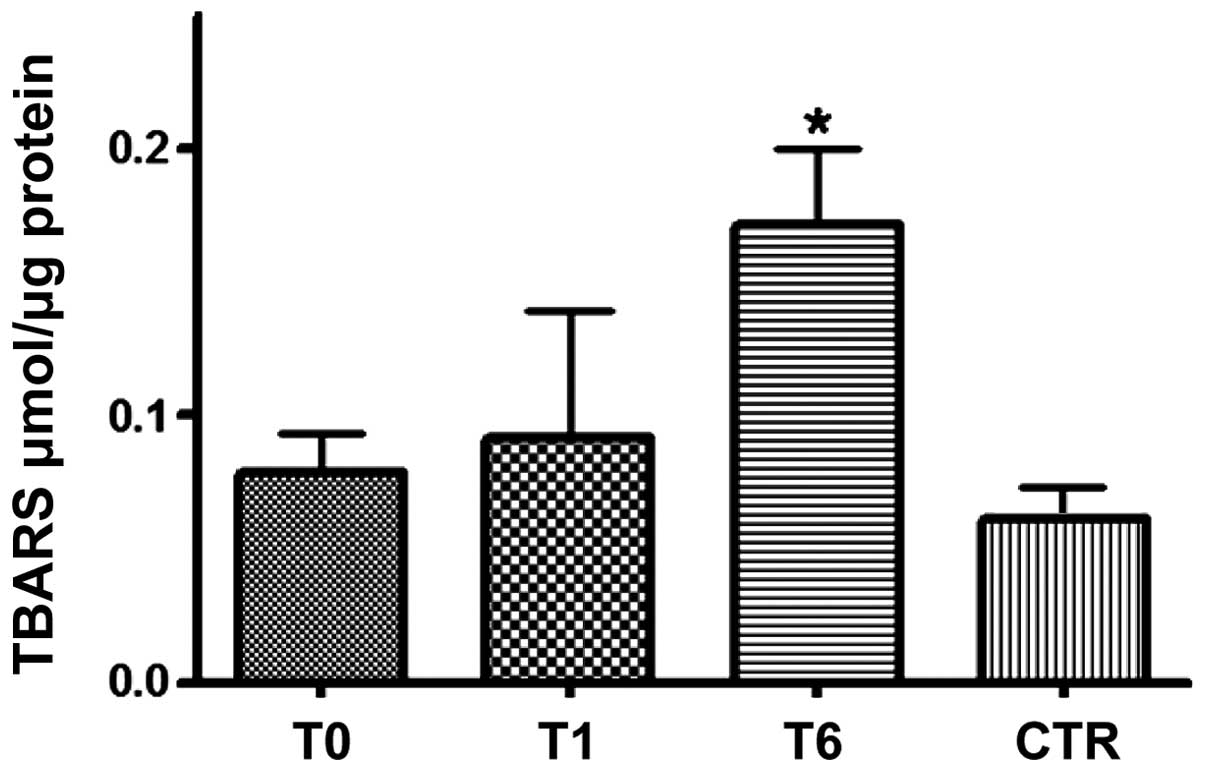
At T0, lower levels of NO (Fig. 2) and marginally higher levels of
TBARS (Fig. 3) were identified in
the CLL patients compared with the healthy controls. Bendamustine
plus rituximab therapy at the T1 timepoint did not significantly
change the serum NO levels (Fig. 2)
but did slightly increase the TBARS level (Fig. 3); however, NO (Fig. 2) and TBARS (Fig. 3) mean serum levels were
significantly (P<0.0001) increased in all responder patients
(24/30) at T6. TBARS but not NO levels exceeded the mean values
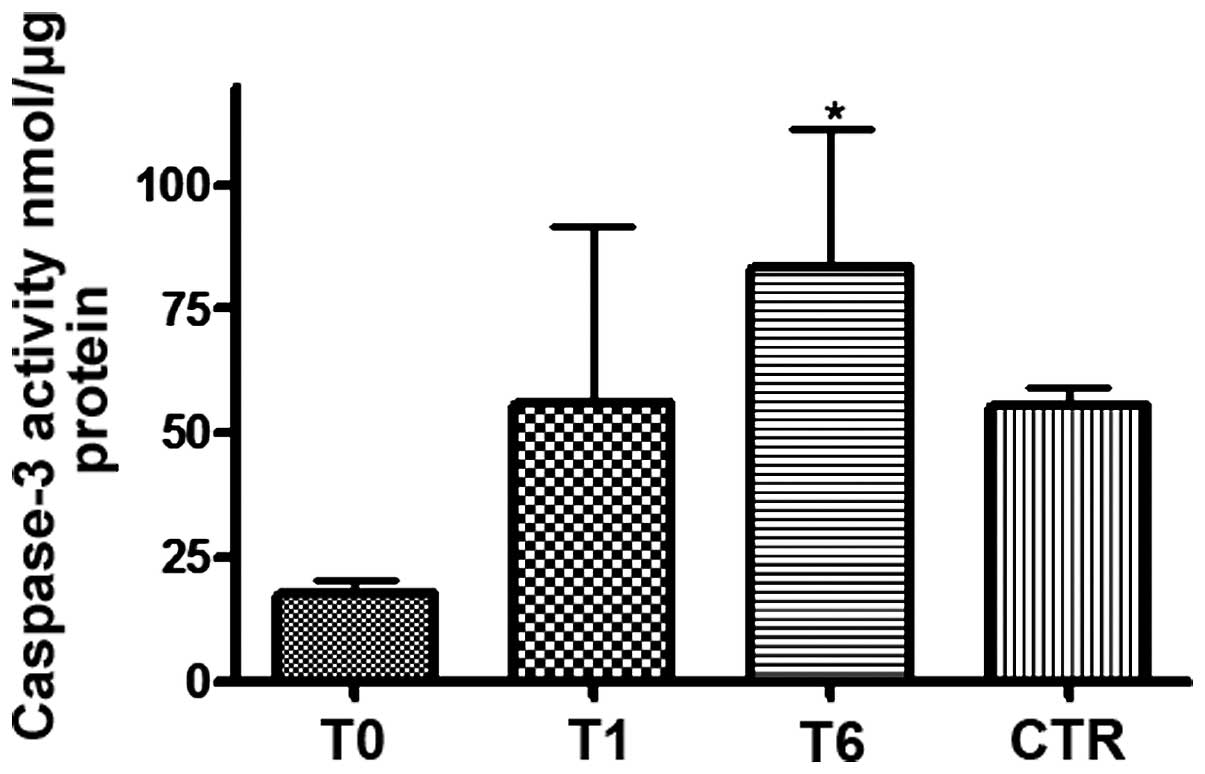
recorded in healthy subjects. In addition, apoptosis was evaluated
by detecting caspase-3 activity in the CLL patients. Prior to
treatment (T0), low caspase-3 activity was identified in the CLL
patients compared with the healthy donors; however, one week after
treatment commenced, an increase in caspase-3 activity was observed
in all of the responder patients (24/30) and an additional increase
was observed six months after the commencement of treatment
(Fig. 4).
Of note, the six patients who appeared to be
resistant to treatment displayed lower caspase-3 activity and TBARS
levels six months after therapy (data not shown). These data
indicate again that the increase in NO, TBARS and caspase-3
activity levels recorded following six months of treatment may be
predictive of a response to therapy.
Discussion
TSPO is a component of the MPTP (with other protein
constituents, such as VDAC) and is involved in a number of
biological processes, including apoptosis, the regulation of
cellular proliferation, porphyrin transport and heme biosynthesis,
immunomodulation, anion transport, and the regulation of
steroidogenesis. In addition, TSPO facilitates the regulation of
the release of apoptotic factors into the cytosol; this correlation
between TSPO, and the occurrence of programmed cell death and
apoptotic onset was identified by studying the role of TSPO in the
MPTP (29). TSPO causes prolonged
opening of the MPTP and the release of apoptotic factors, such as
Smac, cytochrome c and apoptosis-inducing factor
(30,31), from the mitochondria into the
cytosol, resulting in osmotic swelling of the mitochondrial matrix,
and dysregulation of ATP synthesis and oxidative phosphorylation.
