Introduction
Mucosa-associated lymphoid tissue (MALT) lymphoma is
a low-grade B cell lymphoma that was first identified by Isaacson
and Wright (1). Primary pulmonary
lymphoma is rare and accounts for 0.5% of lung tumors, with 72–90%
of pulmonary lymphomas being MALT lymphomas (2,3). The
outcome of pulmonary MALT lymphoma is generally favorable, with a
five-year survival rate of >80% and a median survival time of
>10 years (4). At present, no
consensus exists with regard to treatment, however, simple clinical
monitoring is recommended (5,6). Surgical resection is usually performed
in patients with localized lesions, whereas chemotherapy is
administered to patients with bilateral or extrapulmonary
involvement, relapse or progression (4). Large cell neuroendocrine carcinoma
(LCNEC) is a tumor that was first proposed by Travis et al
(7). LCNEC is also rare, accounting
for 2.4% of lung cancers, and its prognosis is extremely poor with
a five-year survival rate of 15–57%, and 27–67% in patients with
stage I disease according to TNM staging (8,9). Surgical
resection alone is not sufficient for the treatment of LNEC and
thus, adjuvant chemotherapy is recommended after surgery even in
patients with stage IA disease accordign to TNM staging (10). The present study reports a case of a
patient with combined LCNEC and MALT lymphoma that responded well
to chemoradiotherapy. The combination of these two tumors is
extremely rare, and their development was documented over a period
of six years, including the onset of disease. The present study may
therefore be valuable in clarifying the mechanism of the
development of lung cancer.
Case report
A 79-year-old male was referred to Kobe City Medical
Center General Hospital (Kobe, Japan) with an abnormal shadow that
was revealed on a chest X-ray. The patient possessed a history of
cerebral infarction, which occurred at 55 years old, had undergone
a subtotal gastrectomy for gastric cancer at the age of 70, and had
also undergone an aortic arch replacement for thoracic aortic
aneurysm at 75 years old. The patient was an ex-smoker, and had not
experienced apparent asbestos or silica dust exposure. Follow-up
had been performed at Rokko Island Hospital (Kobe, Japan) for the
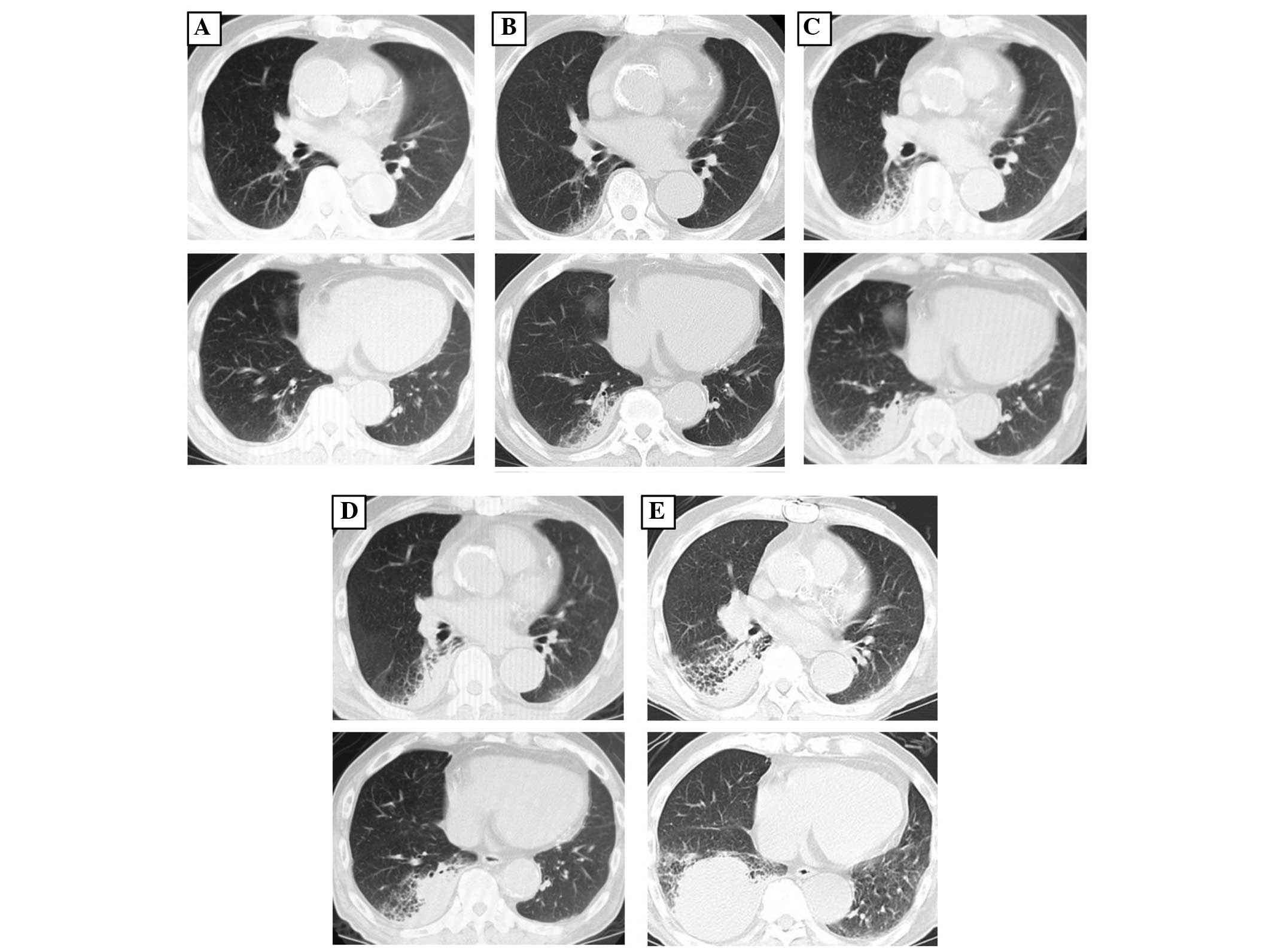
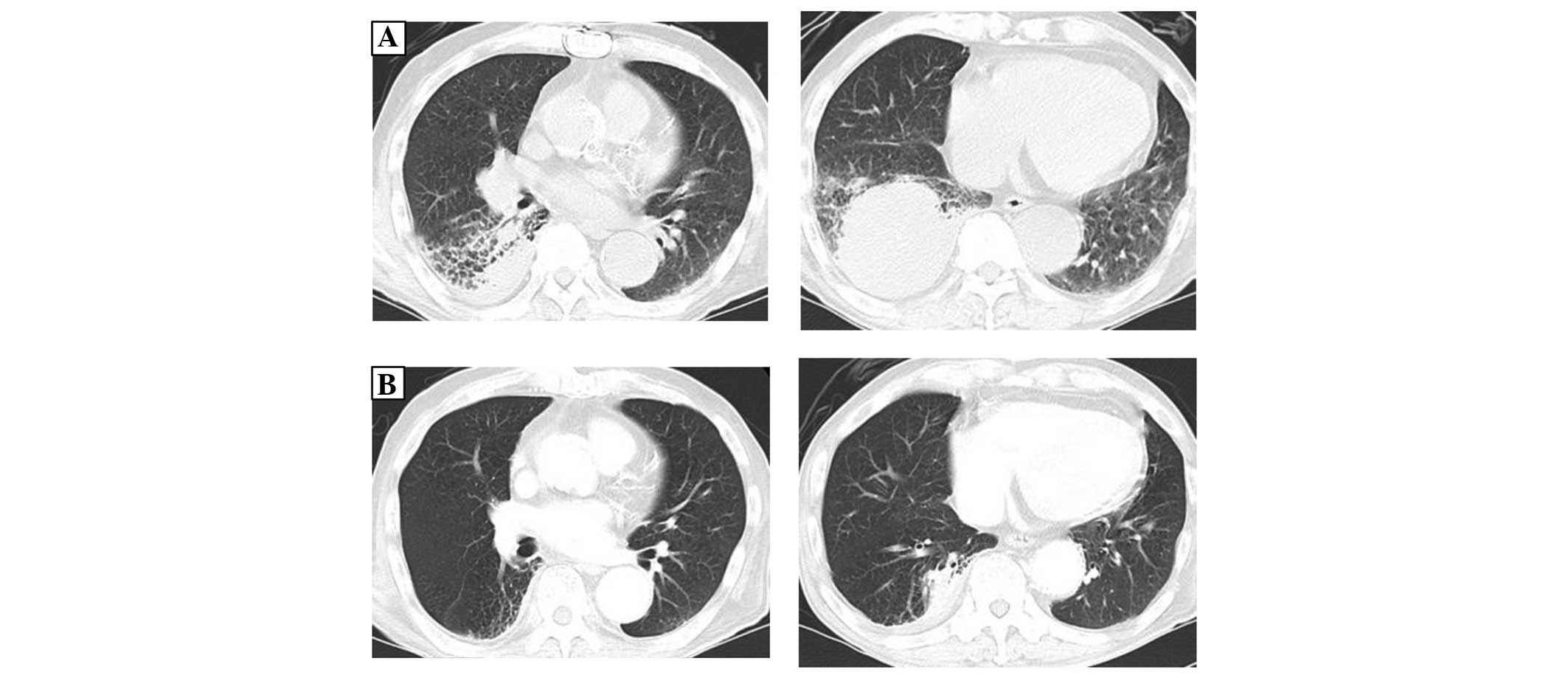
five years prior to the referral (Fig.
