Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of liver cancer, with ~748,300 new liver cancer cases
and 695,900 liver cancer-related mortalities occurring worldwide
(1). The overall five-year survival
rate of HCC is 3% in the USA (2). A
population-based study conducted by Guiu et al reported that
the one-year survival rate of HCC had increased to 32.8% and the
five-year survival rate of HCC had risen to 10.0% over the past
four decades (3). Surgical
resection is the first option for HCC patients who meet the Milan
Criteria (4): (i) one lesion <5
cm; (ii) ≤3 lesions <3 cm; (iii) no extrahepatic manifestations
and (iv) no vascular invasion. However, it is not feasible when
patients present at an advanced stage of the disease (5). Furthermore, conservative treatments,
including chemotherapy, radiotherapy and biotherapy may not achieve
satisfactory curative results (6).
Transcatheter arterial chemoembolization (TACE) is the primary
treatment option for patients with unresectable HCC (7). Approximately 16–55% patients can
benefit from TACE therapy and achieve a low rate of tumor
regression. Llovet et al reported that TACE resulted in
objective responses that were sustained for ≥6 months in 35% of
cases, and was associated with a lower rate of portal-vein invasion
compared with conservative treatment (8). Following TACE therapy, HCC tumor cells
undergo ischemia and necrosis. Healthy liver tissue is inevitably
damaged during the procedure, which may adversely affect the
postoperative recovery of the patient (9). Magnetic resonance imaging (MRI) in
combination with liver-specific contrast agents facilitates the
detection of focal liver disease, and has been demonstrated to be
superior to computed tomography (CT) for this purpose (10). Gadolinium ethoxybenzyl
diethylenetriamine pentaacetic acid (Gd-EOB-DTPA; Primovist), is a
liver-specific, paramagnetic contrast agent developed by
Bayer-Schering Pharma (Berlin, Germany) with combined perfusion and
hepatocyte-selective properties. A number of studies have
demonstrated the reliability of Gd-EOB-DTPA as a non-invasive tool
for estimating overall and regional liver cell function and
viability, through measuring the cytomembrane transporter function
(such organic anion transporting polypeptide 1 and MRP) (11–13).
The present study aimed to investigate the potential utility of
Gd-EOB-DTPA-enhanced MRI in the evaluation of regional liver
function damage in peritumoral regions following TACE therapy.
Materials and methods
Ethics statement
Written, informed consent was obtained from all
patients. The study was conducted in accordance with the
Declaration of Helsinki and approved by the ethics committee of The
Second XiangYa Hospital of Central South University (Changsha,
China).
Patients
A total of 35 HCC patients who underwent
Gd-EOB-DTPA-enhanced MRI of the liver were enrolled in this
prospective study between March 2012 and May 2013. Of the 35
patients, four were excluded from the study due to poor
postoperative clinical status and two were excluded due to poor
breath-holding during MRI examination. The final study population
comprised 29 patients [18 males and 11 females; mean age ± standard
deviation (SD), 49.86±11.05 years; age range, 28–76 years].
The diagnosis of HCC was determined on the basis of
the following criteria: Typical lesions observed on at least one
imaging modality (CT, MRI or ultrasound) with an elevated serum
α-fetoprotein level (>400 ng/ml; n=23) or liver biopsy with
pathological confirmation of HCC (n=12).
Inclusion criteria were as follows: Patients who
could understand the study documents (which contained the study
design, content, background, methods and possible treatment
outcomes), had a single lesion with tumors <10 cm in size; were
categorized as Child-Pugh class A or B prior to surgery and were
receiving TACE therapy for the first time. Exclusion criteria were
as follows: Severe motion artifacts due to poor breath-holding,
profound liver cirrhosis, multiple lesions, tumors >10 cm in
size, obstruction of main or first branch of portal vein,
difficulty in locating the feeding artery during the procedure or
hepatic artery to portal vein shunting. Exclusion criteria for the
use of Gd-EOB-DTPA were as follows: Allergy to Primovist, severe
cardiovascular disease or kidney insufficiency (glomerular
filtration rate <60 ml/min/1.73 m2).
TACE protocol
TACE was performed via the femoral artery under
GELCE 3100 (GE Healthcare, Fairfield, USA) bidirectional digital
subtraction angiography. Using the Seldinger technique (14), a catheter sheathe was inserted into
the femoral artery with the aid of a guide wire. The Yashiro, RH
catheter or Microcatheter (Terumo, Tokyo, Japan) was sent to the
artery feeding the tumor. The Yashiro and RH catheters were the
first option for TACE therapy; when these two catheters were not
effective, the microcatheter was used, as it is smaller than the
Yashiro and RH catheters. However, the microcatheter was not
regularly used, owing to its high cost. Iodized oil (5–30 ml; mean,
16.14±7.04 ml; Guerbet, Paris, France) mixed with chemotherapeutics
(pirarubicin, 10–40 mg; 5-fluorouracil, 250–1,000 mg; cisplatin,
40–80 mg) was injected into the feeding artery of the tumor.
