Introduction
Hepatoid adenocarcinoma of the stomach (HAS) is a
particular type of extrahepatic adenocarcinoma, presenting with
morphological characteristics identical to those of hepatocellular
carcinoma (HCC). A case of α-fetoprotein-producing gastric
adenocarcinoma was reported by Ishikura et al (1) in 1985. The authors suggested the name of
HAS due to the ability of the tumor cells to produce α-fetoprotein
(AFP), which is characteristic of hepatoid adenocarcinoma cells
(1). HAS is reported to occur with
more frequent lymph node and liver metastasis and exhibits a poorer
prognosis than common gastric cancer (CGC) (2). HAS is rare (with a worldwide incidence
of 0.3–1%) of all kinds of gastric cancer (2–4), the
sypmtoms are similar to those of normal gastric cancer (abdominal
discomfort, fullness of anorexia, epigastric pain, voimiting and
weight loss), surgery is the usual treatment option (4–7). The
literature review presented in this study was conducted to
contribute towards the improvement of the diagnosis and treatment
of HAS (3). We report a case of a
72-year-old female patient who sufferd upper abdominal discomfort.
Endoscopical and radiological examination revealed a neoplasm in
the stomach, however serum α-fetoprotein levels were normal.
Written informed consent was obtained from the patient's
family.
Case report
Case presentation
A 72-year-old Chinese female presented to Huashan
Hospital (Shanghai, China) complaining of upper abdominal
discomfort for the previous two months. The patient was admitted to
the General Surgery Department of Huashan Hospital on October 8th,
2011. The results of the physical examinations were unremarkable.
The past medical history revealed nothing significant; the patient
had not previously undergone any surgery and there was no family
history of cancer. The laboratory investigation revealed normal
blood levels following routine tests, and liver and kidney function
were normal. The white blood cell count was 6.8×109/l
(normal range, 4.5–11×109/l), the hematocrit level was
120 g/l (normal range, 110–150 g/l), the red blood cell count was
3.93×1012/l (normal range, 3.5–5.0×1012/l),
alanine aminotransferase levels were 11 U/l (normal range, 0–50
U/l), aspartate aminotransferase level was 18 U/l (normal range,
0–30 U/l), the bilirubin level was 12 µmol/l (normal range,
3.4–20.4 µmol/l), the serum creatinine level was 87 µmol/l (normal
range, 50–130 µmol/l) and the blood urea nitrogen level was 5.3
mmol/l (normal range, 2.5–7.0 mmol/l) In addition, the AFP level
was 4.93 µg/l (normal value, <10 µg/l), carcinoembryonic antigen
(CEA) level 0.69 µg/l (normal range, 0–10 µg/l), a CA125 level of
30.55 U/ml (normal range, 0–35 U/ml) and the CA724 levels of 2.5
U/ml (normal range, 0–8.2 U/ml), were all within the normal ranges.
Furthermore, the hepatitis B and C panels were negative. A
gastroduodenoscopy revealed a large gastric antrum ulcer and,
subsequently, irregular and hyperchromatic nuclei were observed in
the carcinoma cells, which was diagnosed as a poorly differentiated
adenocarcinoma. Computed tomography was performed and mural
thickening of the gastric antrum was observed, as well as a gastric
mass, measuring 10×5 cm in diameter. In addition, massive lymph
node swelling around the greater and lesser curvature of the
stomach, head of pancreas, portal and splenic vein was evident.
Treatment
The patient underwent a distal gastrectomy plus
resection of the small intestine with tumor debulking and
subsequent chemotherapy (150 mg oxaliplatin with 3.5 g fluorouracil
and 300 mg folinic acid, six cycles). For the first two years after
surgery, the patient was followed-up every three-four months, then
every six months thereafter. The patient was without relapse at the
time of writing.
