Introduction
Esophageal cancer (EC) is the sixth leading cause of
cancer-associated death worldwide, and the incidence and mortality
associated with esophageal squamous cell carcinoma (ESCC) is
highest in China in comparison with other countries (1). Current treatment options for cancer are
based on surgery, chemotherapy and radiation therapy. However, the
development of drug resistance and the severe side effects of
chemotherapy remain unresolved problems in clinical oncology and
reduce successful therapeutic outcomes of chemotherapy (2). Therefore, the identification of improved
novel anticancer compounds is required. Artesunate (ART) is a
semi-synthetic derivative of artemisinin extracted from the Chinese
herb Artemisia annua and is a safe and effective
anti-malarial drug (2). In addition
to anti-malarial activity, previously published studies indicate
that artemisinin and its derivatives are active against cells from
a broad spectrum of types of cancer (3–8). Cell
proliferation of >70 cell lines from different tumor types are
inhibited by ART and its associated compound artemisinin (9,10).
However, the effects of ART on the growth, cell cycle, apoptosis,
migration and invasion in ESCC have not yet been reported.
The structural information and biomechanical
properties of cell surface membranes are important indicators for
determining structural changes (11).
The cell membrane acts as the exchange interface between the inside
and outside of the cell (12).
Changes in cell membrane structure can therefore directly influence
the behaviors of cells, in addition to elucidating disease or
differentiation processes (13,14).
However, the effects of ART on the biomechanical properties of cell
surface membranes in ESCC remains unclear. Atomic force microscopy
(AFM) is a powerful tool for obtaining high-resolution
ultrastructural data from biological samples (15), exploring the shape of a single cell
and the properties of the cellular membrane (16,17). In
particular, chemical functionalization of the AFM tip with various
ligands has enabled the mapping of complementary receptors on model
or cellular surfaces (18). AFM is
now frequently used to detect cancer cell membranes in the context
of anticancer drugs (19–21).
In the present study, the effects of ART on cell
proliferation, cell cycle, apoptosis, cell migration, invasion and
cell structure were evaluated. In addition, the cell surface
membranes and biomechanical properties of the KYSE-150 ESCC cell
line were detected using AFM-based single-molecule force
spectroscopy in vitro.
Materials and methods
Cell culture conditions
The SHEE (human esophageal epithelial cell line) and
KYSE-150 (ESCC) cell lines were obtained from the Key Laboratory of
Cellular Physiology, Ministry of Education, Shanxi Medical
University (Taiyuan, China). They were cultured in Dulbecco's
modified Eagle's medium (DMEM)/F12 (GE Healthcare Life Sciences,
Logan, UT, USA) with 10% fetal bovine serum (FBS; GE Healthcare
Life Sciences) and incubated in a humidified atmosphere at 37°C
with 5% CO2. The cells were subcultured every 2–3 days.
Exponentially growing cells were used throughout the study.
Drugs and reagents
ART was purchased from Guilin Pharmaceutical
(Shanghai) Co., Ltd. (Guilin, China). MTT
[3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide]
and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Matrigel Basement Membrane Matrix was
purchased from BD Biosciences (Franklin Lakes, NJ, USA). An annexin
V/propidium iodide (PI) double-staining kit was purchased from
Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Cell culture
flasks, plates, centrifuge tubes and Transwell plates were
purchased from Corning, Inc. (New York, NY, USA).
MTT assay
The MTT assay was used to assess KYSE-150 cell
proliferation following treatment with various concentrations of
ART. Briefly, KYSE-150 cells were seeded into a 96-well plate at a
density of 5×103 cells/well with culture medium, as
described above. Different concentrations of ART (0, 10, 30 or 50
mg/l) were then added to the KYSE-150 cells to yield a final volume
of 200 µl/well. KYSE-150 cell proliferation was measured at 24, 36
and 48 h. Following incubation, 20 µl MTT (5 mg/ml) was added to
each well and the cells were incubated at 37°C for 4 h. The medium
was then removed from each well and replaced with 200 µl DMSO. The
optical density was determined at 490 nm with a microplate reader
(M491 EON1/S-130306F; BioTek Instruments, Inc., Winooski, VT, USA).
Each assay was repeated at least three times.
Cell cycle and apoptosis assay by flow
cytometry
Based on the results of the MTT assay, the most
effective concentration of ART (30 mg/l) was used to assess the
cell cycle distributions and apoptosis rates of KYSE-150 cells
treated with ART, compared with the control group (0 mg/l).
