Introduction
Penile cancer is an extremely rare form of
urological cancer, with an incidence of 0.1–8.3/100,000 males
(1,2). The annual incidence is <1/100,000
males in the USA and in European countries (2). However, it is an important global
health concern due to the higher incidence, up to 10–20%, in
several countries and worldwide (2). The incidence of the disease is highest
in Brazil, Uganda and India, while it is lower in the Jewish and
Muslim communities, in which infants and children are mostly
circumcised. In addition, early circumcision reduces the risk of
penile cancer by three to five times (1).
Predisposing factors for penile cancer include
chronic inflammatory diseases, such as phimosis, balanoposthitis
and balanitis xerotica obliterans, ultraviolet phototherapy,
multiple sexual partners, an early age at first intercourse and a
previous history of condyloma. Smoking and intercourse with a
partner infected with human papilloma virus (HPV) types 6, 11, 16
or 18 are also among the risk factors (3). In addition, the presence of high-risk
HPV DNA is critical in the prognosis of the disease, as this may
reduce the survival rate (4).
Furthermore, defects in tumor suppressor genes, including p53 and
Rb, play an important role in the development of cancer (5). As smegma has been reported to be
likely to exert carcinogenic effects, circumcision has been
considered to reduce the incidence of smegma (6).
Squamous penile cancer accounts for >95% of all
malignant penile cancers. Cutaneous horn and Bowenoid papulosis of
the penis and balanitis xerotica obliterans, also termed lichen
sclerosus, are pre-malignant lesions of penile cancer.
Additionally, penile intraepithelial neoplasia, a form of carcinoma
in-situ, erythroplasia of Queyrat and Bowen’s disease are
potential risk factors for penile cancer (3). Nearly 30% of these conditions result
in invasive cancer. Patients are usually asymptomatic and exhibit a
variable clinical presentation, which may be accompanied by
indurated and growing papules, and pustule warts or ulcerative
tumors. Early symptoms of the disease are mainly itching and
burning in the area of the preputium (3). The present study reports the case of a
39-year-old patient with penile mucinous adenocarcinoma who was
admitted with the complaint of perineal discharge.
Case report
A 39-year-old male patient was admitted to Ataturk
Education and Research Hospital (Izmir, Turkey) due to a complaint
of perineal discharge. The patient’s medical history revealed that
a solid mass had been observed in the perineal area three years
prior to the present study and perineal abscess drainage had been
performed twice. With an additional complaint of stranguria, the
patient also underwent an internal urethrotomy twice for a urethral
stricture. A biopsy specimen was obtained from the area in which
the abscess drainage was applied one month prior to admission. The
medical history also reported that circumcision had been performed
at the age of two. The patient possessed poor penile hygiene and a
history of smoking.
Systemic examination findings were normal.
Urogenital examination revealed a palpable solid flat mass, 8 cm in
size, which originated from the left side of the penis glans. The
mass involved the urethra in the ventral view of the penis and the
spongious body, with an invasion of the left corpus cavernosum,
which advanced through the left side of the radix penis and
scrotum. A continuous perineal induration and hyperemia were
present. An orifice of the urethral fistula in the perineum was
observed. A painful lymphadenopathy, 1.5 cm in size, was found in
the left superficial inguinal lymph node chain. Digital rectal
examination revealed anal edema. Two painless and soft nodules,
each 1 cm in size, were observed in the anterior wall of the
rectum. The testicles were normal. The biochemical results were as
follows: White blood cell count, 6,400/μl (normal range,
4.0–11.0/μl); hemoglobin, 12.9 g/dl (normal range, 12.8–15.5 g/dl);
platelet count, 326,000/l (normal range, 150,000–450,000/l); total
bilirubin, 0.73 mg/dl (normal range, 0.1–1.2 mg/dl); direct
bilirubin, 0.21 mg/dl (normal range, 0.1–0.4 mg/dl); alkaline
phosphatase, 46 U/l (normal range, 44–147 U/l); glucose, 86 mg/dl
(normal range, 70–101 mg/dl); blood urea nitrogen, 13 mg/dl (normal
range, 8–20 mg/dl); creatinine, 1.0 mg/dl (normal range, 0.6–1.2
mg/dl); albumin, 2.7 g/dl (normal range, 3.4–4.7 g/dl); aspartate
aminotransferase, 98 U/l (normal range, 10–41 U/l); alanine
aminotransferase, 30 U/l (normal range, 10–44 U/l); calcium, 7.9
mg/dl (normal range, 8.5–10.5 mg/dl); sodium, 142 mmol/l (normal
range, 134–143 mmol/l); potassium, 3.8 mmol/l (normal range,
3.5–5.5 mmol/l); chlorine, 106 mmol/l (normal range, 98–107
mmol/l); sedimentation rate, 33/h (normal range, <15/h);
carcinoembryonic antigen, 4.88 ng/dl (normal range, <7 ng/dl);
and prostate-specific antigen, 0.7 ng/ml (normal range, <2.5
ng/ml). An angiogram of the fistula discharge revealed culture
positivity for Klebsiella pneumoniae. Susceptibility to
piperacillin + tazobactam, cefoperazone + sulbactam and meropenem
was detected.
