Introduction
Breast cancer is a type of cancer that accounts for
>1.2 million new cases worldwide and 500,000 mortalities
annually, resulting in breast cancer being the most malignant form
of cancer among females (1). Numerous
clinically-used drugs are available for the treatment of cancer,
including breast cancer, but the use of these agents does not
provide optimum effectiveness for the treatment of the disease. The
majority of the drugs result in serious side-effects, which
generates excessive damage to normal cells (2). Therefore, investigating anti-cancer
drugs of plant origin continues to provide novel and significant
possibilities for anti-cancer agents. Numerous types of bioactive
compounds from medicinal plants have been isolated at present and
several of these compounds are currently undergoing further
investigation (3–5).
Plants consumed by primates are considered to be a
promising source of therapeutic agents for the management of human
diseases, including cancer (6).
Previous investigations have been performed on primate-consumed
plants to assess their anti-tumor activity (6). Additional investigations led to the
isolation of kaempferol-3-O-rhamnoside from the leaves of
Schima wallichii Korth, a plant commonly consumed by
primates. Kaempferol-3-O-rhamnoside exhibited inhibitory
activity against MCF-7 breast cancer cell proliferation through the
activation of the caspase cascade pathway (7). In another study, 42 species of
primate-consumed plants that grow in Indonesia were evaluated for
their antiproliferative activity against MCF-7 human breast cell
lines using a MTT bioassay. The results revealed that certain plant
extracts demonstrated strong inhibitory activity against MCF-7 cell
proliferation, and one of these was the extract from the leaves of
Eugenia aquea (E. aquea) (8). The present study aimed to identify the
active compound derived from the leaves of E. aquea,
responsible for the antiproliferative activity against MCF-7 cell
lines, and to examine the pro-apoptotic activity of this
compound.
Materials and methods
Plant materials
The leaves of E. aquea were collected from
Pangandaran Beach Conservation Area (Pangandaran, West Java,
Indonesia). The plant species was then confirmed by the Department
of Biology of Padjadjaran University (Bandung, West Java,
Indonesia). The leaves were dried in the open air, away from direct
sunlight.
Cell culture and treatment
MCF-7 human breast cancer cell lines were purchased
from the American Type Culture Collection (Manassas, VA, USA). The
cell lines were cultured in RPMI-1640 medium (Sigma-Aldrich, St.
Louis, MO, USA) supplemented with 10% fetal bovine serum and
antibiotics, which consisted of 100 units/ml penicillin and 100
µg/ml streptomycin. For the cell treatments, various concentrations
of the sample were added to the cell culture medium. After 24 h,
the cells were released from treatment, the medium was replaced and
the cells were subsequently collected at 24 and 48 h.
Drug sensitivity assays
Cell proliferation analysis was performed using a
MTT assay on cells in the presence of various concentrations of
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone isolated from
Eugenia aquea at various concentrations ranged from 3 – 1342
µM, according to the method described by Abdulah et al
(9). Briefly, 2×104 cells
per 50 µl/well were plated into 96-well plates. Subsequent to the
initial cell seeding, various concentrations of primate-consumed
plant extracts were added and the cells were incubated for 24 h. In
total, 10 µl WST-8 assay cell-counting solution (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was added to each well and
incubated at 37°C for 3 h. Following the addition of 100 µl/well of
1 M HCl, the cell proliferation rate was determined by measuring
absorbance at a wavelength of 450 nm. The absorbance was read using
a microtiter plate reader (BD Biosciences, Franklin Lakes, NJ,
USA).
Extraction and isolation
A total of 736 g of dried leaves of E. aquea
were powdered and extracted using 95% ethanol (3×24 h) at room
temperature and the solvent was evaporated under reduced pressure
at 50°C to yield concentrated extracts. The concentrated extracts
were partitioned using a mixture of n-hexane-water (3:1) to
yield hexane and water layers. The water layer was further
extracted using ethyl acetate to yield ethyl acetate and water
fractions. The n-hexane and ethyl acetate fractions
demonstrated inhibitory activity against the proliferation of MCF-7
cells. The n-hexane fraction was subsequently subjected to
column chromatography over silica gel and eluted with
n-hexane-ethyl acetate mixtures of increasing polarity
(n-hexane to ethyl acetate, 9:1, 8:2, 7:3, 6:4 and 5:5) to
yield seven fractions. Fraction four was repeatedly further
purified via silica gel column chromatography, with the elution of
n-hexane-ethyl acetate mixtures of increasing polarity,
resulting in the isolation of 150.30 mg of a pure active compound,
in the form of yellow crystals. The compound was identified using
an analysis of its spectroscopic data, consisting of ultraviolet
(UV), infrared (IR), mass and nuclear magnetic resonance (NMR)
spectra.