Ultimately, apoptosis and necrotic signaling cascades are activated
and cell death occurs (32),
highlighting the correlation between TSPO and the protection of
cells from apoptosis. Notably, previous studies have identified an
association between TSPO and cancer; for example, it was
demonstrated that TSPO overexpression induced by transfection
protects lymphocytes against ultraviolet light-induced cell
apoptosis (33), and TSPO
expression was correlated with the ability of breast cancer cells
to grow in severe combined immunodeficient mice (34). Furthermore, it was demonstrated that
TSPO positively correlates with proliferation rate but inversely
correlates with spontaneous apoptosis rates in various glioma cell
lines. In patients exhibiting tumors with TSPO overexpression, TSPO
may contribute to a poor prognosis by mediating proliferative
and/or apoptosis-protective effects. However, these functions of
TSPO may be reversed by the administration of TSPO-specific
exogenous ligands in a variety of tumor types (14–18);
furthermore, increased binding capacities of TSPO-specific ligands
have been reported in colorectal cancer (18) and a variety of different tumor types
(14–16). However, TSPO protein overexpression
was only directly determined in astrocytoma and breast cancer. In
addition, a study of 86 astrocytoma patients identified that TSPO
protein expression correlated with the tumor grade (9) and, in a small number of breast cancer
samples and cell lines, TSPO protein expression correlated with
malignant cancer (21).
In the present study, TSPO expression levels were
evaluated in 30 CLL patients treated with bendamustine plus
rituximab. Following six months of treatment, 12/30 (40%) patients
achieved CR, eight (26%) patients achieved PR, six (20%) achieved
no remission (NR) and four (13%) succumbed to the disease. TSPO
levels were higher in leukemic cells compared with the lymphocytes
of healthy individuals at T0; however, six months after treatment
commenced, the present study identified a decrease in the
TSPO/mitochondria ratio in 24/30 CLL patients to resemble that of
the healthy controls. In addition, CR patients exhibited low TSPO
levels at T0 compared with NR patients. Thus, the results of the
present study indicate an inverse trend in TSPO expression levels
in the two groups of patients.
The role of NO varies in cancer biology and is
dependent on a number of factors; NO may be involved in the
promotion or prevention of tumor occurrence, depending on the time
of exposure to NO, the tumor microenvironment and the concentration
of NO. Low concentrations of NO (range, 1–30 nM) produced high
levels of cyclic guanosine monophosphate, promoting tumor
angiogenesis and the proliferation of endothelial cells, and a
wider range of NO concentrations (range, 0–100 nM) corresponded to
an increase in the activity of the proliferative and anti-apoptotic
Akt and Erk-dependent pathways in tumor cells (35,36),
appearing to enhance angiogenesis and protect tumor cells from
apoptosis. Thus, at NO concentrations of 0–100 nM, the molecules
activated by NO are considered to be correlated with poor prognosis
events in cancer. By contrast, higher NO levels (>300 nM)
appeared to promote apoptosis and anti-tumor activity.
In the present study, a spectrophotometric assay was
performed to determine the serum NO levels of CLL patients treated
with bendamustine plus rituximab. Six months after (T6) treatment
commenced, responsive patients exhibited a strong increase in NO
and TBARS levels, as well as a potentiation of caspase-3 activity.
Notably, the six patients who were resistant to treatment exhibited
lower caspase-3 activity and TBARS levels. These data again
indicate that the increase in NO and TBARS levels, as well as
caspase-3 activity levels, recorded at T6 may be predictive of a
response to therapy. Notably, in responsive patients, the increased
NO levels possibly had an anti-tumor and pro-apoptotic role, as
demonstrated by the corresponding increase in caspase-3
activity.
In conclusion, these data indicate that TSPO
expression may be a molecular prognostic marker in CLL patients. In
addition, TSPO may represent a useful therapeutic target for
increasing treatment efficacy in CLL patients.
Abbreviations:
|
CLL
|
chronic lymphocytic leukemia
|
|
CR
|
complete remission
|
|
MPTP
|
mitochondrial permeability transition
pore
|
|
NO
|
nitric oxide
|
|
NR
|
no remission
|
|
PR
|
partial remission
|
|
PBMC
|
peripheral blood mononuclear cells
|
|
TBARS
|
thiobarbituric acid reactive
substances
|
|
TSPO
|
translocator protein
|
|
VDAC
|
voltage-dependent anion channel
|
References
|
1
|
McEnery MW, Snowman AM, Trifiletti RR and
Snyder SH: Isolation of the mitochondrial benzodiazepine receptor:
association with the voltage-dependent anion channel and the
adenine nucleotide carrier. Proc Natl Acad Sci. 89:3170–3174. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Casellas P, Galiegue S and Basile AS:
Peripheral benzodiazepine receptors and mitochondrial function.
Neurochem Int. 40:475–486. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sutter AP, Maaser K, Höpfner M, et al:
Specific ligands of the peripheral benzodiazepine receptor induce
apoptosis and cell cycle arrest in human esophageal cancer cells.
