Introduction
Esophageal cancer is one of the eight most common
cancers and is the sixth leading cause of global cancer mortality.
An estimate indicates that there were 482,300 novel esophageal
cancer cases and 406,800 mortalities due to esophageal cancer in
2008 worldwide (1). The major
histological subtypes of esophageal cancer are adenocarcinoma (AC)
and squamous cell carcinoma (SCC) (2). There is a high prevalence of AC in
Europe, while SCC is the dominant form in Asia (1). The prevalence of esophageal SSC (ESSC)
has risen in developing countries, particularly in China (2–4). A
notable belt of esophageal cancer occurrence, primarily SCC,
extends from Northeast China to the Middle East (3). The high incidence of ESCC is
associated with smoking, obesity, consumption of hot beverages and
red meat, a high alcohol intake, and a low intake of fresh
vegetables and fruit (4).
Furthermore, early diagnosis of esophageal cancer remains to be a
challenge in clinical practice as early esophageal cancer exhibits
no characteristic clinical manifestations and there are no
effective screening tools (5).
Therefore, the majority of patients are at an advanced stage of
disease at the time of diagnosis, and the carcinoma has already
metastasized. Clinically, the mainstays of treatment for esophageal
cancer include surgical resection, radiation therapy and
chemotherapy (6,7). However, chemotherapy and radiotherapy
demonstrate acute and chronic toxicities, which often results in
not only a cessation of therapy, but also a decrease in the quality
life (8,9). For these reasons, esophageal cancer
exhibits a poor prognosis and the five-year survival rate,
subsequent to diagnosis, is <13% (10). Therefore, novel therapeutic
alternatives or agents are urgently required for patients with
esophageal cancer.
Currently, increasing numbers of studies investigate
plants or herbs for antitumor effects (11–13),
as they are generally safe and exhibit no or low toxicity. Saffron
is the flower of Crocus sativus L. and is generally used as
a spice and food colorant. Saffron has also been used as a
traditional medicine in China, India and the Arab world since time
immemorial. Crocetin, the major component of saffron, is a low
molecular weight carotenoid compound (14). Numerous studies have been performed
to indicate the medicinal properties of crocetin, including
antioxidative (15),
antihypertensive (16),
antithrombotic (17),
anti-inflammatory (18),
cardioprotective (19),
hepatoprotective (20) and
neuroprotective (21) effects.
Crocetin also exhibits anticancer and antitumor properties.
Numerous studies have reported that crocetin exhibits an inhibitory
effect on cell proliferation and cytotoxicity, which has been
detected in several malignant cell lines, including human gastric
(22), colon (23) and breast (24) cancer cells, and in in vitro
models. In the benzo(a)pyrene-induced lung carcinoma mouse model,
crocetin significantly reversed the pathological changes (25). In the
1-methyl-3-nitro-1-nitrosoguanidine-induced gastric cancer rat
model, crocetin demonstrated a significant regression of tumor
growth in a dose-dependent manner (22). From these studies, it can be
observed that crocetin possesses considerable anticancer
properties.
Crocetin has exhibited excellent anticancer
properties, while the underlying mechanism remains unclear.
KYSE-150 cells are an esophageal squamous cell carcinoma cell line
and are widely used as an in vitro esophageal cancer model
to study esophageal cancer. In the present study, the mechanism of
the anticancer action of crocetin in the human esophageal squamous
carcinoma KYSE-150 cell line was examined by evaluating its
antiproliferative, proapoptotic and inhibitory effects on
migration. In addition, the intracellular signaling pathway of
apoptosis was also investigated.
Materials and methods
Reagents
Crocetin (C20H24O4;
molecular weight, 328.4) was obtained from MP Biomedicals (Santa
Ana, CA, USA). The crocetin was dissolved in dimethyl sulfoxide
(DMSO) stored at −20°C and then diluted in medium prior to each
experiment. The final DMSO concentration did not exceed 0.1%
throughout the study. MTT, Hoechst 33258 and DMSO were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Propidium iodide (PI) was
obtained from Beijing Dingguo Biotech Co., Ltd (Beijing, China). A
bicinchoninic acid (BCA) Protein Assay kit was purchased from
Beyotime Institute of Bioengineering (Haimen, Jiangsu, China).
Cleaved monoclonal rabbit anti-human caspase 3 antibody (cat. no.
9664) was obtained from Cell Signaling Technology Inc. (Danvers,
MA, USA) and polyclonal rabbit anti-human B-cell
lymphoma-2-associated X protein (Bax) (cat. no. ab7977) and
monoclonal rabbit anti-human β-actin (cat. no. ab179467) antibodies
were purchased from Abcam (Cambridge, UK). Horseradish
peroxidase-conjugated goat anti-rabbit antibodies were obtained
from Wuhan Boster Biological Technology, Ltd. (BA1054-0.5, Wuhan,
Hubei, China).
Cell culture
The esophageal squamous carcinoma KYSE-150 cell line
(Japanese Collection of Research Bioresources Cell Bank, Osaka,
Japan) was grown in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum, 100 units/ml penicillin
and 100 μg/ml streptomycin (Gibco Life Technologies, Carlsbad, CA,
USA). The cells were cultured under an atmosphere of 5%
CO2 and 95% air at 37°C.
Cell proliferation MTT assay
Cell proliferation was measured by an MTT assay as
previously described (26).
