Introduction
Gliomas comprise the majority of primary brain
tumors and are the leading cause of intracranial neoplasm
mortality, resulting in a poor prognosis despite advances in
surgical techniques and concurrent radiochemotherapy regimens
(1–3).
High-grade gliomas exhibit aggressive biological features,
including extensively infiltrative growth and diffuse invasion, and
are frequently recurrent and intractable (3,4).
Radiotherapy, chemotherapy and photodynamic therapy may increase
survival rates (5,6); however, disease recurrence is virtually
inevitable (7). The invasive growth
of gliomas is a long-standing and formidable issue that often leads
to the failure of glioma therapy (3,8). At
present, the biological behavior and characteristics of gliomas,
including infiltrative invasion, should be investigated, rather
than methods of reducing the tumor bulk.
The invasive growth of gliomas is a complex
multistage process that involves changes in the expression levels
of multiple genes and includes various pathophysiological aspects,
including attachment to neighboring cells, aggregate formation,
adhesion to the matrix substratum, migration and invasion into the
three-dimensional cellular microenvironment, which includes tumor
cells, the extracellular matrix, stromal cells, cytokines, immune
cells and is characterized by tissue hypoxia, acidosis and
interstitial high pressure; of these components, the extracellular
matrix key and may be hydrolyzed by ADAM10 during glioma invasion
(9,10). As glioma cells acquire their
infiltrative phenotype, they overexpress various matrix
metalloproteinases (MMPs), which are a family of zinc-dependent
endopeptidases that cleave extracellular matrix components
(11–14). In addition to their role in
extracellular matrix degradation, a number of MMPs and the
associated family of ‘a-disintegrin and metalloproteases’ (ADAMs)
also regulate proliferation, adhesion, migration and metastasis by
initiating the cleavage of cell surface proteins (12–15). Among
these genes, ADAM10 is currently attracting increased attention
(12–14). As a member of the zinc-dependent
protease family, the structural characteristics of ADAM10 form the
basis of its biological functions (13,15,16).
Previous studies revealed that ADAM10 is involved in the
pathogenesis of several types of malignant solid tumors and that it
may be involved in invasive growth (12,17–20).
Therefore, in the present study, a series of assays were designed
and conducted to determine the expression of ADAM10 and its
localization in low- and high-grade gliomas. These results may
provide an insight into the biological roles of ADAM10 in gliomas
with different malignancies, thereby elucidating its underlying
mechanisms, which may provide a promising target for gene therapy
of gliomas.
Materials and methods
Specimen processing
Between May 2007 and July 2010, a total of 43 glioma
specimens were collected from patients hospitalized at the
Department of Neurosurgery (The First Affiliated Hospital of China
Medical University, Shenyang, China) and included in this study.
This study was approved by the ethics committee of The First
Affiliated Hospital of China Medical University and written
informed consent was obtained from all patients. These specimens
included 22 cases of low-grade gliomas and 21 cases of high-grade
gliomas. In addition, 20 meningioma specimens [World Health
Organization (WHO) grade II (21)]
were collected and used as the controls. Each specimen was stored
at −70°C immediately following surgical resection, and the
corresponding patient's information was obtained and registered.
All the specimens were pathologically confirmed by two
neuropathologists according to the 2007 WHO criteria (21). Paraffin-embedded sections were
obtained from the Department of Pathology of The First Affiliated
Hospital of China Medical University and reserved for the
subsequent immunohistochemical assays. The frozen fresh tissue
samples were subjected to reverse transcription-polymerase chain
reaction (RT-PCR) and western blot assays.
RNA extraction and semi-quantitative
RT-PCR
A tumor sample (100 mg) from each patient,
previously stored at −70°C, was used for semi-quantitative RT-PCR
detection according to the following method: The tissue sample was
initially macerated in 1 ml of RNAiso Plus (Takara Bio, Inc., Otsu,
Japan) in a 1.5 ml RNase-free centrifuge tube. Each centrifuge tube
was vortexed for 15 sec to mix the contents and kept still for 5
min. Subsequently, the tubes were centrifuged at 12,000 × g at 4°C
for 5 min. The supernatant from each tube was collected and
transferred to a new 1.5-ml tube and 1/5 of the chloroform volume
was added. Next, the contents were mixed with 0.5 ml isopropanol,
and a white pellet was obtained and washed with 75% ethanol,
following centrifugation at 12,000 × g at 4°C for 15 min, according
to the manufacturer's instructions. The supernatant was removed and
the resulting pellet was then air-dried and dissolved in 40 µl of
RNase-free water. A UV spectrophotometer (NanoDrop ND-1000;
NanoDrop Technologies, Inc., Wilmington, DE, USA) was used to
measure RNA purity and content.