Cell extraction and western blot
analysis
Protein concentrations were determined using a
bicinchoninic acid protein assay kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). In total, 40 µg protein was electrophoresed on
a Mini-PROTEAN TGX Precast Gel (4–20%; Bio-Rad Laboratories,
Hercules, CA, USA) and electro-transferred to a 7×8 cm Hybond
enhanced chemiluminescence membrane (GE Healthcare Life Sciences,
Little Chalfont, UK). Apoptosis-associated proteins were analyzed
by immunoblot analysis using poly(adenosine diphosphate-ribose)
polymerase (PARP) and Akt antibodies at a 1:1,000 dilution (Cell
Signaling Technology, Danvers, MA, USA). β-actin (Sigma-Aldrich)
served as the loading control.
Results
Determination of the structure of
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone
The active compound
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone was isolated from
the leaves of E. aquea as an active compound that
demonstrated inhibitory activity against the proliferation of MCF-7
cells. The compound exhibited strong yellow color, with a melting
point of 125–126°C and a molecular ion peak at m/z
298 in the electron ionization mass spectrum. The molecular ion
peak and 1H and 13C NMR data indicated that
this compound possessed a molecular formula of
C18H18O4.
The UV spectrum exhibited a major absorption band at
λmax 338 nm, indicating MeOH, and a minor band at
λmax 225 nm, which are characteristic for a type of
flavonoids with a structural skeleton of chalcone (10). This hypothesis was supported by the
color reaction, which revealed the color of this compound to be
red-orange in the presence of NaOH. The IR spectrum indicated the
presence of hydroxyl (3304 cm−), conjugated carbonyl
(1628 cm−), and aromatic (1606-1545 cm−)
groups in the molecule, which also supported this hypothesis.
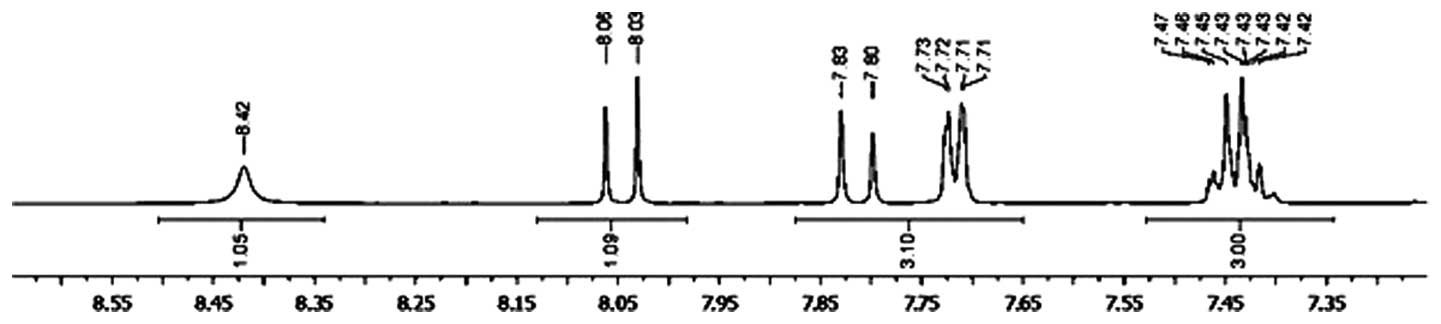
The 1H NMR spectrum of
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone revealed signals at
δ 7.82 (1H; d; J=18.0 Hz) and δ 8.03 (1H; d;
J=18.0 Hz), which is characteristic for the α and β protons
of the O=C-C=C-benzene group of a chalcone skeleton (Fig. 1) (10).