Briefly, the cells were plated in 96-well plates at a density of
1.1×104 cells/well in complete DMEM, and incubated at
37°C. After 24 h, the wells were washed with PBS, and the cells
were incubated with 0, 12.5, 25, 50, 100 or 200 μmol/l crocetin for
48 h. The 96-well plate was gently washed with PBS and then MTT was
added and left for ~4 h. The resulting formazan was dissolved using
DMSO. The product was measured at 570 nm in a microplate reader
(Tecan Austria GmbH, Grödig, Austria). All experiments were
performed at least in triplicate.
Migration of KYSE-150 cells in a
wound-healing assay
An in vitro wound-healing assay was performed
to detect the effect of crocetin on cell migration. Briefly, the
KYSE-150 cells were seeded in 12-well plates at the density of
1.6×105 cells/well, when the cells had grown to 90%
confluency, the cell monolayers were scratch-wounded in a straight
line using a 1,000 μl pipette-tip, providing a wound width of 1 mm.
Subsequently, the cells were washed three times with PBS to remove
the detached cells, and then incubated with 0, 100 or 200 μmol/l
crocetin for 48 h at 37°C. To measure cell migration, a
phase-contrast inverted microscope (Carl Zeiss AG, Oberkochen,
Germany) was used to capture images in four randomly chosen fields
within the wounded region at 0, 24 and 48 h. The migration rate was
calculated as follows: migration rate (%) = (original width -
closure width) / original width × 100%.
Morphological detection of apoptosis
The cells were seeded in a 12-well plate at a
density of 1.2×105 cells/well. Following an overnight
attachment period, the cells were treated using 0, 100 or 200
μmol/l crocetin for 48 h. The cells were then incubated with 1
μg/ml Hoechst 33258 for 10 min at 37°C in a humidified atmosphere
in the dark, and washed three times with PBS. A fluorescent
microscope (Leica Microsystems GmbH, Wetzlar, Germany) was used to
evaluate the nuclear morphology of the cells.
Cell-cycle analysis by flow
cytometry
Subsequent to incubation with 0, 100 or 200 μmol/l
crocetin for 48 h, the KYSE-150 cells were harvested and fixed
overnight in 70% ethanol at 4°C. The fixed cells were washed with
PBS then stained with PI (50 μg/ml) for 30 min at 37°C in the dark.
The stained cells were assessed using flow cytometry (Beckman
Coulter Cell, USA) and analyzed by FlowJo 7.6.5 software (FlowJo,
LLC., Ashland, OR, USA).
Western blot analysis
The KYSE-150 cells were harvested following
incubation with 0, 100 or 200 μmol/l crocetin for 48 h. The cells
were then lysed in ice-cold lysis buffer (1× PBS; 1% NP40; 0.1%
SDS; 5 mm EDTA; 0.5% sodium deoxycholate; 1% PMSF) for 30 min. The
homogenate was centrifuged at 14,000 × g for 15 min at 4°C, the
supernatant extract was gathered and quantified for protein using
the BCA Protein Assay kit. Equal amounts of cell protein were
separated by electrophoresis on 12% SDS-PAGE. The protein was then
transferred to polyvinylidene difluoride membranes, and the
membranes were blocked using 5% bovine serum albumin for 1 h at
room temperature, followed by incubation overnight at 4°C with
primary antibodies for cleaved caspase 3 (1:2,000), Bax (1:1,000)
and β-actin (1:2,000). The membranes were washed three times with
Tris-buffered saline (Guangzhou Whiga Biotechnology Co., Ltd.,
Guangzhou, China) containing 0.05% Tween-20 (Wuhan Boster
Biological Technology, Ltd.), and then incubated with horseradish
peroxidase conjugated anti-rabbit antibodies for 1 h. The protein
bands were visualized using electrochemiluminescence and the band
intensity was measured using Image J 1.46r software (National
Institutes of Health, Bethesda, MA, USA). Each experiment was
repeated at least three times.