1A). Medical attention had been sought at Rokko Island Hospital
three years prior to referral for hemoptysis, and a consolidation
in the right lower lung field was identified at that time (Fig. 1B). The consolidation was followed up
as chronic aspiration pneumonia. Bronchoscopy was performed two
years prior to the current admission as the consolidation adjacent
to the pleura had enlarged (Fig. 1C),
but no specific findings were noted. The consolidation was
considered to be chronic aspiration pneumonia since the size varied
over time, but it had gradually increased in size in the five years
prior to the referral. The patient was referred to Kobe City
Medical Center General Hospital as a novel mass in the right lower
lobe had appeared and rapidly increased in size, which was
accompanied by elevation of soluble interleukin-2 receptor (sIL2R;
Fig. 1D and E).
Physical examination revealed that respiratory
sounds were decreased in the right lower lung. A chest radiograph
revealed a mass and consolidation in the right lower lung. A
computed tomography scan revealed an expanding mass and
consolidation in a region of emphysema adjacent to the pleura in
the right lower lobe (Fig. 1E). A
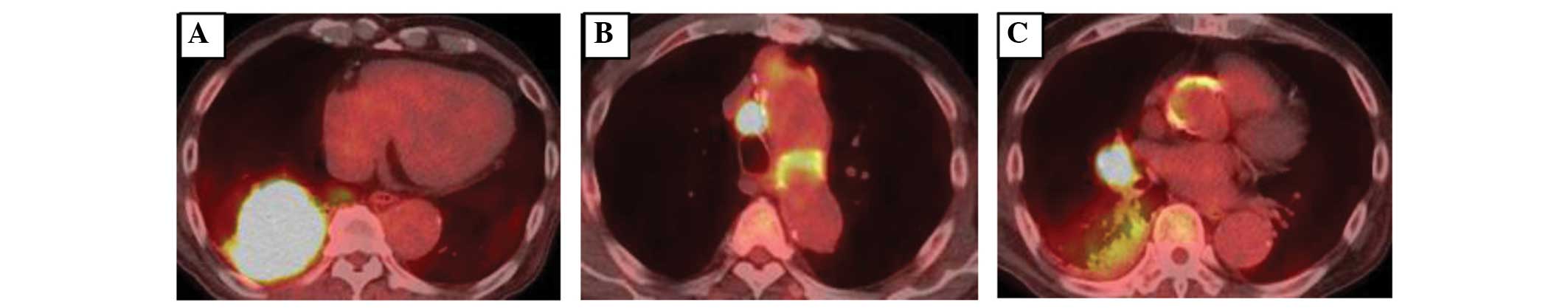
positron emission tomography scan revealed high uptake of
fluorodeoxyglucose in the mass in the right side of the lung
[maximum standardized uptake value (SUVmax), 24.3] and
mediastinal lymph nodes (SUVmax, 18.2) but the uptake
was low in the consolidation region (SUVmax, 3.5)
(Fig. 2). Laboratory examinations
revealed a white blood cell count of 12400 cells/mm3,
comprising 82% neutrophils, a C-reactive protein level of 6.4
mg/dl, a neuron-specific enolase level of 19.2 ng/ml, a
progastrin-releasing peptide level of 33.8 pg/ml, a
carcinoembryonic antigen level of 5.4 ng/ml, a cytokeratin 19
fragment level of 3.4 ng/ml, a squamous cell carcinoma-related
antigen level of 2.1 ng/ml, and a sIL2R level of 1756 units/ml.