Furthermore, proton signals appeared at δ 7.43 (3H; m) and δ
7.71 (2H; dd; J=6.0 and 12.0 Hz), which were assumed
to result from aromatic hydrogens of the 1-substituted benzene
ring, and at δ 13.82 (1H; s), which was derived from a
hydrogen of the hydroxyl group in the benzene ring chelated to a
carbonyl group. The 1H NMR spectrum demonstrating
signals at the downfield region is shown in Fig. 1, and indicated that the compound may
have possessed a 1,2,3,4,5,6-substituted benzene ring A and a
1-substituted benzene ring B, with each of the rings being termed
the A- and B-rings on the left and right of the chalcone skeleton,
respectively. In addition, the 1H NMR spectrum exhibited
two singlet signals at δ 2.10 (3H; s) and 2.15 (3H;
s), revealing two hydrogens belonging to methyl groups that
are attached to a benzene ring, and one methoxyl signal at δ 3.67
(3H; s). These data indicated that the A-ring of this
chalcone may be substituted with two methyl, one methoxyl and two
hydroxyl groups.
The 13C NMR spectrum identified the
presence of 18 carbons, three of which were oxyaryl carbons
revealed by the signals at δ 159.77, 161.76 and 163.20. These
carbon signals indicated the presence of oxygenation in the
chalcone skeleton. The existence of a conjugated carbonyl group was
indicated by the appearance of the signal at δ 193.70. The
13C NMR data supported the assumption of the
substitution pattern of this chalcone compound.
The position of the oxygen groups was determined by
analysis of the 1H multiplicity bond connectivity (HMBC)
data. As indicated in the 1H NMR spectrum, the chalcone
skeleton of 2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone may
possess a hydroxyl group chelated to a carbonyl group, indicating
that the hydroxyl group was attached to C-6′ of the A-ring. In the
HMBC spectrum, a correlation was observed between the proton signal
of the hydroxyl group at δ 13.82 and carbon signals at δ 107.93
(C-3′) and 109.04 (C-1′). The proton signal of one methyl group at
δ 2.10 correlated with the carbon signals at δ 163.70 (C-6′) and
161.76 (C-4′), whereas the signal of another methyl group at δ 2.15
correlated with the carbon signals at δ 161.76 (C-4′) and 159.77
(C-2′). These indicated that two methyl groups were attached to
C-3′ and C-5′, and two oxygen functions were present at C-2′ and
C-4′. Furthermore, the proton signal of methoxyl group at δ 3.67
correlated with the carbon signal at δ 159.77 (C-2′), indicating
that this compound possessed a methoxyl group at C-2′ and a
hydroxyl group at C-4′.
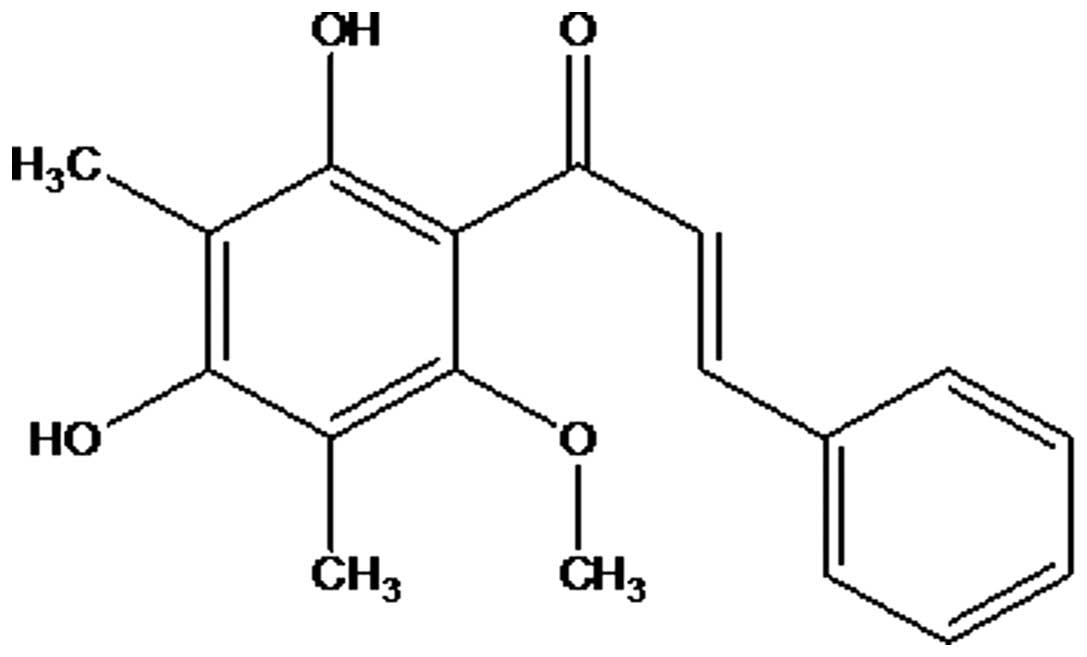
Thus, from the aforementioned accumulated data, the
compound was identified as
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone (Fig. 2), which was confirmed by comparison of
its spectral data with the reported data (11).