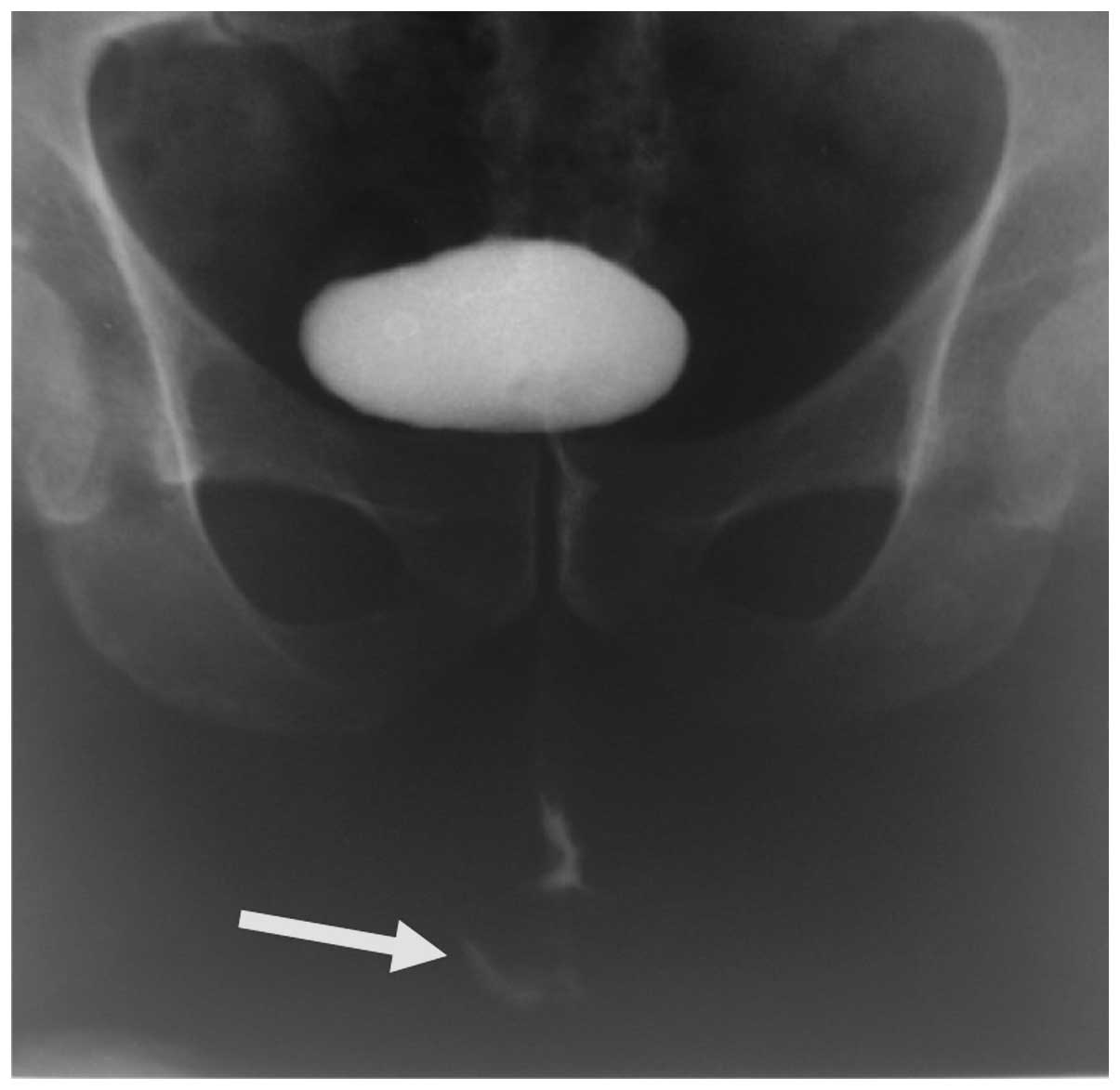
Abdominal ultrasound and thoracoabdominal computed
tomography (CT) produced normal findings. The intravenous
pyelography and cystography results were also normal. A fistula at
bulbar urethral level was found in the retrograde urethrography
(Fig. 1). Rectosigmoidoscopy
demonstrated an internal hemorrhoid, while colonoscopy revealed
normal findings. Upper gastrointestinal endoscopy revealed antral
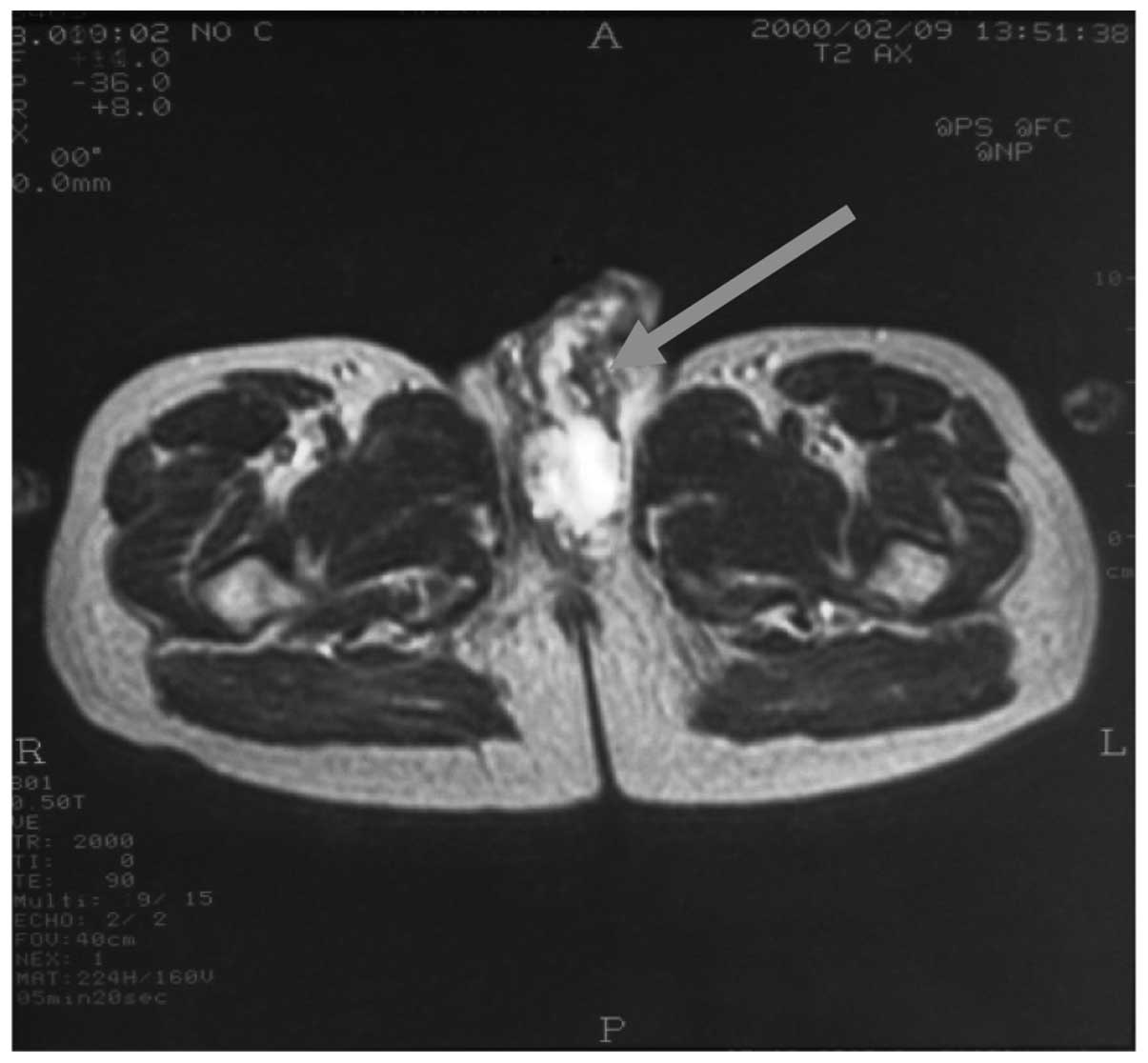
gastritis. Magnetic resonance imaging (MRI) of the pelvis revealed
a heterogeneous mass in the left side of the radix penis and
proximal scrotum. Spongious body involvement was evident. Bulbous
and cavernous portions of the urethra were infiltrated, whereas the
prostate, vesiculae seminalis and testicles were normal (Fig. 2).
Pathological examination of the perineal open biopsy
specimens resulted in the diagnosis of mucinous adenocarcinoma. The