RT-PCR was performed on a GeneAmp PCR System 9700
thermocycler (Applied Biosystems Life Technologies, Foster City,
CA, USA) using a two-step PrimeScript RT-PCR kit (Takara Bio, Inc.)
according to the manufacturer's instructions. Briefly, 1 µg of RNA
from each sample was converted into cDNA in a reverse transcription
reaction. PCR for each gene was performed in a 20 µl reaction
mixture, containing 2 µl cDNA template, 1.6 µl dNTP, 0.4 µl of each
primer, 0.1µl TaKaRa Ex Taq, 2 µl 10X Ex Taq buffer (all reagents
obtained from Takara) and 13.5 µl sterilized distilled water, and
the conditions were as follows: 94°C for 2 min, with cycling
conditions of 94°C for 30 sec, 55°C for 30 sec and 72°C for 2 min,
for a total of 30 cycles, and a final extension step of 10 min. The
amplified DNA product from each specimen was subjected to 1.5%
agarose gel electrophoresis. Subsequently, the samples were
visualized by ethidium bromide staining (Sigma-Aldrich, St. Louis,
MO, USA) and images were captured using Gel Doc 2000 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) under ultraviolet light and
digitized with a Hewlett-Packard Scanjet scanner (Palo Alto, CA,
USA) as a 256-level grayscale image in the gel analysis software,
SigmaGel versino 1.0 (Jandel Scientific, Erkrath, Germany).
Gene-specific primers were designed and synthesized by Takara Bio,
Inc., as listed in Table I. All the
results were normalized against the expression levels of the
housekeeping gene, β-actin. The experiments were performed in
triplicate for each sample.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer sequences | Length (bp) |
|---|
| ADAM10 (F) |
5′-TGGGTCAAAAAGAAAATGGC-3′ | 281 |
| ADAM10 (R) |
5′-CCCAGGTTTCAGTTTGCATT-3′ |
|
| β-actin (F) |
5′-AAATCGTGCGTGACATTAA-3′ | 474 |
| β-actin (R) |
5′-CTCGTCATACTCCTGCCTG-3′ |
|
Western blot analysis
To determine the level of ADAM10 protein expression,
the samples were subjected to sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and western blot analysis, as
described previously (22,23). Equal quantities of tissue lysates were
resolved by SDS-PAGE, transferred onto polyvinylidene fluoride
(PVDF) membranes (Roche Diagnostics, Indianapolis, IN, USA) using
electroblotting, probed with specific primary antibodies, followed
by horseradish peroxidase (HRP)-conjugated secondary antibodies,
and then analyzed. The primary antibodies used were monoclonal
rabbit anti-human ADAM10 (1:1,000; Abcam, Cambridge, UK) and
monoclonal rabbit anti-human β-actin antibody (1:500; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and were detected using
HRP-conjugated mouse anti-rabbit immunoglobulin G antibody (1:500;
Sigma-Aldrich) in blocking solution. The 75 kDa band was used to
analyze changes in ADAM10 expression in all the experiments.
Immunoreactive protein bands were visualized using an enhanced
chemiluminescence reagent (Amersham ECL-PLUS; GE Healthcare Life
Sciences, Little Chalfont, UK), scanned with a FujiFilm LAS300
Intelligent Dark Box scanner (Fujifilm, Tokyo, Japan) and
quantified for pixel density by the optical density (OD) method,
using the Chemi Genius gel imaging system (Chemi ImagerTM 5500;
ProteinSimple, San Jose, CA, USA).
Immunohistochemical assay
ADAM10 protein localization and expression were
determined using an immunohistochemical method. Immunohistochemical
analysis was performed using the Histostain-SP kit (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. The primary antibody (rabbit anti-human ADAM10;
Abcam) was diluted at 1:500 and ADAM10 binding was visualized using
the standard avidin/biotinylated enzyme complex-HRP staining
procedure, with 3,3′-diaminobenzidine as a chromogen and a
monoclonal mouse anti-rabbit immunoglobulin G antibody
(Histostain-SP kit, Invitrogen Life Technologies). The sections
were examined using an optical microscope (BX40; Olympus
Corporation, Tokyo, Japan). Cells that contained brown-yellow
stained granules in the membrane and cytoplasm were considered
positive. Next, five hundred cells in five randomly selected fields
under high magnification were counted. Presence of <5% of
positive cells was classified as (−), then 5–50% as (+), 50–75% as
(++) and >75% as (+++).