Statistical analysis
All data were reported as the mean ± standard error
of the mean and were obtained from at least three independent
experiments. The differences in data were analyzed by one-way
analysis of variance followed by the least significant difference
test, using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Crocetin inhibits the proliferation of
KYSE-15O cells
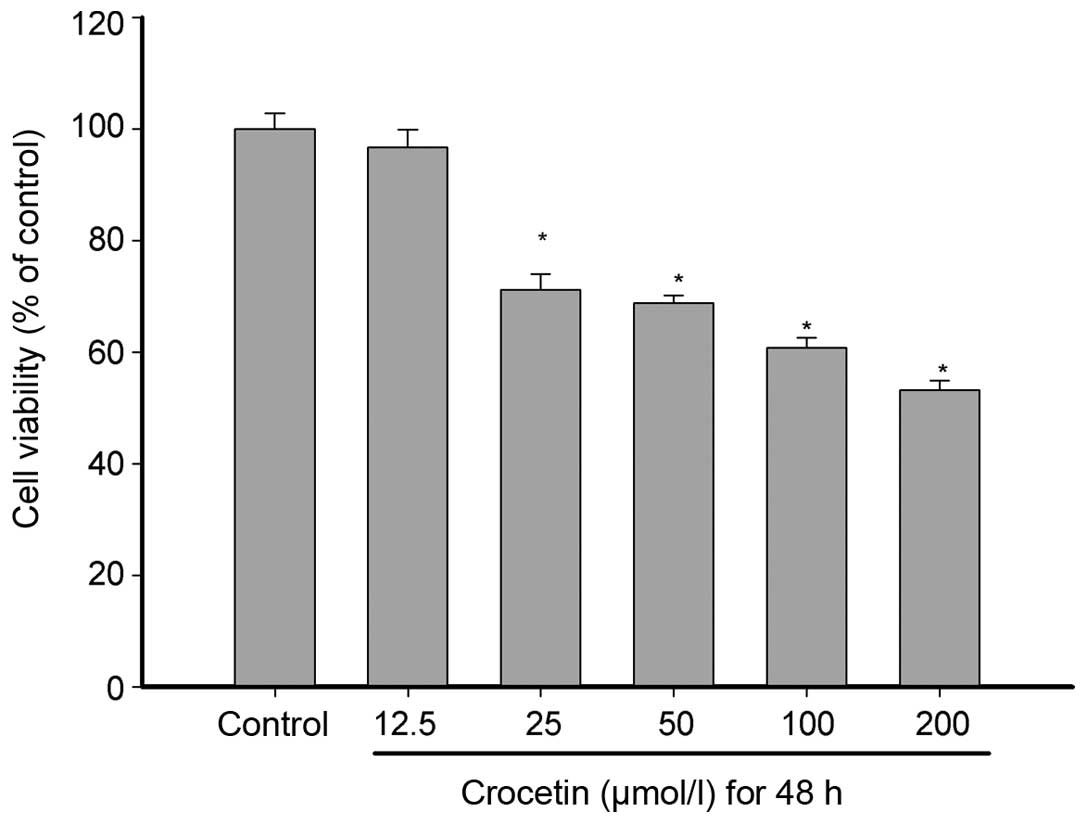
An MTT assay was used to measure the inhibition of
crocetin on KYSE-150 cell proliferation. As shown in Fig. 1, crocetin inhibited the
proliferation of KYSE-150 cells, in a concentration-dependent
manner. Cell proliferation was inhibited by all concentrations of
crocetin, with the exception of the 12.5 μmol/l group. The cell
viability was 96.68, 71.10, 68.76, 60.77 and 53.22% at crocetin
concentrations of 12.5, 25, 50, 100 and 200 μmol/l, respectively,
revealing a notable inhibition of the proliferation of KYSE-150
cells at higher concentrations.
Crocetin induces morphological changes in
KYSE-150 cells
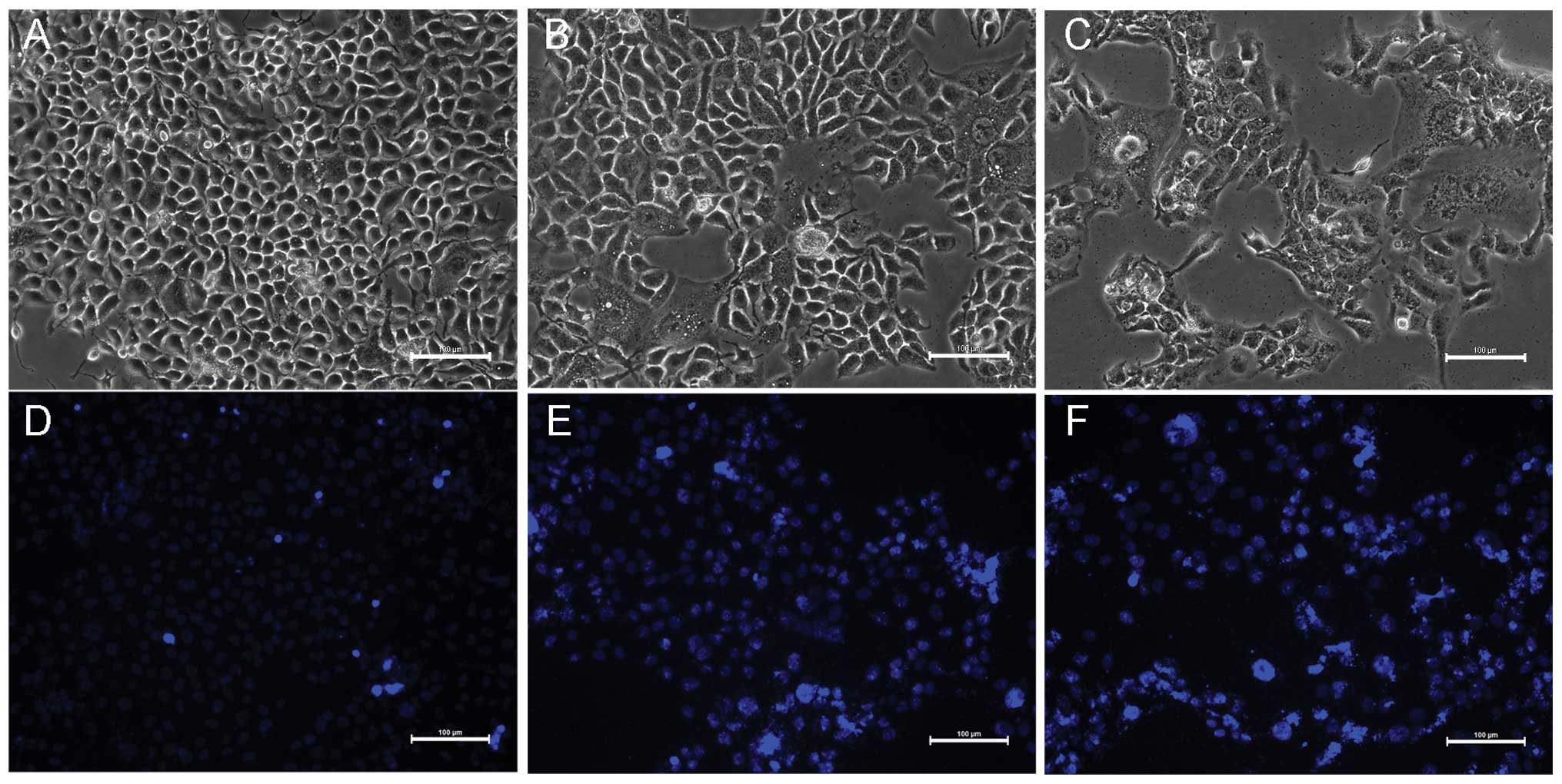
A morphological method was used to observe the
changes in the morphology of the KYSE-150 cells during
crocetin-induced cell death. As shown in Fig. 2A–C, normal KYSE-150 cells possess a
plump cell body, exhibiting a polygonal shape and distinct cell
borders under light microscopy. However, subsequent to 48-h
incubation with concentrations of 100 and 200 μmol/l crocetin, the
morphology of the KYSE-150 cells changed. The cell number was
decreased and the cells became granulated, the cell size reduced,
cytoplasmic vacuolar changes occurred and certain cells even lysed
or became replaced by debris, and cellular detachment was
prominent. This was particularly notable at a 200 μmol/l
concentration of crocetin.