MRI protocol
MRI was performed 3–5 days after TACE therapy using
a 3T superconducting MRI system (Philips, Amsterdam, Netherlands)
with a phased array body coil (SENSE XL Torso; Philips) and the
following imaging parameters: 7 mm section thickness and 3 mm
intersection gap. Three-dimensional T1-weighted turbo field echo
sequence with spectral presaturation inversion recovery fat
suppression [repetition time (TR), 3.0 ms; echo time (TE), 1.35 ms;
field of view, 350×320 mm; matrix, 124×100; flip angle, 10°] was
utilized pre-contrast and post-contrast (15 s, 90 s, 3 min and 20
min after injection of Gd-EOB-DTPA). Respiratory-triggered
T2-weighted fast spin echo sequence with short TI inversion
recovery fat suppression (TR, 1,113 ms; TE, 70 ms; field of view,
350×320 mm; matrix, 268×200; flip angle, 90°) was used prior to
injection of the contrast agent. The contrast agent was used at a
dose of 0.025 mmol/kg body weight and at an injection rate of 2
ml/s by 20 ml saline flush using an intravenous line (via the
cubital vein).
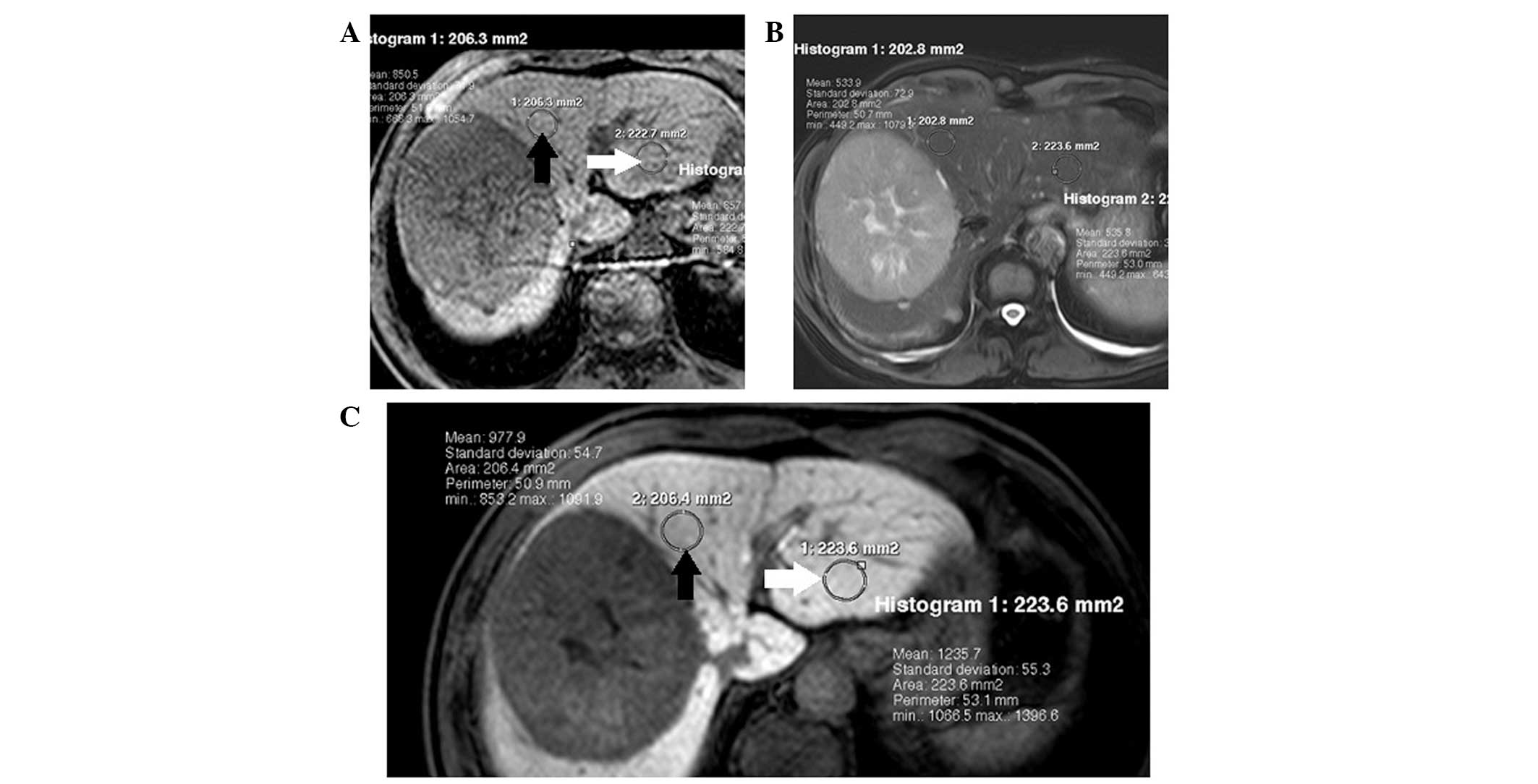
Imaging analysis
In the evaluation of hepatocytic uptake of
Gd-EOB-DTPA, signal intensity (SI) of the region of interest (ROI)
of the liver was measured by one radiologist with 20 years
experience of abdominal imaging, who was blinded to the clinical
data and selection of ROIs. The ROIs were selected to be as large
as possible, avoiding large vessels and biliary ducts, and the
identical location was used prior to and following Gd-EOB-DTPA
administration. Each ROI was circular or oval. Signal to noise
ratio (SNR) was calculated for peritumoral and healthy liver tissue
regions (Fig. 1), by dividing the
SI of the tissue by the standard deviation of the image noise
(background noise outside of the patient’s body).