Briefly, following treatment with ART for 48 h, KYSE-150 cells were
collected at a density of 1×106 cells/ml and washed
twice with phosphate-buffered saline (PBS). For the cell cycle
assay, the cells were washed twice with 500 µl PBS and then fixed
in ice-cold 70% ethanol overnight at 4°C. The cells were
concentrated by removing the ethanol and treated with 0.01%
DNase-free RNase A (Nanjing KeyGen Biotech Co., Ltd.) for 10 min at
37°C. Cellular DNA was stained with 0.05% propidium iodide (PI;
Nanjing KeyGen Biotech Co., Ltd.) for 20 min at 4°C in the dark.
For the apoptosis assay, the cells were suspended in 500 µl binding
buffer (Nanjing KeyGen Biotech Co., Ltd.). Subsequently, 5 µl
annexin V and 5 µl PI was added to each sample for 15 min in the
dark. The cells were analyzed using a FACSCalibur flow cytometer
(BD Biosciences) with CellQuest software (version 5.1; BD
Biosciences). Each sample was run in triplicate.
Cell migration and invasion
assays
KYSE-150 cells were cultured to 85% confluence in
25-cm2 culture flasks and treated with or without 30
mg/l ART for 48 h. For the migration assays, 1×105 cells
in DMEM/F12 were seeded onto an uncoated insert chamber (8 µm;
Corning, Inc.)w. The lower chamber contained culture media with 10%
FBS. Following 24-h culture, invasive cells were stained using 0.1%
crystal violet (Beyotime Institute of Biotechnology, Shanghai,
China) and counted under a microscope (IX71-A12FL/PH; Olympus
Corporation, Tokyo, Japan). Experiments were repeated three times.