Hoechst 33258 is a membrane-permeable DNA dye.
Subsequent to staining with Hoechst 33258, the nuclei of the live
cells became blue, while the apoptotic cell nucleus clearly showed
highly condensed or fragmented chromatin with inhomogeneous blue
fluorescence. Briefly, the cells were stained with 1 μg/ml Hoechst
33258 for 10 min following treatment with crocetin. Crocetin was
demonstrated to induce apoptosis in KYSE-150 cells after 48 h,
particularly at a concentration of 200 μmol/l. As shown in Fig. 2D–F, fluorescence dense particles of
the cell nucleus, nuclear chromatin condensation and fragmentation,
and the formation of apoptotic bodies were observed in the
crocetin-treated cells under fluorescent microscopy. However, no
inhomogenous blue fluorescence in the cell nucleus were observed in
the control group and only a small number of cells exhibited
nuclear chromatin condensation.
Crocetin inhibits the migration of
KYSE-150 cells
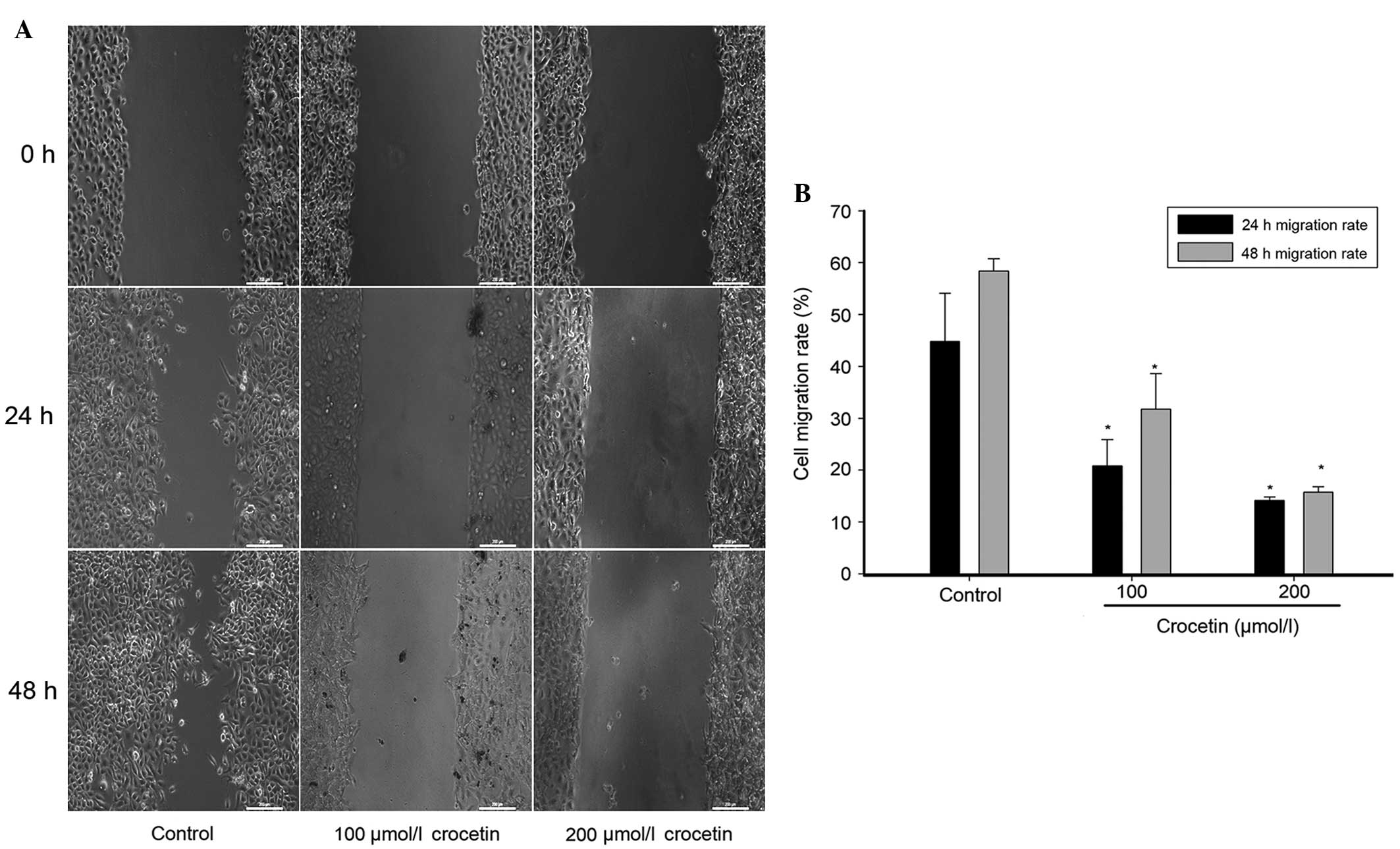
A wound healing assay was performed to measure the
migration capability of KYSE-150 cells. As shown in Fig. 3, after 48 h the untreated KYSE-150
cells had migrated into the wounded area of the cell monolayer,
whereas the migration capability of crocetin-treated cells was
significantly reduced, particularly in the group treated with 200
μmol/l crocetin. The migratory rate at 24 h was 44.83% in the
control group, and 20.82 and 14.15% in the groups treated with 100
and 200 μmol/l crocetin, respectively. The migratory rate at 48 h
was 58.36% in the control group, and 31.78 and 15.71% in the cells
treated with 100 and 200 μmol/l crocetin, respectively. A
statistically significant difference (P<0.05) was identified
between the migratory rates of the crocetin-treated cells and the