The relative SNR in the peritumoral regions was
measured to assess the correlation between hepatocytic uptake of
Gd-EOB-DTPA and potential clinical influencing factors (patients
age, gender, diameter of the tumor, blood supply of the tumor,
Child-Pugh class and quantity of Iodized oil used). Relative SNR =
[(SNRafter - SNRbefore)/SNRbefore]
× 100.
Statistics
The data are presented as the mean ± SD (range) and
were analyzed using SPSS 19.0 software (SPSS Inc., Chicago, IL,
USA). The correlation between clinical factors and relative SNR was
analyzed using Pearson’s correlation coefficient or Spearman’s rank
correlation coefficient. Pearson’s correlation analysis was used to
evaluate the association between the relative SNR of liver
parenchyma and the age of the patient, the diameter of the tumor
and the quantity of iodized oil used for TACE therapy. Spearman’s
rank correlation was used to evaluate the association between the
relative SNR of liver parenchyma and clinical parameters including
gender, blood supply of the tumor and Child-Pugh class. The
quantitative parameter of healthy liver tissue regions vs.
peritumoral regions was calculated using a paired t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Assessment of clinical influencing
factors
Of the 29 patients, 22 were categorized as
Child-Pugh class A and seven as Child-Pugh class B. The quantity of
iodized oil used for individual patients varied from 5–30 ml, with
a mean quantity of 16.14±7.04 ml. A poor blood supply to the tumor
was observed in nine patients, while a rich blood supply was
observed in the remaining 20. The diameter of the tumors ranged
from 3.1–9.9 cm, with a mean diameter of 6.94±2.15 cm. No