Inhibitory activity of
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone against the
proliferation of MCF-7 cells
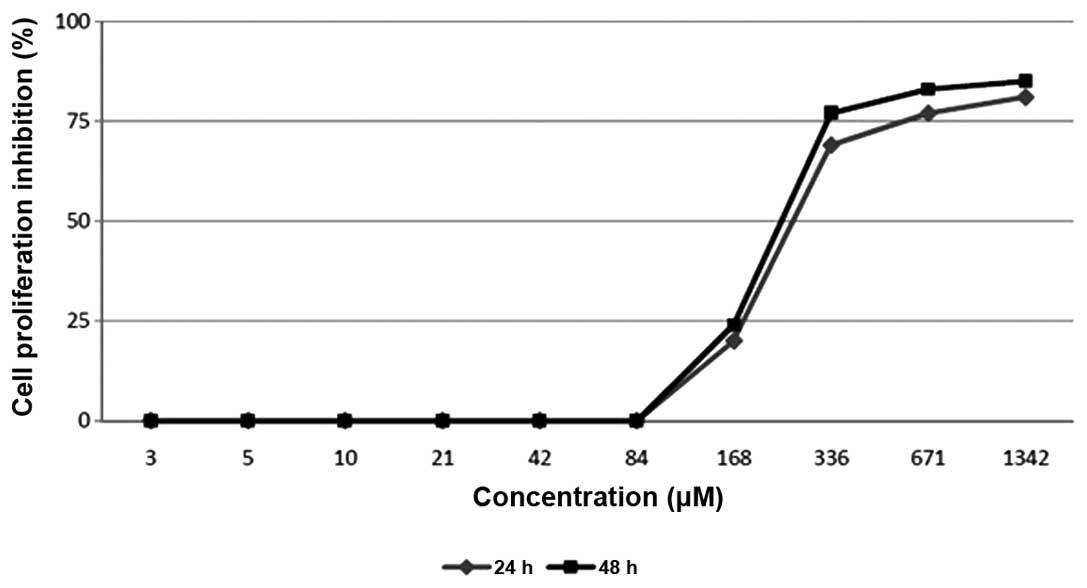
The present study evaluated
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone for its effect on
the proliferation of MCF-7 breast cancer cell lines using an MTT
assay. The evaluation resulted in a dose-dependent manner
inhibition of the compound on the cell proliferation (Fig. 3). The compound was revealed to
strongly inhibit the proliferation of the MCF-7 cell line in the
examinations at 24 and 48 h, demonstrating IC50 values
of 270 µM and 250 µM, respectively.