diagnosis was confirmed by pathological consultation of the same
specimens. The patient underwent total penectomy and inguinal lymph
node dissection. Intraoperative frozen section analysis revealed a
poorly-differentiated mucinous micropapillary tumor originating
from the glans to the radix penis, which infiltrated the corpus
cavernosum and invaded two-thirds of the penile wall. The tumor was
14×3.5 cm in length with a full-thickness tissue depth of 2.5 cm.
One of the inguinal lymph nodes was invaded. However, there was no
metastatic disease, as confirmed by positron emission tomography
(PET)/CT scans. According to the tumor-node-metastasis staging
system, the tumor was staged as T4N1M0.
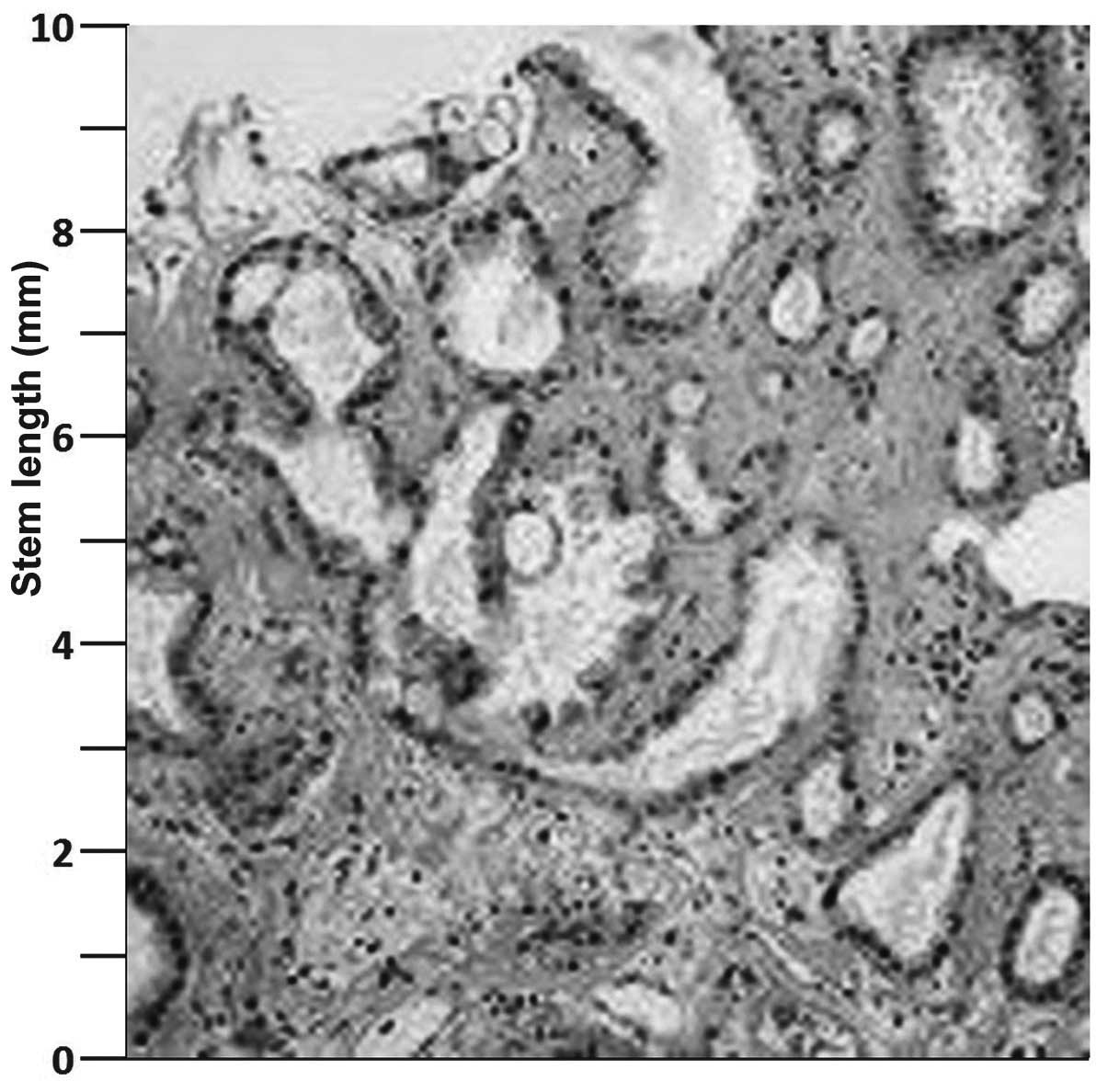
Intraoperative specimens and perineal specimens, in
particular, were analyzed by a pathologist who was experienced in