For the invasion assays, experiments were performed with the
Matrigel-coated Transwell migration chambers; the remainder of the
procedure was performed using the same methods as the migration
assays.
Atomic force microscopy (AFM)
analysis
The 3D morphology and biomechanical properties of
KYSE-150 cells treated with ART (0 or 30 mg/l) and SHEE cells were
detected by AFM. AFM measurements were performed in aqueous
solution at room temperature, using a 5500 atomic force microscope
(Agilent Technologies, Inc., Santa Clara, CA, USA). AFM images were
acquired in tapping mode and in contact mode using Si3N4 tips
(NSC19, 0.68 N/m normal spring constant; Schaefer Technologie GmbH,
Langen, Germany) and gold-coated tips (CSC 38; Schaefer Technologie
GmbH), respectively. The spring constants of the cantilevers used
for AFM force spectroscopy were ~0.1 N/m. Adhesion and elasticity
maps were obtained by recording 16×16 force-distance curves on
areas of a given size (2×2 µm), calculating the adhesion force and
elasticity modulus for each force curve and displaying these values
as gray and colorized scale pixels, respectively. These maps
qualitatively and quantitatively demonstrated the viscoelasticity
of individual cells at the nanoscale level.
Statistical analysis
For statistical analysis, the data were subjected to
an arcsine transformation. The transformed data were then analyzed
by one way analysis of variance, General Linear Model, or Student's