Statistical analysis
Data are presented as the mean ± standard deviation,
and were analyzed by the two-tailed paired t-test with SPSS 18.0
statistical software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of ADAM10 in glioma
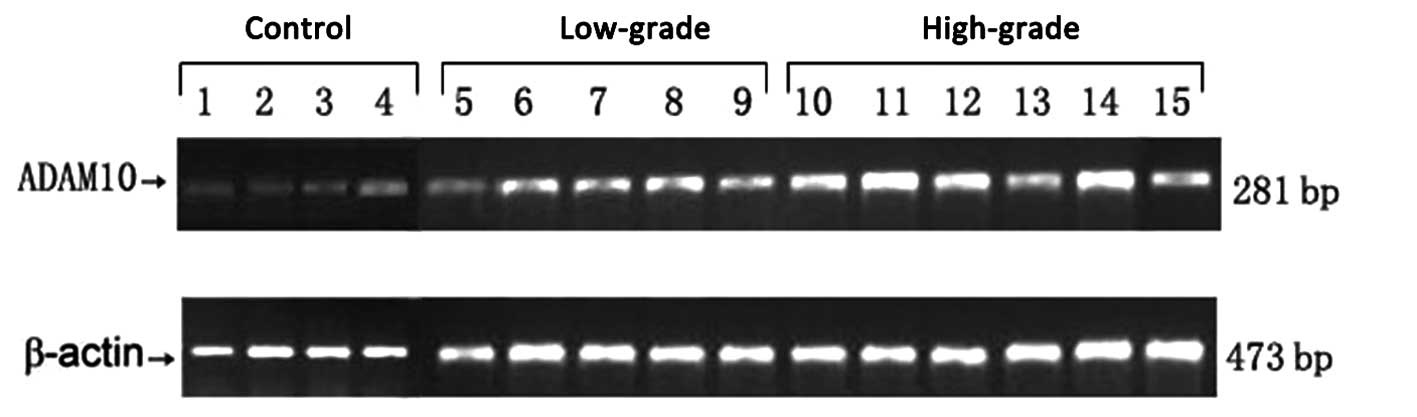
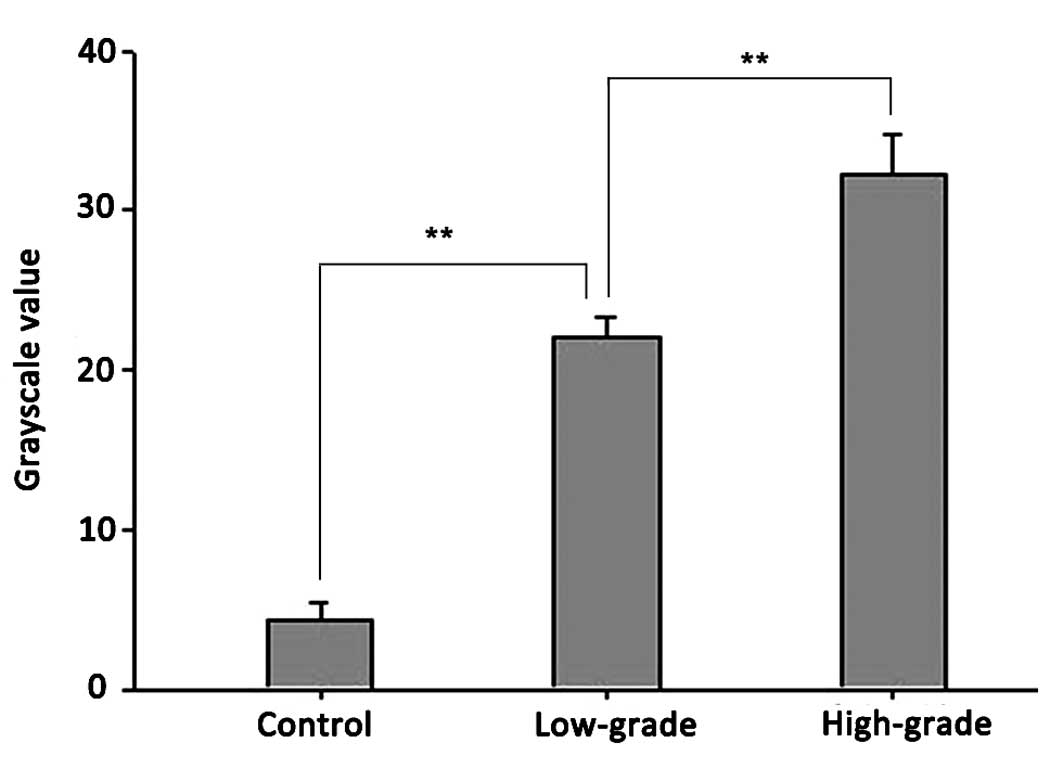
RT-PCR confirmed ADAM10 mRNA expression in the
glioma and control groups. As demonstrated in Figs. 1 and 2,
the relative mRNA expression levels of ADAM10 in the low- and
high-grade groups were 21±1.43 and 32±2.7, respectively, compared
with the control group (4.63 ±1.23). Statistical analysis
demonstrated significant differences between the high- and
low-grade groups (P<0.01) and between the low-grade and control
groups (P<0.01).