Proapoptotic activity of
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone mediated through
PARP protein activation
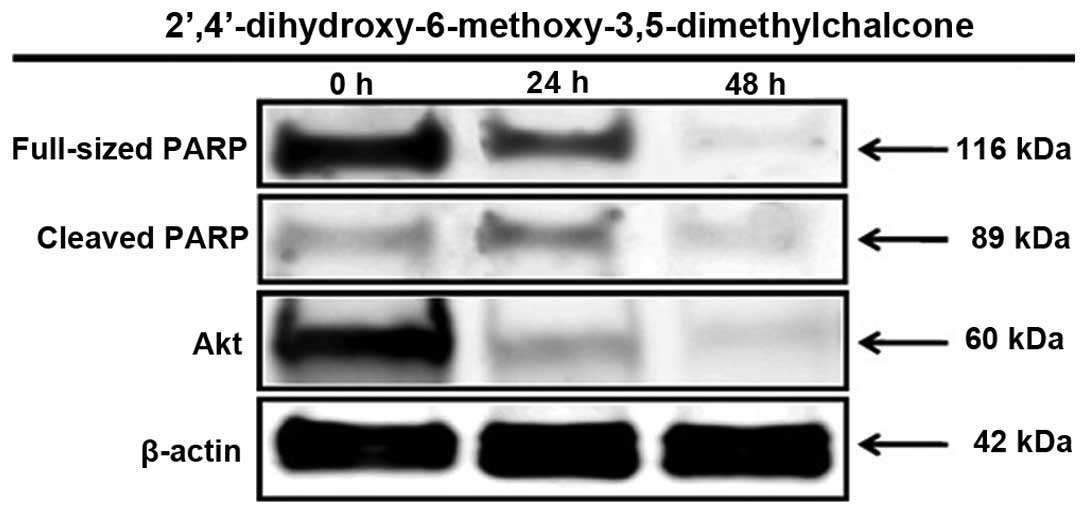
The MTT assay revealed the strong inhibitory
activity of 2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone against
the proliferation of MCF-7 cells. Therefore, the compound was
further examined for its proapoptotic activity through PARP protein
activation within 24 and 48 h of treatment. As can be observed in
Fig. 5, the present study provides evidence that the inhibition of
the proliferation of MCF-7 human breast cancer cells caused by
2′,4′-dihydroxy-6-methoxy-3′,5′-dimethylchalone was mediated by the
induction of apoptosis, marked by PARP protein activation, which is
one of the best biomarkers of apoptosis. Furthermore,
2′,4′-dihydroxy-6-methoxy-3′,5′-dimethylchalone also inhibited the
expression of Akt, the protein that signals cell survival (Fig. 4).
Discussion
Searching for anticancer agents on the basis of
following up plants used by primates is an innovative approach that
possesses high potential for developing novel anticancer drugs or
lead compounds from primate-consumed plants. In a previous study,
it was reported that the extracts of plants ingested by primates
demonstrated strong cytotoxicity in the MCF-7 breast cancer cell
line, and the extract from E. aquea leaves demonstrated
potential as an agent requiring further investigation (8).
The leaves of E. aquea, also known as watery
rose apple or ‘jambu air’ in Indonesia, are a food that is usually
consumed by non-human primates in the Pangandaran Beach Primate
Conservation Area of West Java, Indonesia (8).
The present study aimed to identify a cytotoxic
compound within the leaves of E. aquea, which led to the
isolation of 2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone. This
compound demonstrated a dose-dependent inhibition of the growth of
MCF-7 cells. This evidence was in agreement with the findings of
previous studies (12,13). In addition, the same compound,
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone, isolated from the
buds of Cleistocalyx operculatus significantly inhibits the
growth of human liver cancer SMMC-7721 cells and is able to induce
apoptosis of SMMC-7721 cells in vitro (12). This compound also exerts antitumor
effects in vivo on a solid human tumor xenograft mouse
model, using human liver cancer SMMC-7721 cells (13). Potential hepatoprotective effects,
which may be associated with the attenuation of oxidative stress,
accelerating the antioxidant cascade and inhibition of lipid
peroxidation, have also been demonstrated by
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone (14).
In the present study,
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone was also found to
induce the apoptosis of MCF-7 cells, as indicated by the changes in
the expression levels of PARP, which were analyzed within 24 and 48
h of treatment. The N-terminal fragment of PARP, a 89-kDa peptide
that is cleaved from the 116 kDa full-length PARP protein, was
detected in MCF-7 cells as early as 24 h subsequent to treatment
with 2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone. In addition,
as one of the most important survival signaling pathways in
malignancy, Akt plays a significant role in determining the
chemosensitivity of cancer cells. The survival signaling proteins
were decreased in the MCF-7 cells by treatment with
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone, as demonstrated by
the reduced expression of the Akt protein.