test using SPSS software, version 11.0 (SPSS, Inc., Chicago, IL,
USA) (22). P<0.05 was considered
to indicate a statistically significant difference.
Results
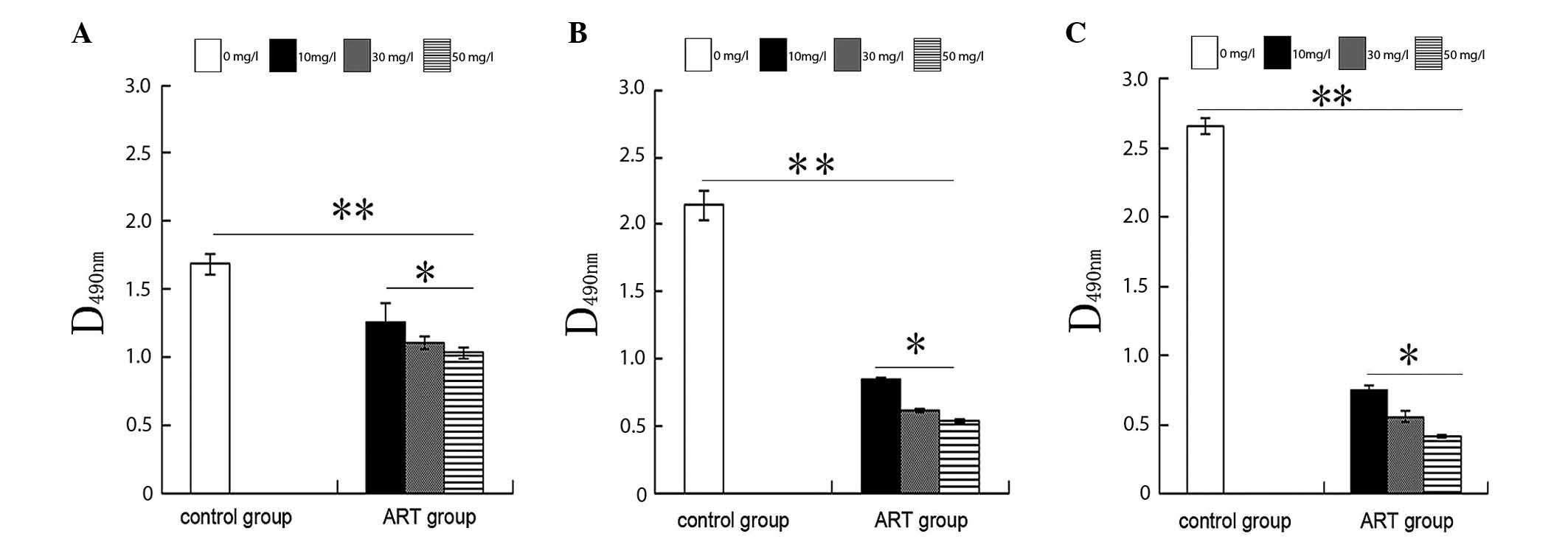
Effects of ART on growth, cell cycle
distribution and levels of apoptosis in ESCC cells
MTT assay and flow cytometry were used to assess
KYSE-150 cell proliferation, cell cycle and apoptosis following
treatment with different concentrations of ART. The proliferation
of the control KYSE-150 cells not treated with ART (0 mg/l ART) was
significantly higher than that of KYSE-150 cells incubated with
other concentrations of ART (10, 30 and 50 mg/l) at all time points
(Fig. 1). Specifically, the
proliferation levels of the 10 mg/l ART-treated group were
significantly higher than those of the 30 and 50 mg/l ART groups at
all time points. There was no significant difference in cell
proliferation between the 30 and 50 mg/l ART groups at all time
points (Fig. 1). ART markedly
inhibited the growth of KYSE-150 cells in a dose- and
time-dependent manner.
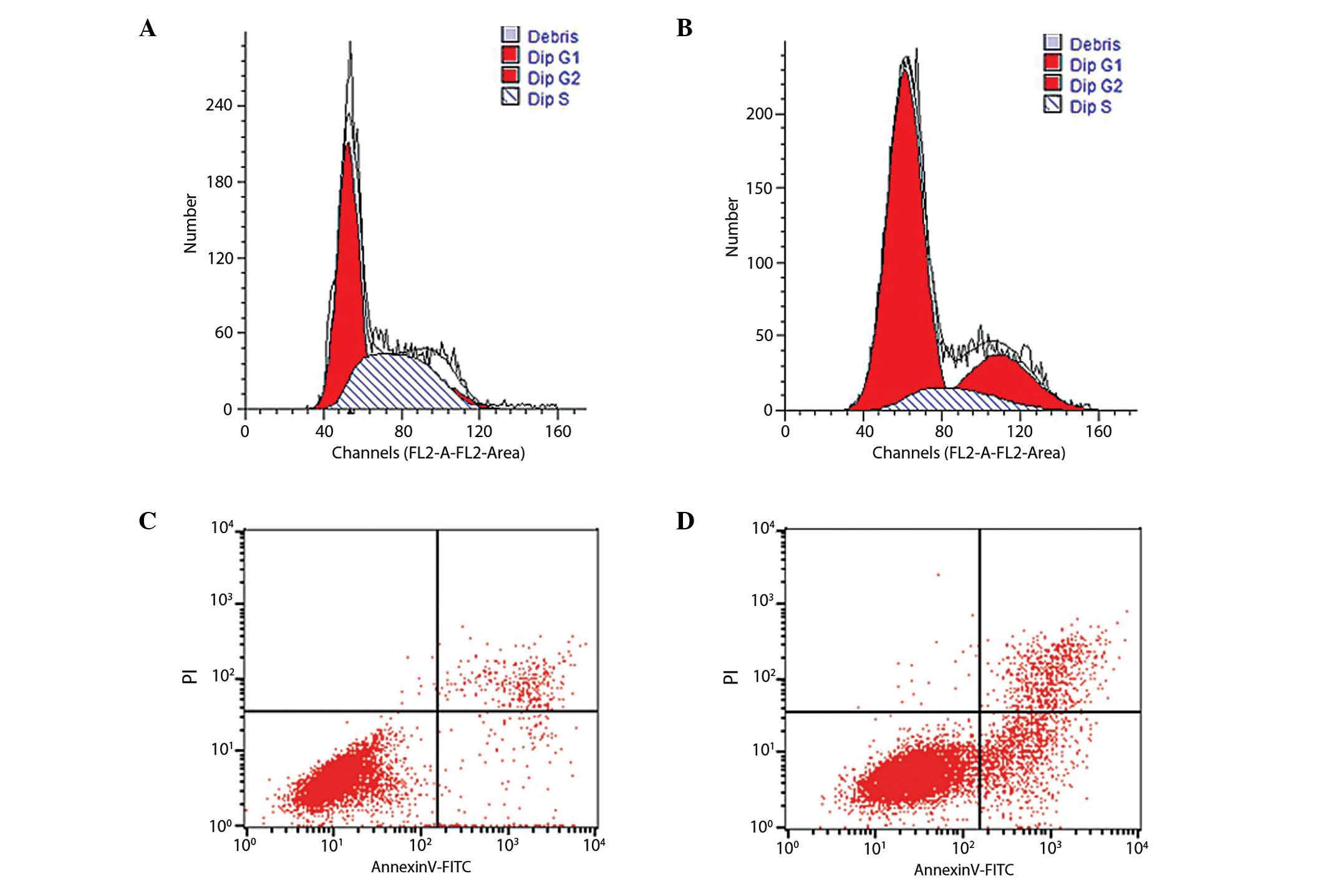
Based on the results of the MTT assay, the most
effective concentration of ART (30 mg/l) was used to measure the
cell cycle distribution and apoptosis rates in KYSE-150 cells for
48 h (Fig. 2). The results
demonstrated that the S phase cell population accounted for
42.5±2.8% in the control group, compared with 11.5±2.7% in the 30
mg/l ART group (Table I). The number
of cells in G0/G1 phase increased from