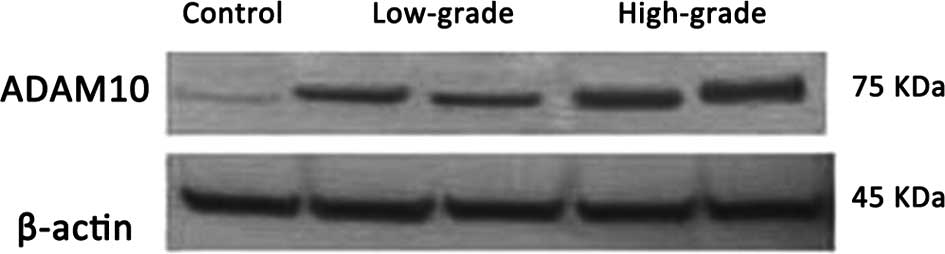
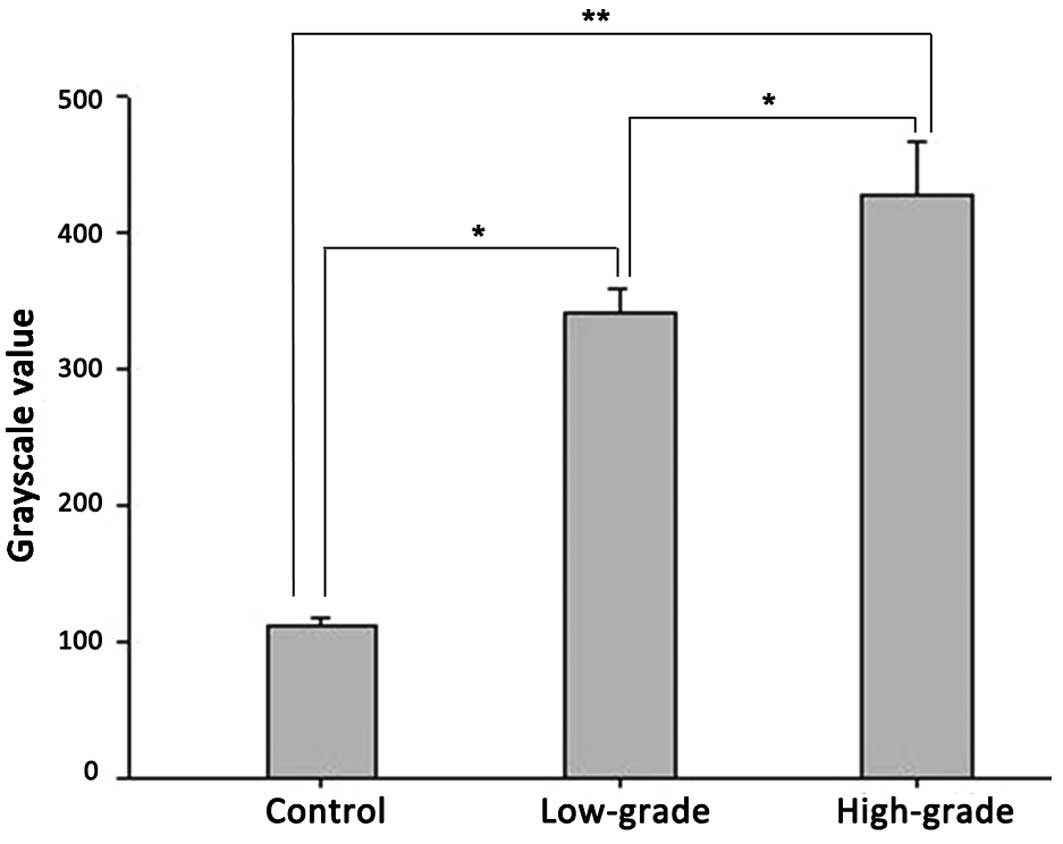
Immunohistochemical and western blot analyses
confirmed ADAM10 protein expression in the controls, and low- and
high-grade gliomas. In the western blot assay, two bands for ADAM10
were observed, in which the 100-kDa band is the ADAM10 protein
precursor, while the 75-kDa band is its active form. The 100-kDa
ADAM10 protein precursor is non-functional and thus was excluded
from the analysis. Analysis of the mean grayscale values of the
mature 75 kDa band indicated that there was a trend of increasing
ADAM10 protein expression correlated with increasing pathological
grade (Figs. 3 and 4; Table II).