In conclusion, the present results indicate that
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone inhibited the growth
of MCF-7 cells through the induction of apoptosis and
downregulation of the Akt pathway. Additional studies are required
to evaluate the toxicity and determine detailed mechanisms of the
antiproliferative action of
2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone to provide a
scientific basis for the chemopreventive and chemotherapeutic
application of 2′,4′-dihydroxy-6-methoxy-3,5-dimethylchalcone for
the management of breast cancer.
Acknowledgements
This study was supported by The Directorate General
of Higher Education of The Ministry of Education and Culture of
Indonesia (Grand-in-Aid for The International Research
Collaborations and Publications, grant no.
430/SP2H/PP/DP2M/VII/2010).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakarkar DM and Deshmukh VN:
Ethnopharmacological review of traditional medicinal plants for
anticancer activity. Int J Pharm Tech Res. 3:298–308. 2011.
|
|
3
|
Kinghorn AD, Farnsworth NR, Soejarto DD,
et al: Novel strategies for the discovery of plant-derived
anticancer agents. Pure Appl Chem. 71:1611–1618. 1999. View Article : Google Scholar
|
|
4
|
Kinghorn AD: The role of pharmacognosy in
modern medicine. Expert Opin Pharmacother. 3:77–79. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kinghorn AD, Farnsworth NR, Soejarto DD,
et al: Novel strategies for the discovery of plant-derived
anticancer agents. Pharm Biol. 41 (Suppl 1):S53–S67. 2003.
View Article : Google Scholar
|
|
6
|
Koshimizu K, Murakami A, Hayashi H,
Ohigashi H, Subarnas A, Gurmaya KJ and Ali A: Biological activities
of edible and medicinal plants from Indonesia and Malaysia.
Proceedings of The Tokyo International Forum on Conservation and
Sustainable Use of Tropical Bioresources. Tokyo. pp. 203–208.
1998;
|
|
7
|
Diantini A, Subarnas A, Lestari K, Halimah
E, et al: Kaempferol-3-O-rhamnoside isolated from the leaves of
Schima wallichii Korth. inhibits MCF-7 breast cancer cell
proliferation through activation of the caspase cascade pathway.
Oncol Lett. 3:1069–1072. 2012.PubMed/NCBI
|
|
8
|
Subarnas A, Diantini A, Abdulah R,
Zuhrotun A, Yamazaki C, Nakazawa M and Koyama H: Antiproliferative
activity of primates-consumed plants against MCF-7 human breast
cancer cell lines. E3 J Med Res. 1:38–43. 2012.
|
|
9
|
Abdulah R, Faried A, Kobayashi K, Yamazaki
C, Suradji EW, Ito K, Suzuki K, Murakami M, Kuwano H and Koyama H:
Selenium enrichment of broccoli sprout extract increases
chemosensitivity and apoptosis of LNCaP prostate cancer cells. BMC
Cancer. 9:4142009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marby TJ, Markham KR and Thomas MB: The
Determination and Interpretation of NMR Spectra of FlavonoidsThe
systematic identification of flavonoids. Springer-Verlag; New York:
pp. 253–273. 1970
|
|
11
|
Amor EC, Villaseñor IM, Yasin A and
Choudhary MI: Prolyl endopeptidase inhibitors from Syzygium
samarangense (Blume) Merr. & L. M. Perry. Z Naturforsch C.
59:86–92. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye CL, Liu JW, Wei DZ, Lu YH and Qian F:
In vitro anti-tumor activity of
2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone against six
established human cancer cell lines. Pharmacol Res. 50:505–510.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye CL, Liu JW, Wei DZ, Lu YH and Qian F:
In vivo antitumor activity by
2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone in a solid human
carcinoma xenograft model. Cancer Chemother Pharmacol. 56:70–74.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu WG, Qian J and Lu YH: Hepatoprotective
effects of 2′,4′-dihy-droxy-6′-methoxy-3′,5′-dimethylchalcone on
CC14-induced acute liver injury in mice. J Agric Food Chem.
59:12821–12829. 2011. View Article : Google Scholar : PubMed/NCBI
|