54±1.5% in the control group to 68.1±0.3% in the ART-treated group,
indicating that ART functions as a negative regulator of the cell
cycle at the G1-to-S phase transition. The apoptosis
rate of the 30 mg/l ART-treated group was significantly increased
at 12.45±0.62% compared with the control group at 4.53±0.58%
(Fig. 2C and D, Table I).
 | Table I.Cell cycle distribution and levels of
apoptosis in KYSE-150 cells prior and subsequent to treatment with
ART. |
Table I.
Cell cycle distribution and levels of
apoptosis in KYSE-150 cells prior and subsequent to treatment with
ART.
|
| Cell cycle
distribution |
|
|---|
|
|
|
|
|---|
| Treatment
Group |
G0/G1 (%) | S (%) | G2 (%) | Apoptosis rate
(%) |
|---|
| Control group (0
mg/l, ART) | 54±1.5 | 42.5±2.8 | 3.5±0.74 | 4.53±0.58 |
| ART-treatment group
(30 mg/l, ART) |
68.1±0.3a |
11.5±2.7a |
20.4±2.53a |
12.45±0.62a |
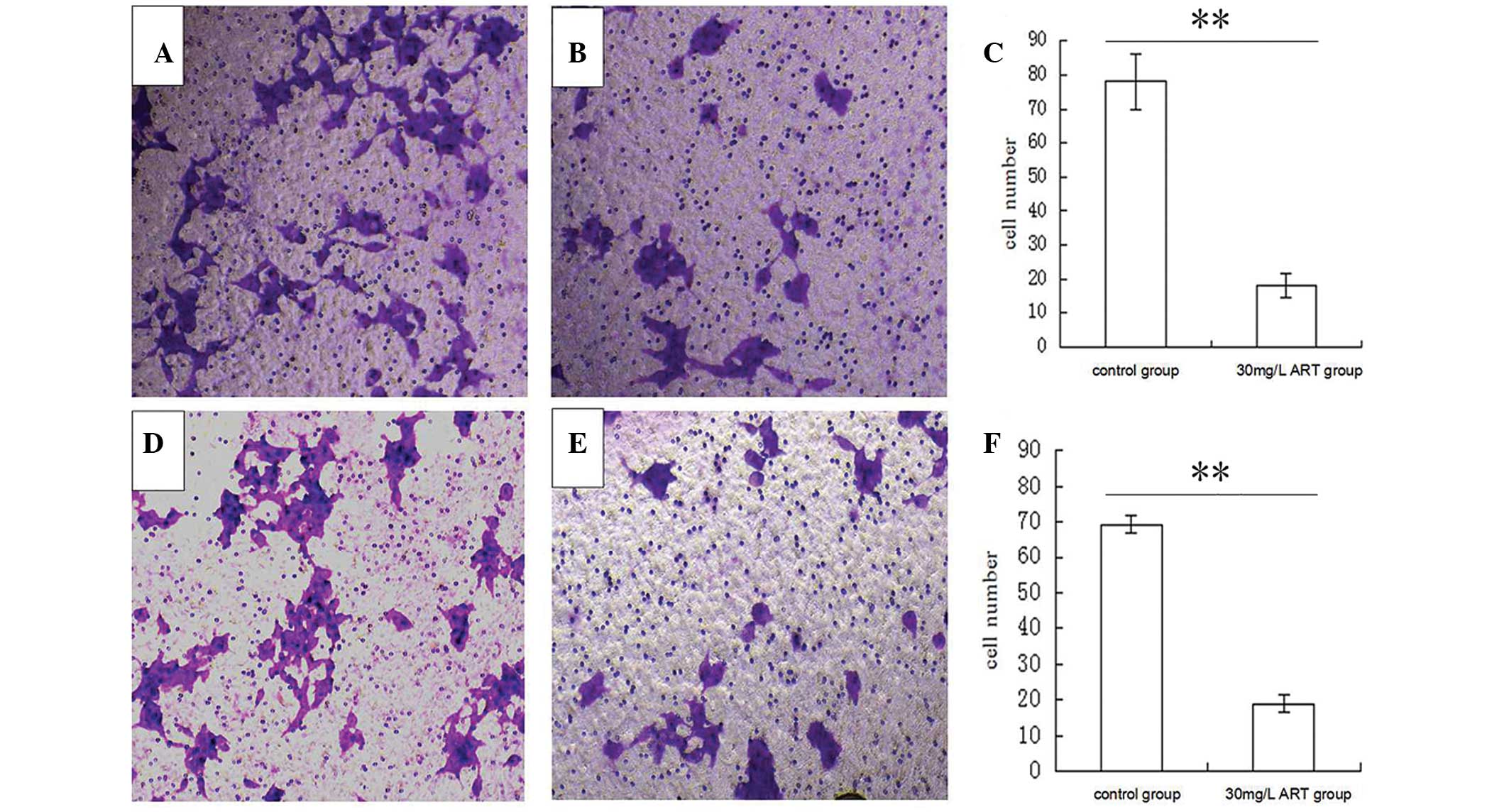
ART mitigates the migration and
invasion of ESCC cells
To further detect whether ART is associated with
ESCC migration and invasion, a Transwell assay was performed to
analyze the effect of ART on the migratory and invasive behavior of
KYSE-150 cells. Compared with the control group (0 mg/l ART),
treatment with 30 mg/l ART resulted in a significant reduction of
cell migration in the KYSE-150 cell line (Fig. 3A–C). In addition, ART suppressed the
normally strong invasive capacity of KYSE-150 cells (Fig. 3D–F). These results indicate that ART