In addition, a significant increase in ADAM10 protein levels was
observed in the low-grade glioma group compared with the non-glioma
control group (P<0.05), while the ADAM10 protein levels in the
high-grade glioma group were significantly higher compared with the
control non-glioma (P<0.01) and low-grade glioma groups
(P<0.05).
 | Table II.Immunohistochemical staining for
a-disintegrin and metalloproteinase 10 in different groups. |
Table II.
Immunohistochemical staining for
a-disintegrin and metalloproteinase 10 in different groups.
| Tissues | Number of positive
cells (n) | Total number of cells
(n) | Percentage of cells
(%) |
|---|
| Control | 47 | 500 | 9.4 |
| Low-grade | 269 | 500 | 53.8 |
| High-grade | 382 | 500 | 76.4 |
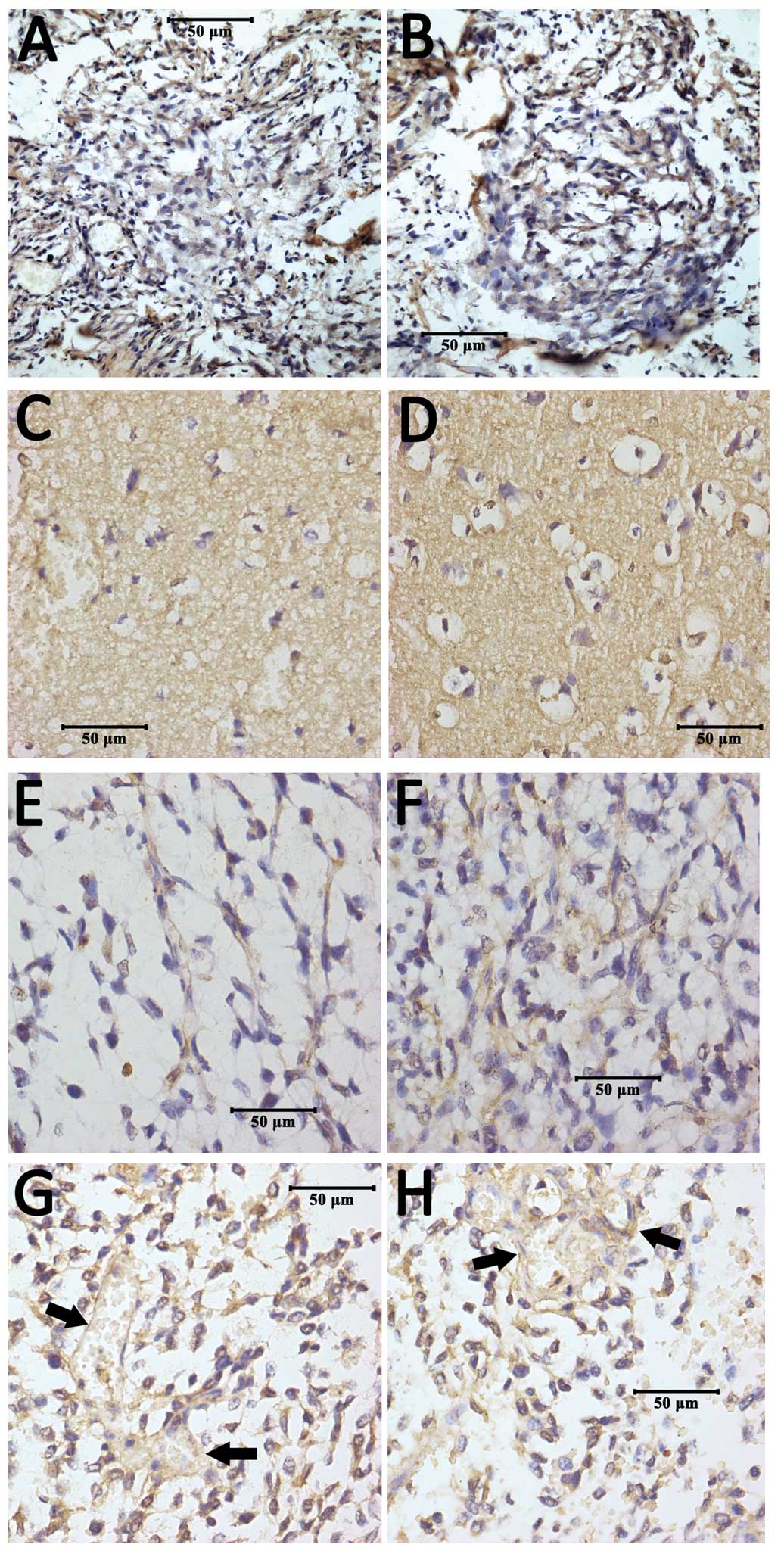
Localization of ADAM10 protein in
tissues
As observed under the light microscope, ADAM10
protein was found to be localized on the cell membrane and blood
vessel walls within tumor tissues (Fig.