Pathological analysis
The pathological analysis was conducted on the
resected specimen, which consisted of stomach tissue, measuring 22
cm in the greater gastric curvature and 15 cm in the lesser
curvature. The serosal surface showed an ill-defined, hemorrhagic,
necrotic mass, measuring 10.5×6×1.5 cm, which invaded into the
whole gastric wall. Sectioning of the mass revealed a gray-white,
homogeneous cut surface with scattered areas of hemorrhage and
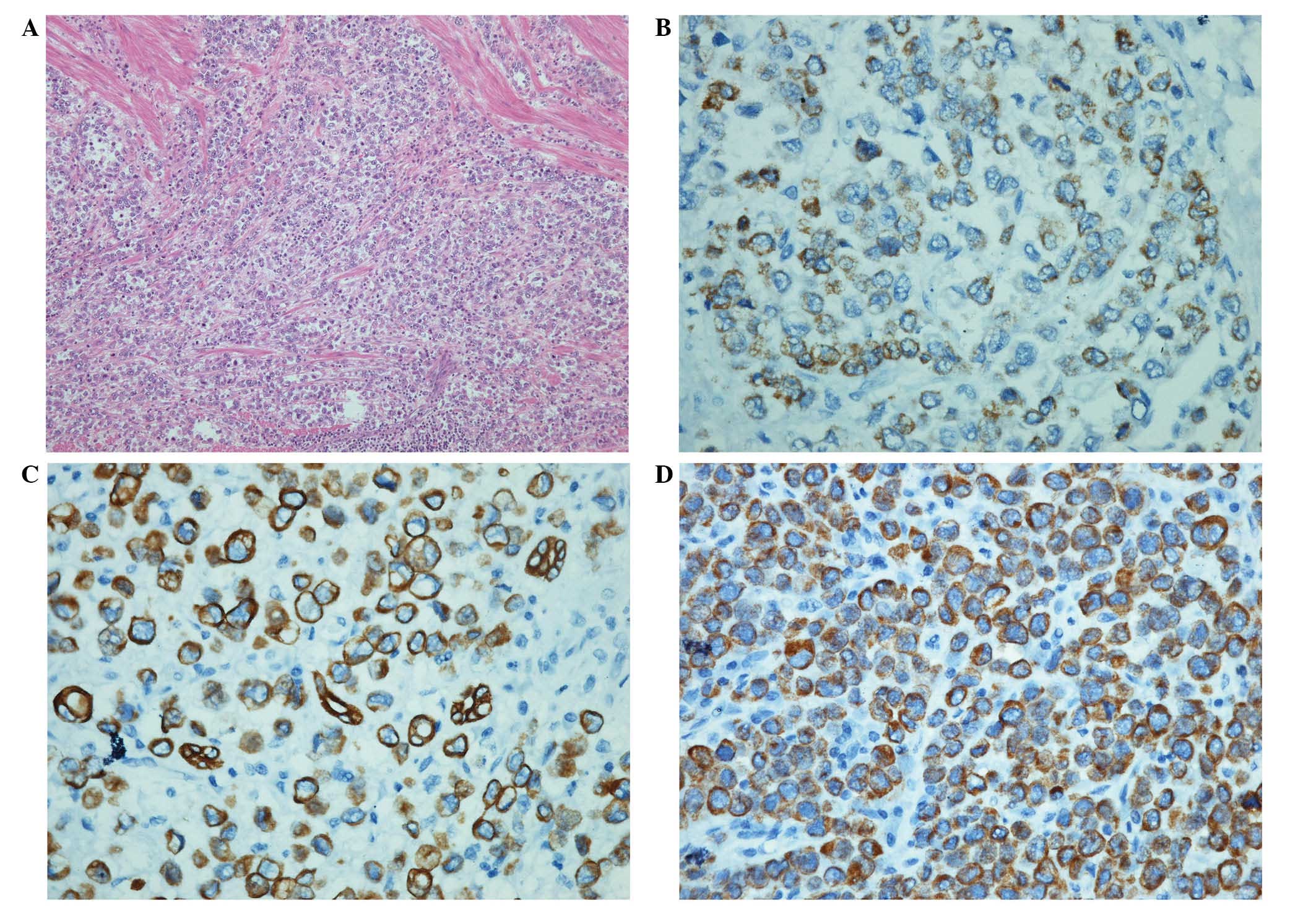
necrosis. Sections of the mass exhibited malignant cells arranged
in a solid to trabecular pattern (Fig.
1A). The cells were polygonal shaped with well-defined
cytoplasmic borders. The cytoplasm was clear to eosinophilic and a
number of the cells exhibited centrally located hyperchromatic
nuclei. Immunohistochemical analysis was performed to further
characterize the tumor. The tumor cells were immunohistochemically
positive for Hep1 (Fig. 1B), CK, CK8
(Fig. 1C), CK18 (Fig. 1D), P53 and Ki67 (50%+), and negative
for Vim, LCA, ChroA, Syn and CerbB-2.