correlation was observed between the blood supply and the diameter
of the tumor (r=0.276, P=0.148). Detailed clinical information is
listed in Table I.
 | Table IClinical data of patients for
estimation. |
Table I
Clinical data of patients for
estimation.
| Age, years | Tumor diameter,
cm | Quantity of iodized
oil used, ml | Child-Pugh score |
|---|
| Mean | 49.86 | 6.94 | 16.14 | 6.21 |
| SD | 11.05 | 2.15 | 7.04 | 0.94 |
| Range | 28–76 | 3.1–9.9 | 5–30 | 5–9 |
Gd-EOB-DTPA uptake in different liver
tissue regions
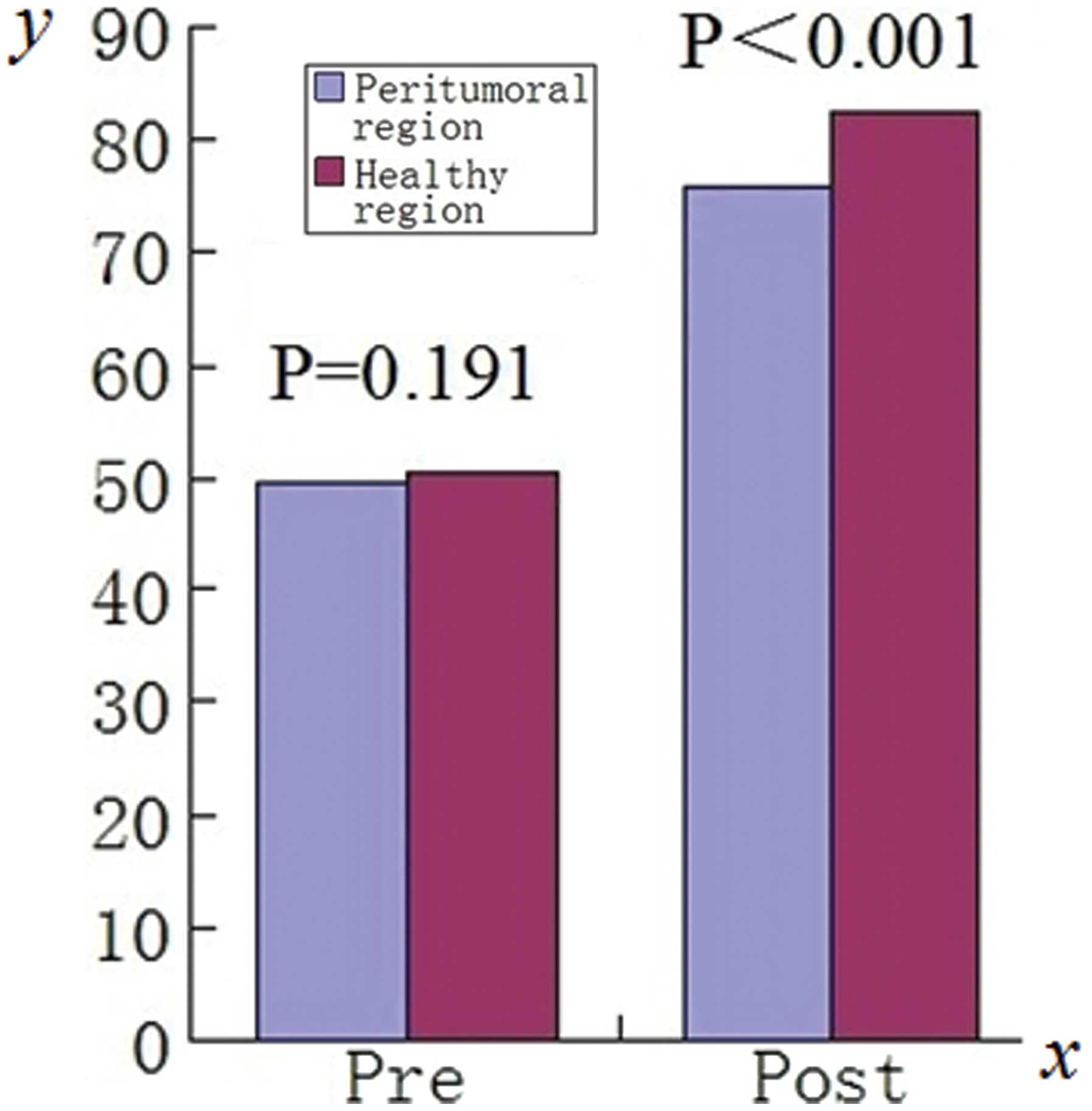
Prior to Gd-EOB-DTPA administration, no significant
difference was observed in the SNR values of healthy liver tissue
regions (50.53±15.99; range, 11.25–83.46) compared with those of
peritumoral regions(49.81±15.85; range, 12.34–81.53;
t=1.341, P=0.191). When measured 20 min after the
administration of Gd-EOB-DTPA, the SNR values of healthy liver
tissue regions (82.55±33.33, range 31.45–153.02) were significantly
higher compared with those of the peritumoral regions (75.77±27.41,
range 31.42–144.49; t=3.732, P<0.001; Fig. 2). Further detail is shown in
Table II. The SNR measured in
healthy liver tissue regions was significantly increased at 20 min
after the administration of Gd-EOB-DTPA compared with that prior to
its administration (t=6.175, P<0.001). In peritumoral
regions, the SNR also exhibited a significant increase when
measured 20 min after Gd-EOB-DTPA administration (t=6.844,
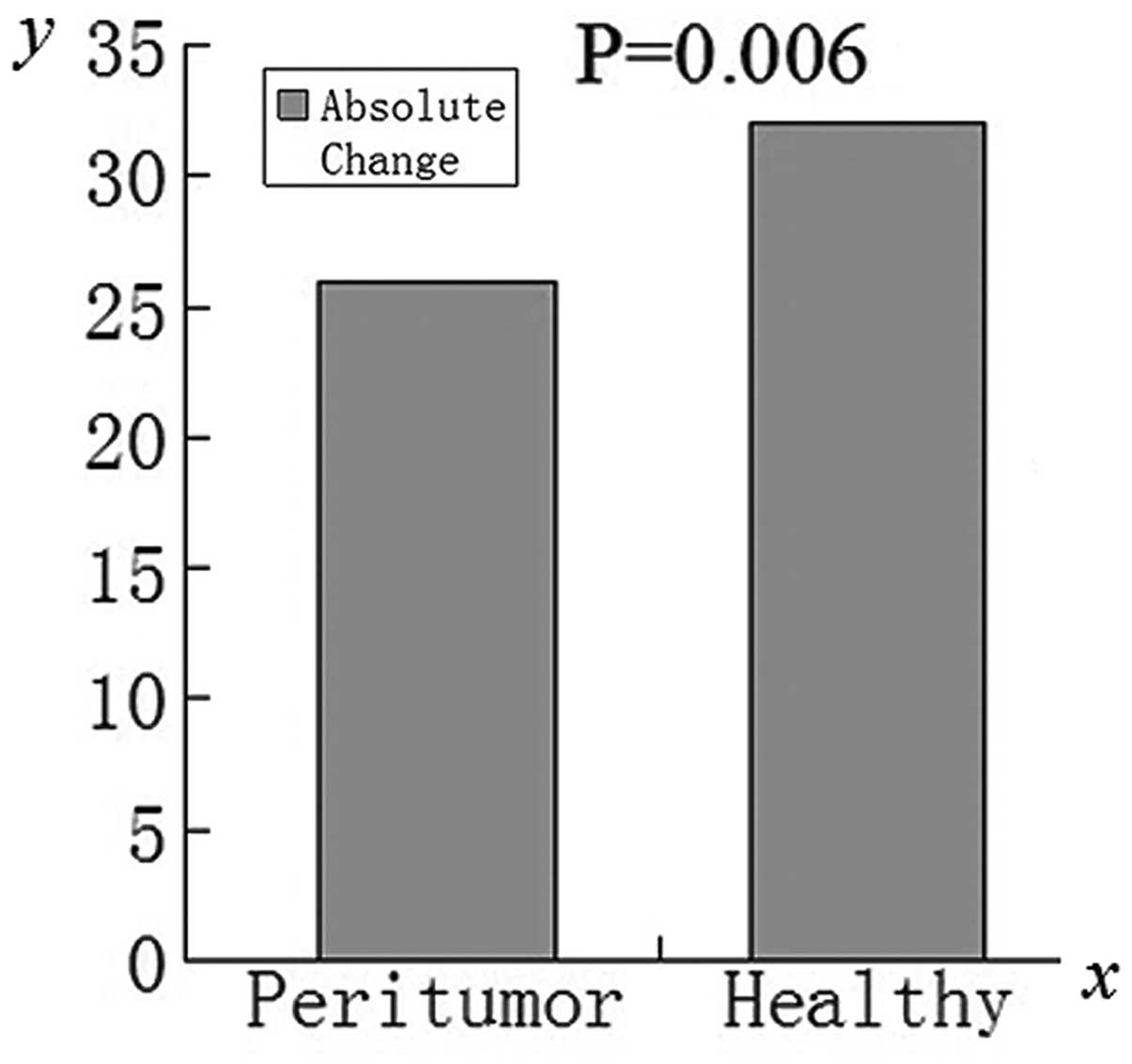
P<0.001) compared with that prior to administration. The
absolute change in SNR for healthy liver tissue regions was
significantly higher (t=3.005, P=0.006) compared with those
of the peritumoral regions (Fig.