reduces cell migration and invasion of ESCC cells in
vitro.
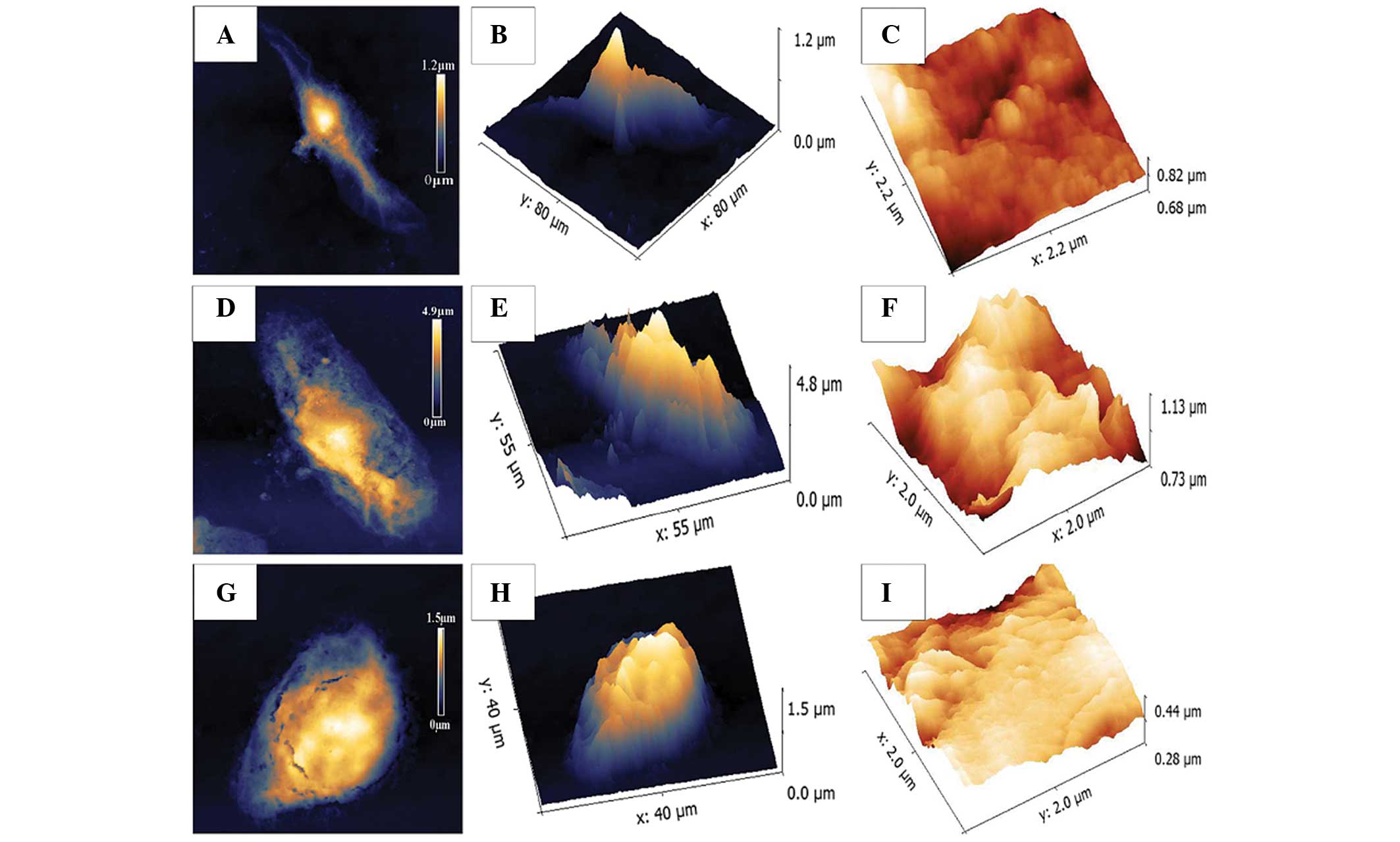
The biomechanical properties of
KYSE-150 cells treated with ART are similar to that of SHEE cells,
detected by AFM
Every cell type possesses a unique structure with
specific mechanical properties that can serve as specific
biomechanical markers. In the present study, AFM was used to
visualize the morphology of SHEE normal esophageal epithelial cells
and KYSE-150 ESCC cells. Fig. 4
demonstrates that without incubation with ART, the KYSE-150 cells
exhibit a spindle-like morphology (Fig.
4B); the KYSE-150 cells treated with 30 mg/l ART were irregular
in shape (Fig. 4E); and the SHEE
normal esophageal epithelial cell line was oval in appearance
(Fig. 4H). The roughness of membrane
ultrastructures of the KYSE-150 cells (Fig. 4C) was similar to that of the SHEE
cells (Fig. 4I), but smoother than
that of the 30 mg/l ART-treated group. The cell membrane of the 30
mg/l ART-treated group was the most irregular and uneven among the
three treatment groups (Fig. 4F).
In the KYSE-150 cell line without ART treatment, the
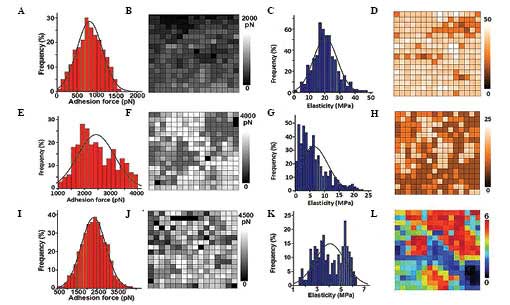
adhesive force was 800±300 pN (Fig. 5A
and B); the elasticity force was 20±7 MPa (Fig. 5C and D); and the average roughness
(Ra) was 0.172±0.025 µm (Table II).
Following 30 mg/l ART treatment in the KYSE-150 cell line, the
adhesive force was 2,400±700 pN (Fig. 5E
and F); the elasticity force was 7±4 MPa (Fig. 5G and H); and the Ra was 1.05±0.088 µm.