control, at 24 and 48 h. This indicates that crocetin inhibits the
migration of KYSE-150 cells.
Crocetin induces cell cycle arrest in
KYSE-150 cells
To elucidate the underlying mechanism of the
inhibitory effect of crocetin on cellular proliferation, the cell
cycle distribution in crocetin-treated cells was determined using
flow cytometry. As shown in Table
I, crocetin significantly increased the number of KYSE-150
cells in the S phase in a concentration-dependent manner. The
percentage of cells in the S phase varied between 12.93% in the
control group and 23.33% in the group treated with 200 μmol/l
crocetin. Concurrently, the number of cells in the G1
phase was markedly reduced, indicating that crocetin inhibits the
proliferation of KYSE-150 cells by inducing cell cycle arrest in
the S phase.
 | Table IEffect of different concentrations of
crocetin on the cell cycle distribution of KYSE-150 cells. |
Table I
Effect of different concentrations of
crocetin on the cell cycle distribution of KYSE-150 cells.
| | Distribution of
cells, % |
|---|
| |
|
|---|
| Groups | Crocetin,
μmol/l |
G0/G1 phase | S phase | G2/M
phase |
|---|
| Control | 0 | 78.23±1.32 | 12.93±0.41 | 7.03±0.31 |
| Crocetin | 100 | 77.33±0.62 | 17.73±0.56a | 4.10±0.29a |
| 200 | 70.00±0.87a | 23.33±0.88a | 6.52±0.20 |
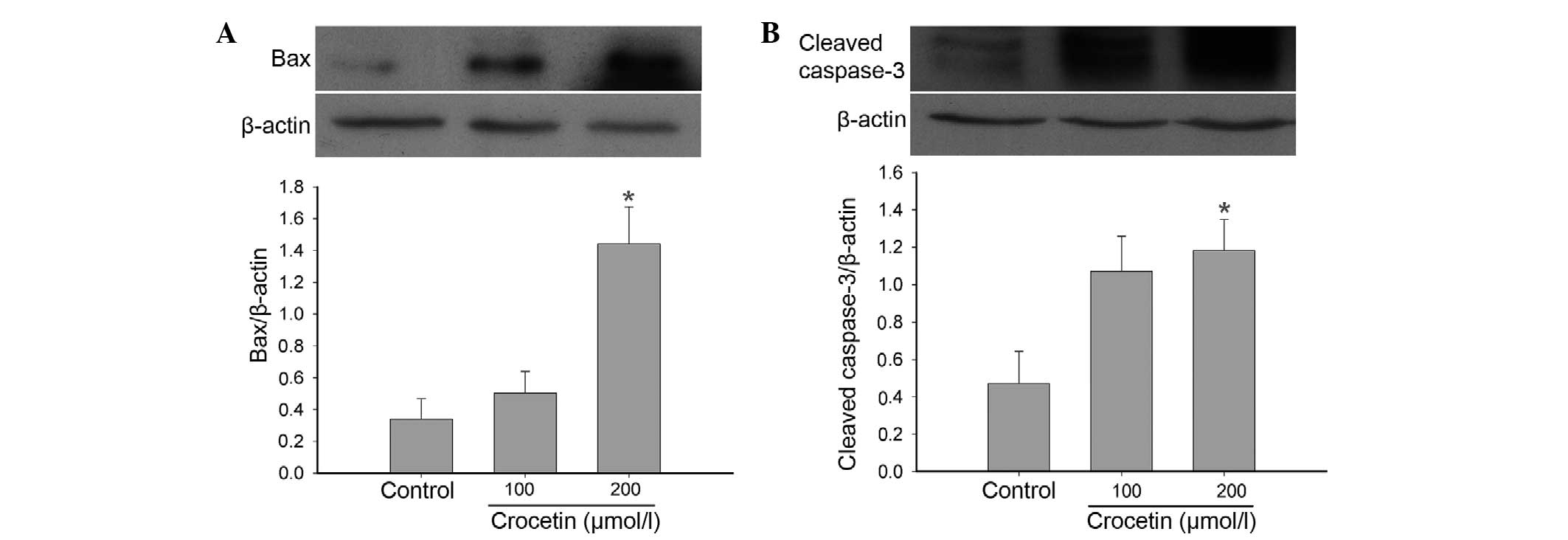
Crocetin induces the expression of
apoptosis-associated proteins
To investigate the underlying mechanism of the
proapoptotic properties of crocetin, the levels of Bax and cleaved
caspase 3 were examined in KYSE-150 cells by western blotting. The
levels of Bax and cleaved caspase 3 were markedly increased in
crocetin-treated cells compared to the control group (Fig. 4).