Int J Cancer. 102:318–327. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zisterer, Campiani G, Nacci V and Williams
DC: Pyrrolo-1,5-benzoxazepines induce apoptosis in HL-60, Jurkat,
and Hut-78 cells: a new class of apoptotic agents. J Pharmacol Exp
Ther. 293:48–59. 2000.PubMed/NCBI
|
|
5
|
Carayon P, Portier M, Dussossoy D, Bord A,
Petitprêtre G, Canat X, Le Fur G and Casellas P: Involvement of
peripheral benzodiazepine receptors in the protection of
hematopoietic cells against oxygen radical damage. Blood.
87:3170–3178. 1996.PubMed/NCBI
|
|
6
|
Austin CJ, Kahlert J, Kassiou M and
Rendina LM: The translocator protein (TSPO): a novel target for
cancer chemotherapy. Int J Biochem Cell Biol. 45:1212–1216. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katz Y, Eitan A and Gavish M: Increase in
peripheral benzodiazepine binding sites in colonic adenocarcinoma.
Oncology. 47:139–142. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cornu P, Benavides J, Scatton B, Hauw JJ
and Philippon J: Increase in omega 3 (peripheral-type
benzodiazepine) binding site densities in different types of human
brain tumours. A quantitative autoradiography study. Acta Neurochir
(Wien). 119:146–152. 1992. View Article : Google Scholar
|
|
9
|
Hardwick M, Fertikh D, Culty M, Li H,
Vidic B and Papadopoulos V: Peripheral-type benzodiazepine receptor
(PBR) in human breast cancer: correlation of breast cancer cell
aggressive phenotype with PBR expression, nuclear localization, and
PBR-mediated cell proliferation and nuclear transport of
cholesterol. Cancer Res. 59:831–842. 1999.PubMed/NCBI
|
|
10
|
Katz Y, Ben-Baruch G, Kloog Y, Menczer J
and Gavish M: Increased density of peripheral
benzodiazepine-binding sites in ovarian carcinomas as compared with
benign ovarian tumours and normal ovaries. Clin Sci (Lond).
78:155–158. 1990.
|
|
11
|
Batra S and Iosif CS: Elevated
concentrations of mitochondrial peripheral benzodiazepine receptors
in ovarian tumors. Int J Oncol. 12:1295–1298. 1998.PubMed/NCBI
|
|
12
|
Venturini I, Alho H, Podkletnova I, Corsi
L, Rybnikova E, Pellicci R, Baraldi M, Pelto-Huikko M, Helén P and
Zeneroli ML: Increased expression of peripheral benzodiazepine
receptors and diazepam binding inhibitor in human tumors sited in
the liver. Life Sci. 65:2223–2231. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papadopoulos V: Peripheral-type
benzodiazepine/diazepam binding inhibitor receptor: biological role
in steroidogenic cell function. Endocr Rev. 14:222–240.
1993.PubMed/NCBI
|
|
14
|
Wu X and Gallo KA: The 18-kDa translocator
protein (TSPO) disrupts mammary epithelial morphogenesis and
promotes breast cancer cell migration. PLoS One. 8:e712582013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beinlich A, Strohmeier R, Kaufmann M and
Kuhl H: Relation of cell proliferation to expression of peripheral
benzodiazepine receptors in human breast cancer cell lines. Biochem
Pharmacol. 60:397–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Landau M, Weizman A, Zoref-Shani E, Beery
E, Wasseman L, Landau O, Gavish M, Brenner S and Nordenberg J:
Antiproliferative and differentiating effects of benzodiazepine
receptor ligands on B16 melanoma cells. Biochem Pharmacol.
56:1029–1034. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikezaki K and Black KL: Stimulation of
cell growth and DNA synthesis by peripheral benzodiazepine. Cancer
Lett. 49:115–120. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maaser K, Höpfner M, Jansen A, et al:
Specific ligands of the peripheral benzodiazepine receptor induce
apoptosis and cell cycle arrest in human colorectal cancer cells.
Br J Cancer. 85:1771–1780. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alexander BE, Roller E and Klotz U:
Characterization of peripheral-type benzodiazepine binding sites on
human lymphocytes and lymphoma cell lines and their role in cell
growth. Biochem Pharmacol. 44:269–274. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Canat X, Carayon P, Bouaboula M, Cahard D,
Shire D, Roque C, Le Fur G and Casellas P: Distribution profile and
properties of peripheral-type benzodiazepine receptors on human
hemopoietic cells. Life Sci. 52:107–118. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O’Brien ER, Kersemans V, Tredwell M, Checa
B, Serres S, Soto MS, Gouverneur V, Leppert D, Anthony DC and
Sibson NR: Glial activation in the early stages of brain
metastasis: TSPO as a diagnostic biomarker. J Nucl Med. 55:275–280.