the field of uro-oncology. Hematoxylin and eosin staining revealed
mucinous islets (Fig. 3). In
addition, large pools of mucin arranged in lobules, separated by
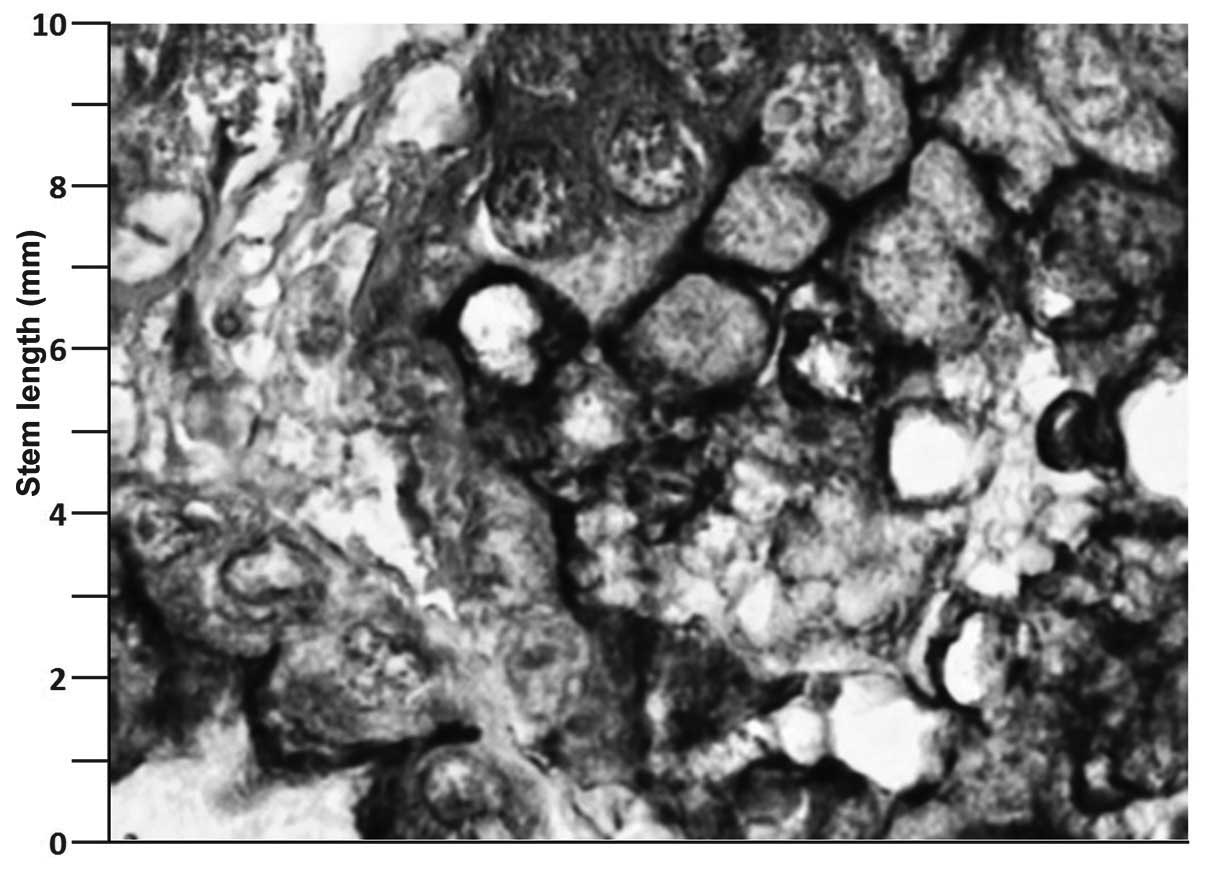
collagenous septae were identified. The mucin 1 antibody was
labeled in the immunohistochemical examination (Fig. 4), which stained strongly, indicating
increased Muc 1 expression.
The patient was treated with pelvic radiotherapy (50
Gy) and systemic neoadjuvant chemotherapy (cisplatin and
paclitaxel) simultaneously. After nine months of follow-up the
patient succumbed to the disease due to widespread metastases.
Written informed consent was obtained from the
patient who participated in the present study. All procedures
followed were in accordance with the ethical standards of the
Atatürk Education and Research Hospital committee on human
experimentation (institutional and national) and with the Helsinki