Discussion
In the previous two decades, natural products have
been becoming a popular area for studies into chemopreventive and
chemotherapeutic agents for the treatment of cancers (11). Crocetin is the main constituent of
saffron, and there have been numerous studies into its anticancer
and antitumor properties. Several hypotheses for these
anticarcinogenic and antitumor effects have been proposed,
including inhibition of nucleic acid synthesis, inhibition of free
radical chain reactions and the generation of reactive oxygen
species through eliminating free radicals, as well as conversion to
vitamin A, which enhances carcinogen metabolism (14,27,28).
However, the exact mechanism of the anticancer and antitumor
effects of crocetin requires additional investigation. Thus, in the
present study, the inhibition of cell proliferation and migration
and the proapoptotic effects of crocetin on KYSE-150 cells were
investigated. To the best of our knowledge, the present study is
the first to report crocetin inducing the inhibition of
proliferation, proapoptotic effects and the inhibition of migration
in the KYSE-150 cell line.
One feature of malignancy is infinite proliferation.
Thus, suppression of cell growth has become an important target in
cancer therapy. There is a growing body of evidence indicating that
crocetin and the analogues of crocetin from various crocus species
can inhibit cancer cell proliferation (29). In the present study, it was found
that crocetin produced a marked reduction in the proliferation of
KYSE-150 cells in a concentration-dependent manner. As it is known
that the infinite proliferation of the malignancy is closely
associated with the cell cycle regulation (30,31),
the cell cycle distribution was detected in the present study in
order to explore the underlying mechanism of crocetin. Cell cycle
progression is monitored by cell cycle checkpoints that ensure the
order and timing of cell cycle transition (32). In addition, the proper replication
and segregation of genetic material to daughter cells is crucial.
Disorder in cell cycle regulation causes the endless proliferation
of cells, leading to cancer (33).
Inducing cell cycle arrest has become a key area of antitumor drug
development and there are numerous chemopreventive drugs used in
the clinic that are based on this principle, such as cisplatin
(34). In the present study,
treatment with crocetin significantly increased the number of
KYSE-150 cells in the S phase. Li et al (23) demonstrated that crocetin induced
cell cycle arrest in the S phase of SW480 cells through decreased
levels of cyclin A and cdk2. However, certain studies have reported
that crocetin induces cell cycle arrest in the G1 or
G2 phase (28,29). The variation in cell lines and
dosages used in these studies may be responsible for this
difference. The exact mechanism of the effects of crocetin requires
further investigation.
Apoptosis, or programmed cell death, is a
gene-regulated phenomenon, and disequilibrium between cell
proliferation and apoptosis is known to cause various diseases,
including cancer. Resisting cell death is another notable
characteristic of cancer (35).
Therefore, the induction of apoptosis in malignant cells is
considered to be an important target for the therapy and prevention
of cancer (36). The proapoptotic
effect of crocetin has been demonstrated in various human tumors,
including gastric, colon, breast, liver and pancreatic cancer cells
(22–24,28,37).
In the present study, following incubation with crocetin for 48 h,
the KYSE-150 cells exhibited wide cytoplasmic vacuole-like areas,
reduced cytoplasm, cell shrinkage, pyknotic nuclei and the
formation of apoptotic bodies, suggesting proapoptotic effects of
crocetin. Additionally, crocetin exhibits no toxic effects towards
non-malignant cells (23). Overall,
this indicates that crocetin meets the requirements of a novel
chemotherapy agent that may effectively target cancer cells, whilst
exhibiting no or negligible toxicity towards non-malignant cells.
Selective induction of apoptosis is one of the major goals of
cancer chemotherapy. To investigate the precise mechanism
responsible for the selectivity of crocetin, the levels of Bax and
cleaved caspase 3 were determined. Sequential activation of
caspases plays a central role in the execution phase of cell
apoptosis, with caspase 3 being the downstream effector of
apoptosis (39,40). Activated caspase 3 is necessary in
the mitochondrial and death receptor-mediated cell apoptosis
pathways. Bax is located in the cytoplasm and once activated, Bax
inserts into the mitochondrial membrane, increasing the membrane
permeability and leading to the release of cytochrome c
(38). Cytochrome c then
binds with apoptotic protease activating factor-1 and ATP to form
an oligomeric apoptosome. The apoptosome binds and cleaves
pro-caspase 9, releasing activated caspase 9, which continues to
activate caspase 3 and ultimately results in an apoptotic cell
(39). In the present study, the
levels of cleaved caspase 3 and Bax were significantly increased.