Discussion
HAS originates from gastric mucosa, exhibiting
morphological features of CGC and hepatoid adenocarcinoma, and has
been classified as a rare subtype of gastric malignant tumors
(4). According to recent studies, the
incidence among gastric cancer cases was 1.5–15% (5,6). The mean
incidence age of HAS has been reported to be 63.5 years of age and
more male patients have been observed, with a male to female ratio
of 2.3:1 (7). The most common tumor
site was identified to be the gastric antrum (7). The diagnosis of HAS is dependent on the
pathological examination.
The formation of HAS may be regarded as consequence
of embryogenesis, as the stomach and liver are derived from the
foregut three to four months following fertilization. Gastric
adenocarcinoma cells may be misdirected during differentiation and
alternatively oriented towards hepatoid cells, the most
characteristic of which are AFP-producing (8). AFP produced by HAS can be distinguished
from AFP-producing gastric cancer (AFPPGC), another distinctive
type of gastric cancer, as it exhibits a higher canavalin binding
rate than that of AFPPGC (>90 vs. <50%) (9). In a study by Ooi et al, the
difference in canavalin binding was hypothesized to be due to the
various origin cells of AFP. In AFPPGC, AFP was secreted by gastric
adenocarcinoma cells, while it was secreted by hepatoid cells in
cases of HAS (10).
As a rare subtype of gastric malignant tumors, HAS
has been reported in individual cases from various therapeutic
centers (1,3,5,6,9). The
elevated serum AFP level is regarded as a significant feature of
HAS (11) and, among the previously
reported HAS patients, 84.8% were observed to have elevated AFP
levels ranging from 10–475000 ng/ml (12).
In a number of previously reported cases, the
pathological features of HAS were as follows: i) HAS cells were
large and polygonal with prominent nucleoli and abundant cytoplasm.
The polygonal tumor cells were eosinophilous under hematoxylin and
eosin staining, forming myeloid or cord structures, separated by
sinusoidal capillaries (3,14); ii) trabecular and intestinal-like
structures may be identified in HAS cells and were defined as two
pathological subtypes (7,12,14). In
these subtypes, hyaline particles may be observed, indicated by
positive periodic acid-Schiff staining (3,13); iii)
steatosis and biliation were identified in a number of HAS cells
(11,12); iv) ultra microstructure of the HAS
cells showed microvilli differentiation, originating from
gastrointestinal epithelial cells (3,8,13); and v) characteristic
immunohistochemical staining, as reported in the literature,
consisted of positive AFP expression in the majority of HAC
patients (91.6%) and positive CEA staining observed in 78.7% cases
(1,10,14).
Additionally, cells were commonly observed to be positive for
α1-antitrypsin and α1-antichymotrysin following immunohistochemical
analysis (13,14).
The clinical symptoms are not specific enough to
accurately determine the diagnosis of HAS, as similar symptoms
including nausea, loss of appetite and epigastric distress may be
present in a variety of malignant tumors of the digestive system.
Therefore, in gastric tumor cases with an elevated serum AFP level,
the possibility of HAS must be considered. Preoperative computed
tomography and β-ultrasound of the liver are routinely recommended
for the exclusion of HCC, hepatic metastasis, teratoma and other
diseases that may also produce AFP (4,5,7). Histologically, the hepatoid structure
identified in the gastric adenocarcinoma region may contribute
significantly to the diagnosis (3–5,7). Immunohistochemical analysis may exhibit
positive staining for epithelial membrane antigen and CEA, which
may aid in the exclusion of gastric metastasis of HCC (4,8,10,11). New
molecular markers, including palate, lung, and nasal epithelium
clone protein and GATA4, may allow the differentiation of gastric
hepatoid adenocarcinoma, HCC and CGC (15,16).
Previous studies have reported that HAS frequently
occurred with liver metastasis and exhibited a shorter interval
between gastrectomy and liver metastasis than that of CGC, leading
to a poorer prognosis (17,18). As reported by Liu et al, the
one-, three- and five-year survival rates of HAS were 30, 13 and
9%, respectively, compared with 95, 57 and 38%, respectively in the
CGC group (19). The poorer prognosis
indicated a more aggressive biological behavior of HAS compared
with that of CGC; the mechanism of this was proposed to be due to
the immunosuppressive and protease-inhibitory properties, which may
enhance the invasiveness of the tumor (20).