In the control SHEE cells, the adhesive force was 2,300±600 pN
(Fig. 5I and J); the elasticity force
was 4±1 MPa (Fig. 5K and L); and the
Ra was 0.183±0.026 µm. Following incubation with ART (30 mg/l) for
48 h, the adhesive force and elasticity of KYSE-150 cells was
similar to that of the SHEE normal esophageal epithelial cell line
(Table II).
 | Table II.Biomechanical properties of SHEE and
KYSE150 cells prior and subsequent to treatment with ART. |
Table II.
Biomechanical properties of SHEE and
KYSE150 cells prior and subsequent to treatment with ART.
| Variable | Ra of cytomembrane
(μm) | Adhesive force
(pN) | Elasticity
(MPa) |
|---|
| 0 mg/l ART | 0.172±0.025 | 800±300 | 20±7 |
| 30 mg/l group |
1.05±0.088a |
2400±700a | 7±4a |
| SHEE | 0.183±0.026 |
2300±600a | 4±1a |
Discussion
In the present study, the effect of the
anti-malarial agent ART on ESCC cells was analyzed. Compared with
the control group, ART treatment increased the Ra of the cell
membrane in addition to the adhesive force. The cell membrane
contains numerous different biological macromolecules, including
certain sugars, proteins and lipids. These macromolecules transfer
signals to cells, form an exchange interface between the inside and
the outside of the cell and maintain the integrity and
functionality of the cell membrane (12,23).
Changes in cell membrane structure can therefore directly influence
the cytoskeleton (13,14).
The cytoskeleton is mainly composed of microtubules,
microfilaments and intermediate filaments and determines the cell
morphology and biomechanical characteristics under different
physiological statuses (24). A
previous study demonstrated that chromosome stability and cell
proliferation are influenced when the cytoskeleton is altered
(25). In addition, previous studies
have indicated that ART inhibits DNA synthesis and cell growth in
several tumor cell lines in vitro (9,10,26,27). The
results of the present study for the MTT assay, cell cycle and AFM
detection revealed that ART may damage the cytoskeleton to affect
signal transduction, DNA synthesis and inhibit cell migration. The
results presented in the current study also suggest that ART is a
negative regulator of the cell cycle, preventing G1-to-S
phase transition. Cell elasticity may reflect alterations in the
cytoskeleton and be associated with cell deformation (28), which is inevitable in tumor cell
migration and invasion (29). The
results of the AFM detection indicated that ART may inhibit the
migration and invasion of KYSE-150 cells through suppressing cell
elasticity and increasing adhesive force.
Apoptosis serves an important function in
maintaining cell homeostasis, and the dysfunction of apoptotic
signaling has been implicated in cancer (30). Apoptosis has been studied as a
possible target mechanism for anticancer therapy and the
cytoskeleton participates in this process; thus, damage of actin in
the cytoskeleton may promote apoptosis (31). The results of the apoptosis assay and
AFM detection in the present study indicated that ART may increase
the apoptosis rate by altering the cytoskeleton of KYSE-150
cells.
In the present study, it was demonstrated that ART
is an effective drug for anti-ESCC. ART has the potential ability
to inhibit the proliferation, migration and invasion of ESCC cells
by affecting their cell morphology and structure.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272694 and
81071625) to Professor Xiaolong Cheng; the National Natural Science
Foundation of China (grant no. 81201956) to Ms. Jing Liu; the
Science and Technology Innovation Fund of Shanxi Medical University
(grant no. 01201309) to Dr Ruyi Shi; and the Youth Research Fund of
Shanxi Medical University (grant no. Q02201202) to Dr Ruyi Shi.
References
|
1
|
Brown LM, Devesa SS and Chow WH: Incidence
of adenocarcinoma of the esophagus among white Americans by sex,
stage, and age. J Natl Cancer Inst. 100:1184–1187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
White NJ: Qinghaosu (artemisinin): the
price of success. Science. 320:330–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Youns M, Efferth T, Reichling J,
Fellenberg K, Bauer A and Hoheisel JD: Gene expression profiling
identifies novel key players involved in the cytotoxic effect of
Artesunate on pancreatic cancer cells. Biochem Pharmacol.
78:273–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou J, Wang D, Zhang R and Wang H:
Experimental therapy of hepatoma with artemisinin and its
derivatives: in vitro and in vivo activity, chemosensitization, and
mechanisms of action. Clin Cancer Res. 14:5519–5530. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li LN, Zhang HD, Yuan SJ, Yang DX, Wang L
and Sun ZX: Differential sensitivity of colorectal cancer cell
lines to artesunate is associated with expression of beta-catenin
and E-cadherin. Eur J Pharmacol. 588:1–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nam W, Tak J, Ryu JK, Jung M, Yook JI, Kim
HJ and Cha IH: Effects of artemisinin and its derivatives on growth
inhibition and apoptosis of oral cancer cells. Head Neck.