3).
 | Table IISignal to noise ratio in healthy and
peritumoral liver tissue before and 20 min after Gd-EOB-DTPA
administration. |
Table II
Signal to noise ratio in healthy and
peritumoral liver tissue before and 20 min after Gd-EOB-DTPA
administration.
| Before
administrationa | 20 min after
administrationb |
|---|
|
|
|
|---|
| Healthy region | Peritumoral
region | | Healthy region | Peritumoral
region | |
|---|
| Mean | 50.53 | 49.81 | | 82.55 | 75.77 | |
| SD | 15.99 | 15.85 | | 33.33 | 27.41 | |
| Range | 11.25–83.46 | 12.34–81.53 | P=0.191 | 31.45–153.02 | 31.42–144.49 | P<0.001 |
Association between relative SNR and its
potential influencing factors
The relative SNR did not correlate with the age
(r=0.151, P=0.434), gender (r=−0.381, P=0.055) or Child-Pugh class
(r=0.106, P=0.584) of the patient. Additionally, no correlation was
observed between the SNR and the blood supply (r=0.241, P=0.209) or
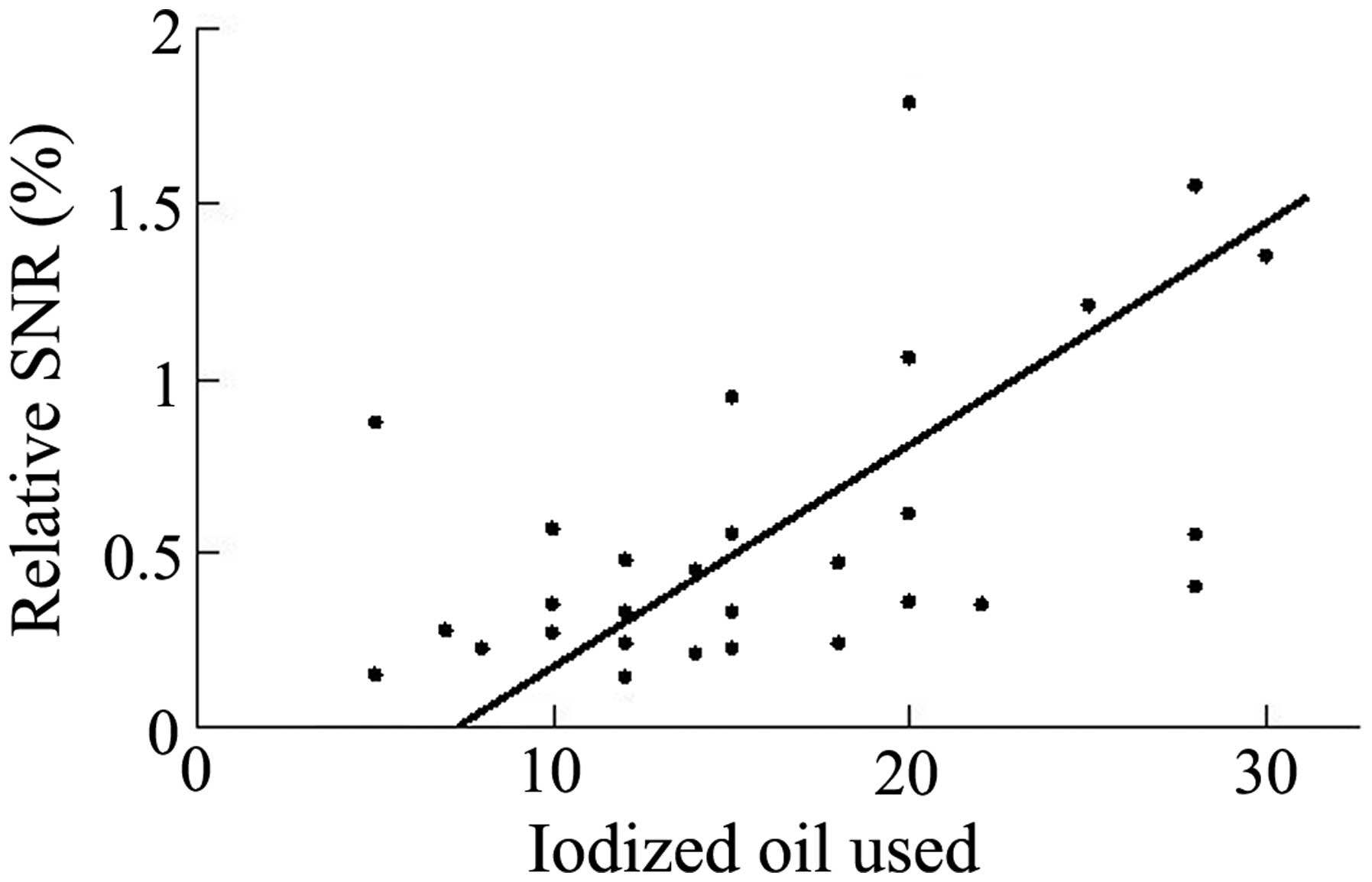
the diameter (r=0.226, P=0.238) of the tumor. Relative SNR,
measured 20 min following Gd-EOB-DTPA administration, was observed
to correlate only with the quantity of iodized oil used during TACE
therapy (r=0.528, P=0.003; Fig. 4).