5). Cells which contained brown-yellow granules in the membrane
and cytoplasm were considered to be positive for ADAM10 protein
expression. The positive rate of ADAM10 protein expression in the
control non-glioma group was only 9.4% of cells, while 53.8% of
cells in the low-grade and 76.4% of cells in the high-grade glioma
groups were deemed positive for ADAM10 protein expression.
Therefore, these observations indicated a malignancy-dependent
pattern for ADAM10 mRNA and protein expression.
Discussion
ADAM10, which is secreted as a precursor protein, is
a member of the ADAM transmembrane protein family and has four
potential functional domains: proteolytic, adhesion, fusion and
intracellular signaling domains (13,15,16,18,24,25).
Previous studies have reported that a number of ADAMs are involved
in the biological processes of malignant tumor cells, including
proliferation and invasive growth (26–30). In
addition, ADAM10 has been found to be highly expressed in local
secretory cells in prostate cancer and expressed in the basal cells
of benign glands, while aberrant ADAM10 expression has been
demonstrated to correlate with osteosarcoma progression (17,31,32). A
previous study has demonstrated that ADAM10 promotes the
proliferation of gastric cancer cells by mediating ectodomain
shedding and, therefore, activating epidermal growth factor
receptor ligands (25). Other studies
have demonstrated that ADAM10 plays a role in the hydrolysis of
placental type IV collagen, an important extracellular matrix
component, further indicating that ADAM10 is involved in cancer
invasion and metastasis (33).
In the present study, ADAM10 expression was assessed
in different grades of gliomas at the transcriptional and
translational levels, while the association between glioma
malignancy and ADAM10 expression was investigated. ADAM10
expression was measured in controls (meningioma) using RT-PCR and
western blot analysis. The present study demonstrated that ADAM10
expression was significantly higher with increasing malignancy
grade. The statistical analysis revealed that there were
significant differences among the high- and low-grade groups and
the control in a malignancy-dependent pattern, indicating that
ADAM10 expression is closely associated with glioma malignancy. In
accordance with previous studies on the role of ADAM family members
in other malignancies, the present study revealed that ADAM10 may
also be involved in glioma growth and invasiveness. The
semi-quantitative RT-PCR and western blot analysis demonstrated
that the expression level of ADAM10 is highest in high-grade
gliomas, and relatively higher in low-grade gliomas compared with
the controls. In the control benign brain tumors (meningiomas),
ADAM10 was not significantly expressed, as confirmed by the
immunohistochemical assay (Table
II). Although the specific mechanism of ADAM10 in glioma
biology remains unclear, immunohistochemical staining for ADAM10
may provide new insights. As Fig. 5
shows, positive staining was mainly observed on the cell membrane,
further confirming that ADAM10 is a transmembrane protein. The
results of the present study demonstrated that the higher the tumor
grade, the higher the ADAM10 expression level, and thus the more
aggressive the tumor. In accordance with existing evidence
(12–20,28,31,33,34),
one hypothesis is that ADAM10 promotes the invasive growth of
glioma cells by hydrolyzing extracellular matrix collagen. This
hydrolysis may transmit intercellular signals and promote
subsequent ectodomain EGFR ligand shedding, thus stimulating
intracellular relevant genes to increase tumor cell proliferation.
However, this hypothesis requires further verification.
The present study also revealed that ADAM10 protein
was present in the blood vessel walls of glioma tissues but the
implication of this finding is unclear at present. However, a
previous study suggested that ADAM10 modulates the connections
between vessel wall cells by regulating vascular endothelial
cadherin, a cell-adhesion glycoprotein that regulates blood vessel
permeability, implying a pathological mechanism for the edema
formation that can occur in glioma (32). In addition, a previous study reported
that ADAM10 may be associated with peripheral migration of glioma
cells (34). Based on these findings,
ADAM10 may be involved in the regulation of tumor vascular
permeability, which is likely to be one of the mechanisms
responsible for the formation of evident peritumoral edema in
gliomas.