29:335–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu WM, Gravett AM and Dalgleish AG: The
antimalarial agent artesunate possesses anticancer properties that
can be enhanced by combination strategies. Int J Cancer.
128:1471–1480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rinner B, Siegl V, Pürstner P, Efferth T,
Brem B, Greger H and Pfragner R: Activity of novel plant extracts
against medullary thyroid carcinoma cells. Anticancer Res.
24:495–500. 2004.PubMed/NCBI
|
|
9
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Oncol. 18:767–773. 2001.PubMed/NCBI
|
|
10
|
Efferth T, Sauerbrey A, Olbrich A, et al:
Molecular modes of action of artesunate in tumor cell lines. Mol
Pharmacol. 64:382–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Evans EA and Calderwood DA: Forces and
bond dynamics in cell adhesion. Science. 316:1148–1153. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Puech PH, Poole K, Knebel D and Muller DJ:
A new technical approach to quantify cell-cell adhesion forces by
AFM. Ultramicroscopy. 106:637–644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sato K, Adachi T, Ueda D, Hojo M and
Tomita Y: Measurement of local strain on cell membrane at
initiation point of calcium signaling response to applied
mechanical stimulus in osteoblastic cells. J Biomech. 40:1246–1255.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Voïtchovsky K, Antoranz Contera S,
Kamihira M, Watts A and Ryan JF: Differential stiffness and lipid
mobility in the leaflets of purple membranes. Biophys J.
90:2075–2085. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hörber JK and Miles MJ: Scanning probe
evolution in biology. Science. 302:1002–1005. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alonso JL and Goldmann WH: Feeling the
forces: atomic force microscopy in cell biology. Life Sci.
72:2553–2560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lesniewska E, Milhiet PE, Giocondi MC and
Le Grimellec C: Atomic force microscope imaging of cells and
membranes. Methods Cell Biol. 68:51–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugimoto Y, Pou P, Abe M, Jelinek P, Pérez
R, Morital S and Custance O: Chemical identification of individual
surface atoms by atomic force microscopy. Nature. 446:64–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soofi SS, Last JA, Liliensiek SJ, Nealey
PF and Murphy CJ: The elastic modulus of Matrigel as determined by
atomic force microscopy. J Struct Biol. 167:216–219. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kondra S, Laishram J, Ban J, et al:
Integration of confocal and atomic force microscopy images. J
Neurosci Methods. 177:94–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Wan Z, Liu W, et al: Atomic force
microscope study of tumor cell membranes following treatment with
anti-cancer drugs. Biosens Bioelectron. 25:721–727. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Snedecor GW and Cochran WG: Statistical
Methods. 7th. Iowa State University Press; Ames, IA, USA: pp.
32–43. 1980
|
|
23
|
Geiger B, Bershadsky A, Pankov R and
Yamada KM: Transmembrane crosstalk between the extracellular matrix
– cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2:793–805. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fuchs E and Cleveland DW: A structural
scaffolding of intermediate filaments in health and disease.
Science. 279:514–519. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saunders WS, Shuster M, Huang X, Gharaibeh
B, Enyenihi AH, Petersen I and Gollin SM: Chromosomal instability
and cytoskeletal defects in oral cancer cells. Proc Natl Acad Sci
USA. 97:303–308. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Efferth T, Benakis A, Romero MR, et al:
Enhancement of cytotoxicity of artemisinins toward cancer cells by
ferrous iron. Free Radic Biol Med. 37:998–1009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Posner GH, McRiner AJ, Paik IH, et al:
Anticancer and anti-malarial efficacy and safety of
artemisinin-derived trioxane dimers in rodents. J Med Chem.
47:1299–1301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fletcher DA and Mullins RD: Cell mechanics
and the cytoskeleton. Nature. 463:485–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar S and Weaver VM: Mechanics,
malignancy, and metastasis: the force journey of a tumor cell.
Cancer Metastasis Rev. 28:113–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mahoney JA and Rosen A: Apoptosis and
autoimmunity. Curr Opin Immunol. 17:583–588. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gourlay CW and Ayscough KR: A role for
actin in aging and apoptosis. Biochem Soc Trans. 33:1260–1264.
2005. View Article : Google Scholar : PubMed/NCBI
|