Therefore, it was deduced that crocetin exerts its proapoptotic
effects by increasing the levels of proapoptotic proteins.
Metastasis is one of the most common features of
malignancy, and is characterized by the ability of cancer cells to
invade into surrounding tissues, permeate blood or lymphatic
vessels and extravasate into a distant environment, which is
primarily responsible for the poor prognosis of esophageal cancer
(41). It has been reported that
>50% of patients with esophageal cancer possess an incurable
metastatic disease at the time of diagnosis (42). Therefore, it is imperative to
explore the underlying mechanism of metastasis and develop an
effective chemopreventive agent to halt the metastasis of
esophageal cancer. Numerous studies have revealed that carcinoma
cell metastasis demonstrates a close association with the loss of
cell-cell adhesion, downregulation of the extracellular matrix,
production of chemotactic factors and angiogenesis (43–45).
There is a growing body of evidence indicating that crocetin may
downregulate matrix metalloproteinases and intercellular adhesion
molecule-1, and inhibit angiogenesis (46,47).
The present study confirmed that crocetin treatment inhibited
KYSE-150 cell migration in a concentration-dependent manner.
Although the underlying mechanism of the crocetin-mediated
suppression of KYSE-150 cell migration has yet to be elucidated, it
can be speculated, based on these aforementioned studies, that
crocetin inhibits carcinoma cell migration via multiple pathways
rather than a single and specific pathway.
Although the exact mechanism for the anticancer
properties of crocetin requires additional investigation, the
present results confirmed that crocetin exhibits anticancer
properties through three pathways as follows: the inhibition of
cell proliferation by blocking the cell cycle progression between S
and G2 phase; the induction of apoptosis by increasing
the activity of the proapoptotic protein Bax and the activation of
caspase 3 levels; and the inhibition of carcinoma cell migration.
In summary, crocetin may be considered to be a promising
chemopreventive agent for the treatment of esophageal cancer.
Acknowledgements
This study was supported by the National Key
Development Program for Basic Research of China (grant no.,
2006cb500700), the Science and Technology Innovation Project of
Guangdong Education Department (grant no., 2012KJCX0089) and the
Guangdong Medical Research Foundation of Guangdong Province (grant
no., A2010222).
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cook MB, Chow WH and Devesa SS:
Oesophageal cancer incidence in the United States by race, sex, and
histologic type, 1977–2005. Br J Cancer. 101:855–859. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szumiło J: Epidemiology and risk factors
of the esophageal squamous cell carcinoma. Pol Merkur Lekarski.
26:82–85. 2009.(In Polish).
|
|
4
|
Rubenstein JH and Chen JW: Epidemiology of
gastroesophageal reflux disease. Gastroenterol Clin North Am.
43:1–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaur P, Kim MP and Dunkin BJ: Esophageal
cancer: Recent advances in screening, targeted therapy, and
management. J Carcinog. 13:112014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith TJ, Ryan LM, Douglass HO Jr, et al:
Combined chemoradiotherapy vs. radiotherapy alone for early stage
squamous cell carcinoma of the esophagus: a study of the Eastern
Cooperative Oncology Group. Int J Radiat Oncol Biol Phys.
42:269–276. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tepper J, Krasna MJ, Niedzwiecki D, et al:
Phase III trial of trimodality therapy with cisplatin,
fluorouracil, radiotherapy, and surgery compared with surgery alone
for esophageal cancer: CALGB 9781. J Clin Oncol. 26:1086–1092.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blazeby JM, Farndon JR, Donovan J and
Alderson D: A prospective longitudinal study examining the quality
of life of patients with eso-phageal carcinoma. Cancer.
88:1781–1787. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blazeby JM, Williams MH, Brookes ST, et
al: Quality of life measurement in patients with oesophageal
cancer. Gut. 37:505–508. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim T, Grobmyer SR, Smith R, et al:
Esophageal cancer - the five year survivors. J Surg Oncol.
103:179–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin Y, Peng N, Li J, et al: Herbal
compound triptolide synergistically enhanced antitumor activity of
amino-terminal fragment of urokinase. Mol Cancer. 12:542013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Lu A, Meng F, et al: Inhibitory
effects of lupeal acetate of Cortex periplocae on
N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis.
Oncol Lett. 4:231–236. 2012.PubMed/NCBI
|
|
13
|
Rasul A, Yu B, Khan M, et al: Magnolol, a
natural compound, induces apoptosis of SGC-7901 human gastric
adenocarcinoma cells via themitochondrial and PI3K/Akt signaling
pathways. Int J Oncol. 40:1153–1161. 2012.
|
|
14
|
Bolhassani A, Khavari A and Bathaie SZ:
Saffron and natural carotenoids: Biochemical activities and
anti-tumor effects. Biochim Biophys Acta. 1845:20–30. 2014.