The key biological function of ADAM10 and other ADAM
family members is their enzymatic activity as metalloproteinases
(11,13–15,18,24,26,34).
More specifically, ADAM10 is involved in the intramembrane
proteolysis process, whereby it mediates ectodomain shedding of
various membrane-bound receptors, adhesion molecules, growth
factors and cytokines (35). For
instance, ADAM10 is involved in regulating the ectodomain shedding
of Notch, human epidermal growth factor receptor 2, CD44,
interleukin-6 receptor, amyloid precursor protein and certain
cadherins (18). Considering the
infiltrative and invasive growth characteristics of gliomas
(3,4),
the expression of ADAM10 is expected to correlate with not only the
pathological grades, but also the aggressiveness of gliomas to a
certain extent. In other words, the more malignant the glioma is,
the more invasive it may be. Thus, the malignancy-dependent pattern
of ADAM10 expression in gliomas may indicate that ADAM10 plays an
important role in glioma invasion and may subsequently be used to
predict clinical intractability and poor outcome.
In conclusion, the present study demonstrated that
ADAM10 expression is closely associated with glioma grade in a
malignancy-dependent pattern, indirectly indicating an important
biological role for ADAM10 in glioma growth and development,
particularly the invasiveness of gliomas. The expression of ADAM10
in the blood vessel walls of tumors may be associated with the
formation of peritumoral edema. Further evidence is required to
prove these hypotheses in future studies; however, the present
study indicates that ADAM10 may be a promising target for glioma
therapy.
Acknowledgements
This study was supported by grants from the Liaoning
Provincial Natural Science Foundation of China (No. 2013021075 to
Bo Qiu) and the Fund for Scientific Research of the First Hospital
of China Medical University (No. fsfh1304 to Bo Qiu).
References
|
1
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, et al:
European Organisation for Research and Treatment of Cancer Brain
Tumor and Radiotherapy Groups; National Cancer Institute of Canada
Clinical Trials Group: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qiu B, Zhang D, Wang Y, et al:
Interleukin-6 is overexpressed and augments invasiveness of human
glioma stem cells in vitro. Clin Exp Metastasis. 30:1009–1018.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiu B, Zhang D, Tao J, Tie X, Wu A and
Wang Y: Human brain glioma stem cells are more invasive than their
differentiated progeny cells in vitro. J Clin Neurosci. 19:130–134.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stylli SS, Howes M, MacGregor L, Rajendra
P and Kaye AH: Photodynamic therapy of brain tumours: evaluation of
porphyrin uptake versus clinical outcome. J Clin Neurosci.
11:584–596. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stylli SS, Kaye AH, MacGregor L, Howes M
and Rajendra P: Photodynamic therapy of high grade glioma - long
term survival. J Clin Neurosci. 12:389–398. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park DM and Rich JN: Biology of glioma
cancer stem cells. Mol Cells. 28:7–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neurocentric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coniglio SJ and Segall JE: Review:
molecular mechanism of microglia stimulated glioblastoma invasion.
Matrix Biol. 32:372–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaczarek E, Zapf S, Bouterfa H, Tonn JC,
Westphal M and Giese A: Dissecting glioma invasion: interrelation
of adhesion, migration and intercellular contacts determine the
invasive phenotype. Int J Dev Neurosci. 17:625–641. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stamenkovic I: Extracellular matrix
remodelling: the role of matrix metalloproteinases. J Pathol.
200:448–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bulstrode H, Jones LM, Siney EJ, et al:
A-Disintegrin and Metalloprotease (ADAM) 10 and 17 promote
self-renewal of brain tumor sphere forming cells. Cancer Lett.
326:79–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edwards DR, Handsley MM and Pennington CJ:
The ADAM metalloproteinases. Mol Aspects Med. 29:258–289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pruessmeyer J and Ludwig A: The good, the
bad and the ugly substrates for ADAM10 and ADAM17 in brain
pathology, inflammation and cancer. Semin Cell Dev Biol.