Further detail is shown in Table
III.
 | Table IIICorrelation between relative SNR in
peritumoral regions and its influencing factors. |
Table III
Correlation between relative SNR in
peritumoral regions and its influencing factors.
| Age | Gender | Tumor diameter | Blood supply | Child-Pugh
class | Iodized oil
dosage |
|---|
| Relative SNR | r=0.151 | r=−0.381 | r=0.226 | r=0.241 | r=0.106 | r=0.528 |
| P=0.434 | P=0.055 | P=0.238 | P=0.209 | P=0.584 | P=0.003a |
Discussion
As TACE therapy may damage normal liver tissue,
patients with pre-existing liver dysfunction commonly experience
hepatic failure following TACE therapy (15). A previous study conducted by Chen
et al suggested that the liver function after TACE therapy
was significantly decreased compared with the preoperative status
(16). Injuries caused by TACE
therapy may affect the selection of the surgical procedure for
patients subsequently requiring tumor resection (17). Reliable estimation of regional liver
function in the preoperative and postoperative periods is crucial,
particularly for patients at high risk of hepatic failure. Shimizu
et al (18) evaluated
regional liver function in a rat ischemia-reperfusion model by
Gd-EOB-DTPA-enhanced MRI. Ischemic lobes were visualized as areas
of high signal intensity in the hepatobiliary region. A
retrospective study conducted by Yamada et al (19) suggested that liver function,
corresponding to the plasma disappearance rate of indocyanine
green, could be estimated by Gd-EOB-DTPA-enhanced MRI; this may
improve the assessment of segmental liver function. The current
study aimed to evaluate the impairment of regional liver function
to provide guidance for the prevention and treatment of hepatic
insufficiency in the selected patients, subsequent to TACE
therapy.
Hepatocytic uptake of Gd-EOB-DTPA is hypothesized to
be regulated by an active membrane transport system such as OATP,
particularly OATP1B1 and OATP1B3, which require adenosine
triphosphate (ATP) for their activity (20). These two transporters are also able
to transport bilirubin (21).
Bilirubin is the main pigment in human bile and thus, it may be
used to indicate the quality of liver function. As these two
transporters are also able to transport serum bilirubin, the uptake
of Gd-EOB-DTPA by hepatocytes is associated with the serum levels
of bilirubin. As the deposition of embolic agents can significantly
reduce the blood supply of the hepatic artery, the peritumoral
liver tissue is inevitably damaged during the TACE procedure by
ischemia and hypoxia of hepatocytes (22,23).
Such injury may cause a decrease in ATP production, leading to the
functional limitation of transporters, which may be the reason for
the reduction in uptake of Gd-EOB-DTPA (24). In addition, it has been reported
that decreased expression of OATP1B1 and OATP1B3 transporters may
lead to reduced Gd-EOB-DTPA uptake by hepatocytes in rat models
(25).
Several studies have proposed that insufficient
liver parenchymal enhancement in Gd-EOB-DTPA-enhanced imaging may
indicate liver dysfunction (26–28). A
cohort study conducted by Utsunomiya et al (29) reported that utilization of
Gd-EOB-DTPA with MRI may provide a potential method for the
estimation of regional liver function. Goshima et al
(30) demonstrated that the
liver-to-spleen volumetric ratio and contrast enhancement index on
Gd-EOB-DTPA-enhanced MRI may be reliable biomarkers for determining
the stage of hepatic fibrosis. Takao et al (31) reported that, following Gd-EOB-DTPA
administration, the signal intensity in the bile duct may indicate
underlying liver function. All of the abovementioned studies
indicated that Gd-EOB-DTPA-enhanced MRI could be used to evaluate
liver function, which is similar to the findings of the present
study.
As proposed by previous studies, the current study
used SNR to quantify the absolute and dynamic contrast enhancement
due to its function as a surrogate marker of hepatocytic
Gd-EOB-DTPA uptake (32).
Quantification of functional impairment is reflected by the SNR
value calculated for peritumoral regions and healthy liver tissue
regions. The SNR values calculated for peritumoral regions and
non-involved liver tissue showed no statistically significant
difference on T1 unenhanced scans. However, the liver tissue in the
peritumoral region exhibited significantly lower Gd-EOB-DTPA uptake
compared with a similar region in healthy liver tissue 20 min after
Gd-EOB-DTPA administration, indicating regional hepatocyte
impairment caused by TACE therapy. It may be argued that the
observed reduction in uptake in peritumoral regions may be due to
the presence of tumor tissue. However, T2-weighted imaging of the
same region revealed no significantly increased signal intensity,
as would be exhibited by tumor tissue. Therefore, it is probable
that this decreased signal intensity is due to ischemia following
TACE therapy, and not the presence of tumor tissue.