Declaration of 1975, as revised in 2008.
Discussion
To the best of our knowledge, the present case is
the first case of primary mucinous adenocarcinoma of the penis in
the literature. Metastatic carcinoma of the penis, one of the rare
forms of penile tumors, was excluded by assessing the possible
sites of origin by thoracic CT, abdominopelvic MRI and PET/CT. In
particular, gastrointestinal tumors are likely to metastasize to
the penis, but metastatic carcinoma of the penis may also arise
from bladder, prostate and kidney tumors (4,5). In
the present patient, endoscopic examination of the gastrointestinal
system and biopsy specimens revealed no pathology, with the
exception of antral gastritis and hemorrhoids. A mucinous carcinoma
originating from the penis glans to the radix penis with inguinal
lymph node metastasis suggested primary mucinous
adenocarcinoma.
The underlying mechanism and histopathogenesis of
mucinous cancer of the penis has yet to be elucidated. Squamous
cell carcinoma, the most common form of penile cancer, relies upon
the malignant transformation of a pre-malignant lesion (7). These tumors indicate damaged DNA
differentiation in the healthy cellular cycle. Possible mechanisms
that induce malignant transformation include chronic irritation,
smoking and poor penile hygiene. Processes in chronic inflammation
may stimulate metaplasia and differentiation. One-third of penile
squamous cell carcinoma is associated with the malignant
transformation of pre-malignant penile lesions (8). Furthermore, viral infections,
including HPV, may cause penile cancer through DNA damage. In
addition, defects in tumor suppressor genes, including p53,
interrupt the cellular cycle and cause apoptosis (4,5). Loss
of such genes contributes to DNA damage and accelerates malignant
transformation.