2014. View Article : Google Scholar
|
|
22
|
Beinlich A, Strohmeier R, Kaufmann M and
Kuhl H: Specific binding of benzodiazepines to human breast cancer
cell lines. Life Sci. 65:2099–2108. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hardwick M, Fertikh D, Culty M, Li H,
Vidic B and Papadopoulos V: Peripheral-type benzodiazepine receptor
(PBR) in human breast cancer: correlation of breast cancer cell
aggressive phenotype with PBR expression, nuclear localization, and
PBR-mediated cell proliferation and nuclear transport of
cholesterol. Cancer Res. 59:831–842. 1999.PubMed/NCBI
|
|
24
|
Chiorazzi N, Rai KR and Ferrarini M:
Chronic lymphocytic leukemia. N Engl J Med. 352:804–815. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheson BD, Bennett JM, Grever M, Kay N,
Keating MJ, O’Brien S and Rai KR: National Cancer
Institute-sponsored Working Group guidelines for chronic
lymphocytic leukemia: revised guidelines for diagnosis and
treatment. Blood. 87:4990–4997. 1996.PubMed/NCBI
|
|
26
|
Correale P, Campoccia G, Tsang KY, et al:
Recruitment of dendritic cells and enhanced antigen specific
immune-reactivity in cancer patients treated with hr-GM-CSF
(Molgramostim) and hr-IL-2: results from a phase Ib clinical trial.
Eur J Cancer. 37:892–902. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gomez-Monterrey I, Campiglia P,
Scognamiglio I, Vanacore D, Dicitore A, Lombardi A, Caraglia M,
Novellino E and Stiuso P: DTNQ-Pro, a mimetic dipeptide, sensitizes
human colon cancer cells to 5-fluorouracil treatment. J Amino
Acids. 2013:5090562013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tenore GC, Manfra M, Stiuso P, Coppola L,
Russo M, Ritieni A and Campiglia P: Polyphenolic pattern and in
vitro cardioprotective properties of typical red wines from
vineyards cultivated in Scafati (Salerno, Italy). Food Chem.
140:803–809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Veenman L and Gavish M: The role of 18 kDa
mitochondrial translocator protein (TSPO) in programmed cell death,
and effects of steroids on TSPO expression. Curr Mol Med.
12:398–412. 2012.PubMed/NCBI
|
|
30
|
Veenman L, Papadopoulos V and Gavish M:
Channel-like functions of the 18-kDa translocator protein (TSPO):
regulation of apoptosis and steroidogenesis as part of the
host-defense response. Curr Pharm Des. 13:2385–2405. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Parker MA, Bazan HE, Marcheselli V,
Rodriguez de Turco EB and Bazan NG: Platelet-activating factor
induces permeability transition and cytochrome c release in
isolated brain mitochondria. J Neurosci Res. 69:39–50. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papadopoulos V, Baraldi M, Guilarte TR, et
al: Translocator protein (18 kDa): new nomenclature for the
peripheral-type benzodiazepine receptor based on its structure and
molecular function. Trends Pharmacol Sci. 27:402–409. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stoebner P E, Carayon P, Casellas P,
Portier M, Lavabre-Bertrand T, Cuq P, Cano JP, Meynadier J and
Meunier L: Transient protection by peripheral benzodiazepine
receptors during the early events of ultraviolet light-induced
apoptosis. Cell Death Differ. 8:747–753. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hardwick M, Rone J, Han Z, Haddad B and
Papadopoulos V: Peripheral-type benzodiazepine receptor levels
correlate with the ability of human breast cancer MDA-MB-231 cell
line to grow in SCID mice. Int J Cancer. 94:322–327. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ridnour LA, Thomas DD, Switzer C, et al:
Molecular mechanisms for discrete nitric oxide levels in cancer.
Nitric Oxide. 19:73–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Prueitt RL, Boersma BJ, Howe TM, et al:
Inflammation and IGF-I activate the Akt pathway in breast cancer.
Int J Cancer. 120:796–805. 2007. View Article : Google Scholar
|