Notably, relative SNR in the peritumoral region only
correlated with the quantity of iodized oil used. The age, gender
and Child-Pugh class of the patient, and the diameter and blood
supply of the tumor did not correlate with relative SNR. This
indicates that the dosage of iodized oil used in TACE therapy may
determine the subsequent level of functional impairment of the
liver, and must be considered when performing the procedure. The
most likely cause of hepatocytic impairment is infarction of the
hepatic artery by iodized oil, which leads to hypoperfusion of the
peritumoral liver tissue (33). As
the liver has a characteristic double blood supply, recovery from
this impairment may be possible in the long term (34).
The present study had several limitations. Firstly,
the number of patients included in this single center study was
relatively small (n=29), which limited the power of the data
analysis. Secondly, histological confirmation of regional hepatic
damage was not included. The impairment of the liver tissue may
have been caused by procedure-related cell death, parenchymal
hypoperfusion and infarction of the liver tissue; histological
examination is required to determine the actual cause of regional
hepatic damage at a cellular level. Finally, as the patients may
have had different underlying diseases, a bias was introduced by
the potential varying disease mechanisms. In future studies,
homogenous patient populations should be utilized.
In conclusion, the present data demonstrates an
apparent decrease in Gd-EOB-DTPA uptake in peritumoral liver
regions compared with healthy liver tissue regions 20 min after
Gd-EOB-DTPA administration, indicating the existence of regional
hepatocytic injury caused by TACE therapy. Gd-EOB-DTPA-enhanced MRI
may therefore represent an effective, non-invasive tool for
evaluating regional liver function impairment following TACE
therapy. Furthermore, an association was observed between the
relative SNR and the quantity of iodized oil used, indicating that
the dosage of oil used may impact the subsequent level of
functional liver impairment. Further study should be concerned with
the timing of liver function recovery after TACE therapy using
Gd-EOB-DTPA-enhanced MRI.
Abbreviations:
|
TACE
|
transcatheter arterial
chemoembolization
|
|
HCC
|
hepatocellular carcinoma
|
|
Gd-EOB-DTPA
|
gadolinium ethoxybenzyl
diethylenetriamine pentaacetic acid
|
|
MRI
|
magnetic resonance imaging
|
|
CT
|
computed tomography
|
|
ROI
|
region of interest
|
|
SI
|
signal intensity
|
|
SNR
|
signal to noise ratio
|
|
SD
|
standard deviation
|
|
ICG-PDR
|
plasma disappearance rate of
indocyanine green
|
|
OATP
|
organic anion transporting
polypeptide
|
References
|
1
|
Liu M, Liu J, Wang L, et al: Association
of serum microRNA expression in hepatocellular carcinomas treated
with transarterial chemoembolization and patient survival. Plos
One. 9:e1093472014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parikh JG, Kulkarni A and Johns C:
α-smooth muscle actin-positive fibroblasts correlate with poor
survival in hepatocellular carcinoma. Oncol Lett. 7:573–575.
2014.PubMed/NCBI
|
|
3
|
Guiu B, Minello A, Cottet V, et al: A
30-Year, Population-Based Study Shows Improved Management and
Prognosis of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol.
8:986–991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Odisio BC, Galastri F, Avritscher R, et
al: Hepatocellular carcinomas within the Milan Criteria: predictors
of histologic necrosis after drug-eluting beads transarterial
chemoembolization. Cardiovasc Intervent Radiol. 37:1018–1026. 2014.
View Article : Google Scholar
|
|
5
|
Le PH, Huang SC, Lim SN, et al: Complex IV
subunit 1 defect predicts postoperative survival in hepatocellular
carcinoma. Oncol Lett. 7:1430–1438. 2014.PubMed/NCBI
|
|
6
|
Choi WJ, Jeong WK, Kim Y, et al:
Assessment of treatment success and short-term effectiveness using
C-arm CT immediately after hepatic chemoembolization of HCC.
Hepatogastroenterology. 61:1353–1358. 2014.PubMed/NCBI
|
|
7
|
Livraghi T, Mäkisalo H and Line PD:
Treatment options in hepatocellular carcinoma today. Scand J Surg.
100:22–29. 2011.PubMed/NCBI
|
|
8
|
Llovet JL, Real MI, Montaña X, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: a
randomised controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schuette K, Bornschein J, Milbradt O,
Mohnike K, Stuebs P and Malfertheiner P: Transarterial
chemoembolization (TACE) does have little impact on hepatic
metabolic function in patients with hepatocelluar carcinoma (HCC).
J Clin Oncol. 29:e145272011.
|
|
10
|
Di Martino M, Marin D, Guerrisi A, et al:
Intraindividual comparison of gadoxetate disodium-enhanced MR
imaging and 64-section multidetector CT in the detection of
hepatocellular carcinoma in patients with cirrhosis. Radiol.
256:806–816. 2010. View Article : Google Scholar
|
|
11
|
Nilsson H, Blomqvist L, Douglas L, Nordell
A, et al: Gd-EOB-DTPA-enhanced MRI for the assessment of liver
function and volume in liver cirrhosis. Brit J Radiol.
86:201206532013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Katsube T, Okada M, Kumano S, et al:
Estimation of liver function using T1 mapping on
Gd-EOB-DTPA-enhanced magnetic resonance imaging. Invest Radiol.