|
|
15
|
Ordoudi SA, Befani CD, Nenadis N, et al:
Further examination of antiradical properties of Crocus sativus
stigmas extract rich in crocins. J Agric Food Chem. 57:3080–3086.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higashino S, Sasaki Y, Giddings JC, et al:
Crocetin, a carotenoid from Gardenia jasminoides Ellis, protects
against hypertension and cerebral thrombogenesis in stroke-prone
spontaneously hypertensive rats. Phytother Res. 28:1315–1319. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsantarliotou MP, Poutahidis T, Markala D,
et al: Crocetin administration ameliorates endotoxin-induced
disseminated intravascular coagulation in rabbits. Blood Coagul
Fibrinolysis. 24:305–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nam KN, Park YM, Jung HJ, et al:
Anti-inflammatory effects of crocin and crocetin in rat brain
microglial cells. Eur J Pharmacol. 648:110–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai J, Yi FF, Bian ZY, et al: Crocetin
protects against cardiac hypertrophy by blocking MEK-ERK1/2
signalling pathway. J Cell Mol Med. 13:909–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang R, Vernon K, Thomas A, et al:
Crocetin reduces activation of hepatic apoptotic pathways and
improves survival in experimental hemorrhagic shock. JPEN J
Parenter Enteral Nutr. 35:107–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan MB, Hoda MN, Ishrat T, et al:
Neuroprotective efficacy of Nardostachys jatamansi and crocetin in
conjunction with selenium in cognitive impairment. Neurol Sci.
33:1011–1020. 2012. View Article : Google Scholar
|
|
22
|
Bathaie SZ, Hoshyar R, Miri H and
Sadeghizadeh M: Anticancer effects of crocetin in both human
adenocarcinoma gastric cancer cells and rat model of gastric
cancer. Biochem Cell Biol. 91:397–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li CY, Huang WF, Wang QL, et al: Crocetin
induces cytotoxicity in colon cancer cells via p53-independent
mechanisms. Asian Pac J Cancer Prev. 13:3757–3761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chryssanthi DG, Dedes PG, Karamanos NK, et
al: Crocetin inhibits invasiveness of MDA-MB-231 breast cancer
cells via downregulation of matrix metalloproteinases. Planta Med.
77:146–151. 2011. View Article : Google Scholar
|
|
25
|
Magesh V, DurgaBhavani K, Senthilnathan P,
et al: In vivo protective effect of crocetin on
benzo(a)pyrene-induced lung cancer in Swiss albino mice. Phytother
Res. 23:533–539. 2009. View
Article : Google Scholar
|
|
26
|
Song EL, Hou YP, Yu SP, et al: EFEMP1
expression promotes angiogenesis and accelerates the growth of
cervical cancer in vivo. Gynecol Oncol. 121:174–180. 2011.
View Article : Google Scholar
|
|
27
|
Gutheil WG, Reed G, Ray A, et al:
Crocetin: an agent derived from saffron for prevention and therapy
for cancer. Curr Pharm Biotechnol. 13:173–179. 2012. View Article : Google Scholar
|
|
28
|
Dhar A, Mehta S, Dhar G, et al: Crocetin
inhibits pancreatic cancer cell proliferation and tumor progression
in a xenograft mouse model. Mol Cancer Ther. 8:315–323. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong YJ, Shi F, Zheng XL, et al: Crocetin
induces cytotoxicity and enhances vincristine-induced cancer cell
death via p53-dependent and -independent mechanisms. Acta Pharmacol
Sin. 32:1529–1536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nurse P: A long twentieth century of the
cell cycle and beyond. Cell. 100:71–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nurse P, Masui Y and Hartwell L:
Understanding the cell cycle. Nat Med. 4:1103–1106. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dehay C and Kennedy H: Cell-cycle control
and cortical development. Nat Rev Neurosci. 8:438–450. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chu G: Cellular responses to cisplatin.
The roles of DNA-binding proteins and DNA repair. J Biol Chem.
269:787–790. 1994.PubMed/NCBI
|
|
35
|
Warner TF: Apoptosis. Lancet. 2:12521972.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wyllie AH: The biology of cell death in
tumours. Anticancer Res. 5:131–136. 1985.PubMed/NCBI
|
|
37
|
Amin A, Hamza AA, Bajbouj K, et al:
Saffron: a potential candidate for a novel anticancer drug against
hepatocellular carcinoma. Hepatology. 54:857–867. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo X, Budihardjo I, Zou H, et al: Bid, a
Bcl2 interacting protein, mediates cytochrome c release from
mitochondria in response to activation of cell surface death
receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li P, Nijhawan D and Wang X: Mitochondrial
activation of apoptosis. Cell. 116(Suppl 2): S57–S59. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Javle M, Ailawadhi S, Yang GY, et al:
Palliation of malignant dysphagia in esophageal cancer: a
literature-based review. J Support Oncol. 4:365–373.
3792006.PubMed/NCBI
|
|
43
|
Martin TA: The role of tight junctions in
cancer metastasis. Semin Cell Dev Biol. 36C:224–231. 2014.
View Article : Google Scholar
|
|
44
|
Liotta LA, Rao CN and Barsky SH: Tumor
invasion and the extracellular matrix. Lab Invest. 49:636–649.
1983.PubMed/NCBI
|
|
45
|
Mahadevan V and Hart IR: Metastasis and
angiogenesis. Acta Oncol. 29:97–103. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xiang M, Qian ZY, Zhou CH, et al: Crocetin
inhibits leukocyte adherence to vascular endothelial cells induced
by AGEs. J Ethnopharmacol. 107:25–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Umigai N, Tanaka J, Tsuruma K, et al:
Crocetin, a carotenoid derivative, inhibits VEGF-induced
angiogenesis via suppression of p38 phosphorylation. Curr Neurovasc
Res. 9:102–109. 2012. View Article : Google Scholar : PubMed/NCBI
|