Due to the limited number of reported cases, there
is currently a lack of understanding surrounding HAS. Therefore,
further clinical studies and information are required for
clinicians and pathologists to improve the diagnosis and treatment
for this subtype of gastric cancer.
References
|
1
|
Ishikura H, Fukasawa Y, Ogasawara K, et
al: An AFP-producing gastric carcinoma with features of hepatic
differentiation: A case report. Cancer. 56:840–848. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inoue M, Sano T, Kuchiba A, et al:
Long-term results of gastrectomy for alpha-fetoprotein-producing
gastric cancer. Br J Surg. 97:1056–1061. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zuo W, Dai G, Liu L and Zuo H:
Pathological character and clinical analysis of the hepatoid
adenocarcinoma of stomach: A report of 11 cases. Chin Clin Ocol.
14:923–926. 2009.[(In Chinese)].
|
|
4
|
Chang YC, Nagasue N, Abe S, et al:
Comparison between the clinicopathologic features of AFP-positive
and AFP-negative gastric cancers. Am J Gastroenterol. 87:321–325.
1992.PubMed/NCBI
|
|
5
|
Baek SK, Han SW, Oh DY, et al:
Clinicopathologic characteristics and treatment outcomes of
hepatoid adenocarcinoma of the stomach, a rare but unique subtype
of gastric cancer. BMC Gastroenterol. 11:562011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye M, Tao F, Liu F and Sun AJ: Hepatoid
adenocarcinoma of the stomach: a report of three cases. World J
Gastroenterol. 19:4437–4442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su JS, Chen YT, Wang RC, et al:
Clinicopathological characteristics in the differential diagnosis
of hepatoid adenocarcinoma: a literature review. World J
Gastroenterol. 19:321–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gitlin D, Perricelli A and Gitlin GM:
Synthesis of -fetoprotein by liver, yolk sac, and gastrointestinal
tract of the human conceptus. Cancer Res. 32:979–982.
1972.PubMed/NCBI
|
|
9
|
Bakir T, Aliyazicioglu Y, Bektas A, et al:
Hepatoid adenocarcinoma of the stomach: report of five cases and
review of literature. Acta Gastroenterol Belg. 69:330–337.
2006.PubMed/NCBI
|
|
10
|
Ooi A, Nakanishi I, Sakamoto N, et al:
Alpha-fetoprotein (AFP)-producing gastric carcinoma. Is it hepatoid
differentiation? Cancer. 65:1741–1747. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kinjo T, Taniguchi H, Kushima R, et al:
Histologic and immunohistochemical analyses of
α-fetoprotein-producing cancer of the stomach. Am J Surg Pathol.
36:56–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishikura H, Kirimoto K, Shamoto M, et al:
Hepatoid adenocarcinomas of the stomach. An analysis of seven
cases. Cancer. 58:119–126. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Plaza JA, Vitellas K and Frankel WL:
Hepatoid adenocarcinoma of the stomach. Ann Diagn Pathol.
8:137–141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sentani K, Oue N, Sakamoto N, et al: Gene
expression profiling with microarray and SAGE identifies PLUNC as a
marker for hepatoid adenocarcinoma of the stomach. Mod Pathol.
21:464–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamura N and Kishimoto T: Epigenetic
regulation of GATA4 expression by histone modification in
AFP-producing gastric adenocarcinoma. Exp Mol Pathol. 93:35–39.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagai E, Ueyama T, Yao T, et al: Hepatoid
adenocarcinoma of the stomach: A clinicopathologic and
immunohistochemical analysis. Cancer. 72:1827–1835. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu X, Cheng Y, Sheng W, et al: Analysis
of clinicopathologic features and prognostic factors in hepatoid
adenocarcinoma of the stomach. Am J Surg Pathol. 34:1465–1471.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Sheng W and Wang Y: An analysis of
clinicopathological features and prognosis by comparing hepatoid
adenocarcinoma of the stomach with AFP-producing gastric cancer. J
Surg Oncol. 106:299–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kono K, Amemiya H, Sekikawa T, et al:
Clinicopathologic features of gastric cancers producing
alpha-fetoprotein. Dig Surg. 19:359–365. 2002. View Article : Google Scholar : PubMed/NCBI
|