To the best of our knowledge, mucinous
adenocarcinoma of the penis has not yet been defined in the
literature. However, there have been case studies on mucinous
metaplasia, which is considered to be a predisposing factor for
penile cancer (9). In addition, a
previous study has reported the presence of mucin-producing cells
presenting with metaplasia in the preputium in the literature
(9). Another study demonstrated a
higher incidence of acid-mucin cells in the preputium compared with
other areas (10). The underlying
mechanism of mucinous transformation remains unknown due to the
limited number of studies in the literature. Mucinous metaplasia of
the penis is a rare condition with unknown origin (9) that is usually observed in the elderly
and has been reported to be associated with chronic balanitis or
Zoon’s balanitis (11), which is
often overlooked in clinical practice. Only eight cases are
available in the literature (9,10,12–14).
It is hypothesized that mucinous metaplasia may initially present
as a pre-malignant lesion of penile mucinous adenocarcinoma and is
associated with malignant transformation due to continuous chronic
irritation. However, the exact association between Zoon’s
balanitis, mucinous metaplasia and adenocarcinoma remains
unknown.
In conclusion, to the best of our knowledge, the
present case is the first case of primary mucinous adenocarcinoma
of the penis in the literature. The patient was treated with pelvic
radiotherapy (50 Gy) and systemic neoadjuvant chemotherapy
(cisplatin and paclitaxel) simultaneously. However, after nine
months of follow-up the patient succumbed to the disease due to
widespread metastases. Although the histopathological mechanism is
indefinite, mucinous metaplasia is considered likely to be a risk
factor for penile cancer. Therefore, patients with mucinous
metaplasia should be closely followed for cancer development.
References
|
1
|
Barnholtz-Sloan JS, Maldonado JL, Pow-Sang
J and Giuliano AR: Incidence trends in primary malignant penile
cancer. Urol Oncol. 25:361–367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Whelan SL, Ferlay J, et al:
Cancer Incidence in Five Continents. VIII:http://www.iarc.fr/en/Publications/PDFs-online/Cancer-Epidemiology/IARC-ScientificPublication-No.-155"ref-label" rowspan="1" colspan="1">
3
|
Pizzocaro G, Algaba F, Horenblas S, et al:
EAU penile cancer guidelines 2009. Eur Urol. 57:1002–1012. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bezerra AL, Lopes A, Santiago GH, et al:
Human papillomavirus as a prognostic factor in carcinoma of the
penis: analysis of 82 patients treated with amputation and
bilateral lymphadenectomy. Cancer. 91:2315–2321. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kayes O, Ahmed HU, Arya M and Minhas S:
Molecular and genetic pathways in penile cancer. Lancet Oncol.
8:420–429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Howe RS and Hodges FM: The
carcinogenicity of smegma: debunking a myth. Eur Acad Dermatol
Venereol. 20:1046–1054. 2006. View Article : Google Scholar
|
|
7
|
Velazquez EF and Cubilla AL: Lichen
sclerosus in 68 patients with squamous cell carcinoma of the penis:
frequent atypias and correlation with special carcinoma variants
suggests a precancerous role. Am Surg Pathol. 27:1448–1453. 2003.
View Article : Google Scholar
|
|
8
|
Ranganath R, Singh SS and Sateeshan B:
Sarcomatoid carcinoma of the penis: clinicopathologic features.
Indian J Urol. 24:267–268. 2008. View Article : Google Scholar
|
|
9
|
Ruiz-Genao DP, Daudén-Tello E, Adrados M,
Fraga J and García-Díez A: Mucinous metaplasia of the glans penis.
Histopathology. 44:90–91. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Val-Bernal JF and Hernández-Nieto E:
Benign mucinous metaplasia of the penis. A lesion resembling
extramammary Paget’s disease. J Cutan Pathol. 27:76–79. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
García-Abós M, Fraga J and Daudén E:
Mucinous metaplasia of the penis associated with Zoon’s balanitis.
Actas Dermosifiliogr. 101:362–364. 2010. View Article : Google Scholar
|
|
12
|
Fang AW, Whittaker MA and Theaker JM:
Mucinous metaplasia of the penis. Histopathology. 40:177–179. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tong PL, Delaney TA and Beer TW:
Erythematous shiny plaque over the glans penis. Arch Dermatol.
147:735–740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai JP, Cowen EW, Jere B, et al: Zoon’s
balanitis with mucinous metaplasia: A case report and review of
literature. Open J Clin Diag. 3:33–36. 2013. View Article : Google Scholar
|