20:164–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huovila AP, Turner AJ, Pelto-Huikko M,
Kӓrkkӓinen I and Ortiz RM: Shedding light on ADAM
metalloproteinases. Trends Biochem Sci. 30:413–422. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seals DF and Courtneidge SA: The ADAMs
family of metalloproteases: multidomain proteins with multiple
functions. Genes Dev. 17:7–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao R, Ni D, Tian Y, Ni B and Wang A:
Aberrant ADAM10 expression correlates with osteosarcoma
progression. Eur J Med Res. 19:92014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Armanious H, Gelebart P, Anand M, Belch A
and Lai R: Constitutive activation of metalloproteinase ADAM10 in
mantle cell lymphoma promotes cell growth and activates the
TNFα/NFκB pathway. Blood. 117:6237–6246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Liu S, Liu K, et al: A
disintegrin and metalloprotease (ADAM)10 is highly expressed in
hepatocellular carcinoma and is associated with tumour progression.
J Int Med Res. 42:611–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko SY, Lin SC, Wong YK, Liu CJ, Chang KW
and Liu TY: Increase of disintergin metalloprotease 10 (ADAM10)
expression in oral squamous cell carcinoma. Cancer Lett. 245:33–43.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kleihues P, Louis DN, Scheithauer BW, et
al: The WHO classification of tumors of the nervous system. J
Neuropathol Exp Neurol. 61:215–229. 2002.PubMed/NCBI
|
|
22
|
Qiu B, Li X, Sun X, et al: Overexpression
of aquaporin-1 aggravates hippocampal damage in mouse traumatic
brain injury models. Mol Med Rep. 9:916–922. 2014.PubMed/NCBI
|
|
23
|
Qiu B, Sun X, Zhang D, Wang Y, Tao J and
Ou S: TRAIL and paclitaxel synergize to kill U87 cells and
U87-derived stem-like cells in vitro. Int J Mol Sci. 13:9142–9156.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wolfsberg TG and White JM: ADAMs in
fertilization and development. Dev Biol. 180:389–401. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshimura T, Tomita T, Dixon MF, Axon AT,
Robinson PA and Crabtree JE: ADAMs (a disintegrin and
metalloproteinase) messenger RNA expression in Helicobacter
pylori-infected, normal, and neoplastic gastric mucosa. J Infect
Dis. 185:332–340. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rocks N, Paulissen G, El Hour M, et al:
Emerging roles of ADAM and ADAMTS metalloproteinases in cancer.
Biochimie. 90:369–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wildeboer D, Naus S, Amy Sang QX, Bartsch
JW and Pagenstecher A: Metalloproteinase disintegrins ADAM8 and
ADAM19 are highly regulated in human primary brain tumors and their
expression levels and activities are associated with invasiveness.
J Neuropathol Exp Neurol. 65:516–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kodama T, Ikeda E, Okada A, et al: ADAM12
is selectively overexpressed in human glioblastomas and is
associated with glioblastoma cell proliferation and shedding of
heparin-binding epidermal growth factor. Am J Pathol.
165:1743–1753. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
D'Abaco GM, Ng K, Paradiso L, Godde NJ,
Kaye A and Novak U: ADAM22, expressed in normal brain but not in
high-grade gliomas, inhibits cellular proliferation via the
disintegrin domain. Neurosurgery. 58:179–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng X, Jiang F, Katakowski M, et al:
Inhibition of ADAM17 reduces hypoxia-induced brain tumor cell
invasiveness. Cancer Sci. 98:674–684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McCulloch DR, Akl P, Samaratunga H,
Herington AC and Odorico DM: Expression of the disintegrin
metalloprotease, ADAM-10, in prostate cancer and its regulation by
dihydrotestosterone, insulin-like growth factor I, and epidermal
growth factor in the prostate cancer cell model LNCaP. Clin Cancer
Res. 10:314–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schulz B, Pruessmeyer J, Maretzky T, et
al: ADAM10 regulates endothelial permeability and T-Cell
transmigration by proteolysis of vascular endothelial cadherin.
Circ Res. 102:1192–1201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Millichip MI, Dallas DJ, Wu E, Dale S and
McKie N: The metallo-disintegrin ADAM10 (MADM) from bovine kidney
has type IV collagenase activity in vitro. Biochem Biophys Res
Commun. 245:594–598. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kohutek ZA, diPierro CG, Redpath GT and
Hussaini IM: ADAM-10-mediated N-cadherin cleavage is protein kinase
C-alpha dependent and promotes glioblastoma cell migration. J
Neurosci. 29:4605–4615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang CL, Jiang FQ, Xu F and Jiang GX:
ADAM10 overexpression confers resistance to doxorubicin-induced
apoptosis in hepatocellular carcinoma. Tumour Biol. 33:1535–1541.
2012. View Article : Google Scholar : PubMed/NCBI
|