46:277–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bae KE, Kim SY, Lee SS, et al: Assessment
of hepatic function with Gd-EOB-DTPA-enhanced hepatic MRI. Dig Dis.
30:617–622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pancholy SB, Sanghvi KA and Patel TM:
Radial artery access technique evaluation trial: randomized
comparison of seldinger versus modified seldinger technique for
arterial access for transradial catheterization. Catheter
Cardiovasc Interv. 80:288–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang YS, Chiang JH, Wu JC, Chang FY and
Lee SD: Risk of hepatic failure after transcatheter arterial
chemoembolization for hepatocellular carcinoma: predictive value of
the monoethylglycinexylidide test. Am J Gastroenterol.
97:1223–1227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Zhang HB, Li ZQ, et al:
Indocyanine green clearance in evaluating the recovery of liver
reserve function after superselective transarterial
chemoembolization. Hepatob Pancreat Dis. 12:656–660. 2013.
View Article : Google Scholar
|
|
17
|
Min YW, Lee JH, Gwak GY, et al: Long-term
survival after surgical resection for huge hepatocellular
carcinoma: comparison with transarterial chemoembolization after
propensity score matching. J Gastroen Hepatol. 29:1043–1048. 2014.
View Article : Google Scholar
|
|
18
|
Shimizu J, Dono K, Gotoh M, et al:
Evaluation of regional liver function by
gadolinium-EOB-DTPA-enhanced MR imaging. Dig Dis Sci. 44:1330–1337.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamada A, Hara T, Li F, et al:
Quantitative evaluation of liver function with use of gadoxetate
disodium-enhanced MR imaging. Radiology. 260:727–733. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leonhardt M, Keiser M, Oswald S, et al:
Hepatic uptake of the magnetic resonance imaging contrast agent
Gd-EOB-DTPA: role of human organic anion transporters. Drug Metab
Dispos. 38:1024–1028. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, He YJ, Gan Z, et al: OATP1B1
polymorphism is a major determinant of serum bilirubin level but
not associated with rifampicin-mediated bilirubin elevation. Clin
Exp Pharmacol Physiol. 34:1240–1244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lao XM, Wang D, Shi M, et al: Changes in
hepatitis B virus DNA levels and liver function after transcatheter
arterial chemoembolization of hepatocellular carcinoma. Hepatol
Res. 41:553–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saito M, Seo Y, Yano Y, et al: Short-term
reductions in non-protein respiratory quotient and prealbumin can
be associated with the long-term deterioration of liver function
after transcatheter arterial chemoembolization in patients with
hepatocellular carcinoma. J Gastroenterol. 47:704–714. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schuhmann-Giampieri G, Schmitt-Willich H,
Press WR, et al: Preclinial evaluation of Gd-EOB-DTPA as a contrast
agent in MR imaging of the hepatobiliary system. Radiology.
183:59–64. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Planchamp C, Montet X, Frossard JL, et al:
Magnetic resonance imaging with hepatospecific contrast agents in
cirrhotic rat livers. Invest Radiol. 40:187–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HY, Choi JY, Park CH, et al: Clinical
factors predictive of insufficient liver enhancement on the
hepatocyte-phase of Gd-EOB-DTPA-enhanced magnetic resonance imaging
in patients with liver cirrhosis. J Gastroenterol. 48:1180–1187.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanki A, Tamada T, Higaki A, et al:
Hepatic parenchymal enhancement at Gd-EOB-DTPA-enhanced MR imaging:
correlation with morphological grading of severity in cirrhosis and
chronic hepatitis. Magn Reson Imaging. 30:356–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Motosugi U, Ichikawa T, Sou H, et al:
Liver parenchymal enhancement of hepatocyte-phase images in
Gd-EOB-DTPA-enhanced MR imaging: which biological markers of the
liver function affect the enhancement? J Magn Reson Imaging.
30:1042–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Utsunomiya T, Shimada M, Hanaoka J, et al:
Possible utility of MRI using Gd-EOB-DTPA for estimating liver
functional reserve. J Gastroenterol. 47:470–476. 2012. View Article : Google Scholar
|
|
30
|
Goshima S, Kanematsu M, Watanabe H, et al:
Gd-EOB-DTPA-enhanced MR imaging: prediction of hepatic fibrosis
stages using liver contrast enhancement index and liver-to-spleen
volumetric ratio. J Magn Reson Imaging. 36:1148–1153. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takao H, Akai H, Tajima T, et al: MR
imaging of the biliary tract with Gd-EOB-DTPA: effect of liver
function on signal intensity. Eur J Radiol. 77:325–329. 2011.
View Article : Google Scholar
|
|
32
|
Bickelhaupt S, Studer P, Kim-Fuchs C, et
al: Gadoxetate uptake as a possible marker of hepatocyte damage
after liver resection-preliminary data. Clin Radiol. 68:1121–1127.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goel A, Mehta N, Guy J, et al: Hepatic
artery and biliary complications in liver transplant recipients
undergoing pretransplant transarterial chemoembolization. Liver
Transpl. 20:1221–1228. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Negi SS and Chaudhary A: Analysis of
abnormal recovery pattern of liver function tests after surgical
repair of bile duct strictures. J Gastroenterol Hepatol.
20:1533–1537. 2005. View Article : Google Scholar : PubMed/NCBI
|