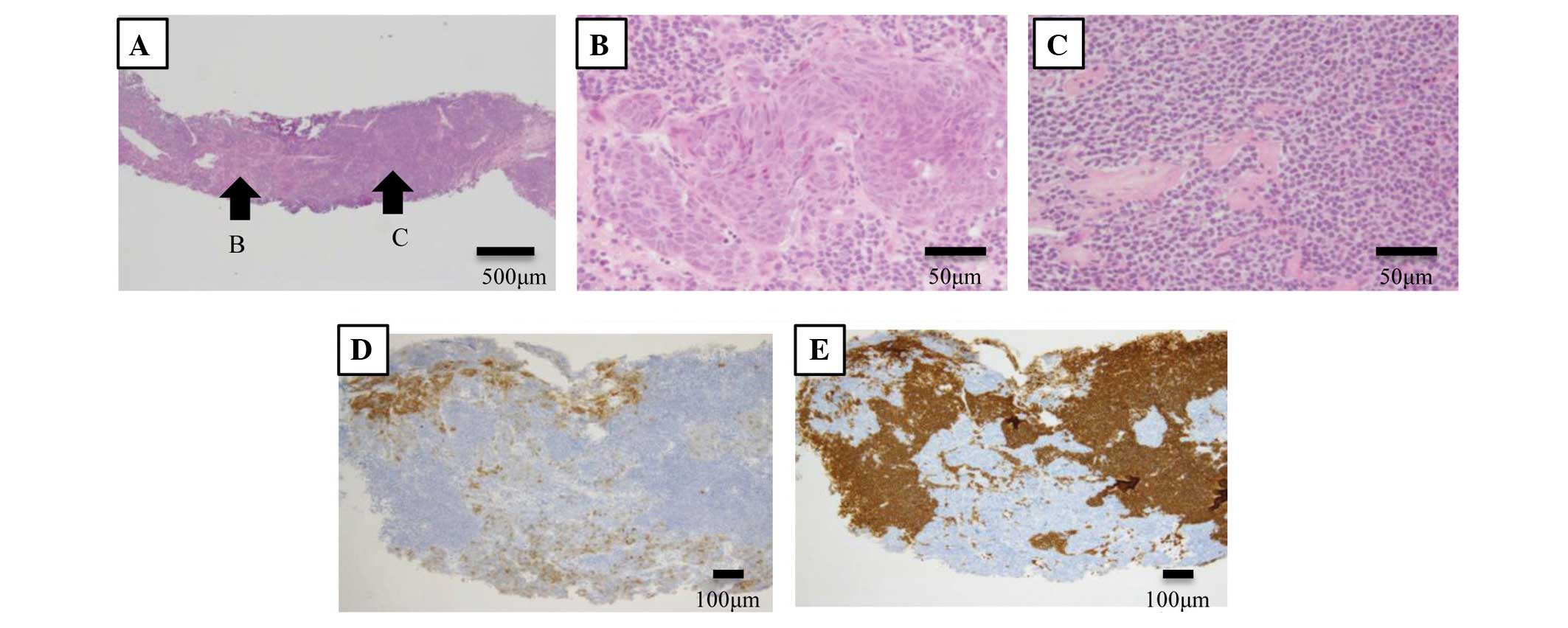
Histological examination of repeat bronchoscopic and echo-guided
biopsies of the right mass and consolidation revealed two types of
abnormal cells (Fig. 3A). One cell
type was carcinoma exhibiting a rosette pattern (Fig. 3B), which was positive for cytokeratin
and cluster of differentiation (CD)56 expression, while the other
cell type was aggregated small lymphocytes (Fig. 3C) that were CD20-positive but
CD3-negative. Certain regions were positive for CD56 expression,
while other areas in the tumor were positive for CD20 expression
(Fig. 3D and E), which was considered
a mix of neuroendocrine carcinoma, either LCNEC or small cell
carcinoma, and MALT lymphoma. Endobronchial ultrasound-guided
transbronchial needle aspiration was performed on the swollen 4R
lymph node to determine the stage of the lesion. The aspiration
revealed a medium to large-sized endocrine carcinoma, with a
rosette pattern that was diffusely cytokeratin-positive and did not
express CD20 and CD56. Therefore, it was hypothesized that the
lesion resulted from the metastasis of LCNEC. The patient was
diagnosed with LCNEC at a clinical tumor-node-metastasis stage
(cTNM) of cT2bN2M0 (stage 3A) and MALT lymphoma at Ann Arbor stage
1E.
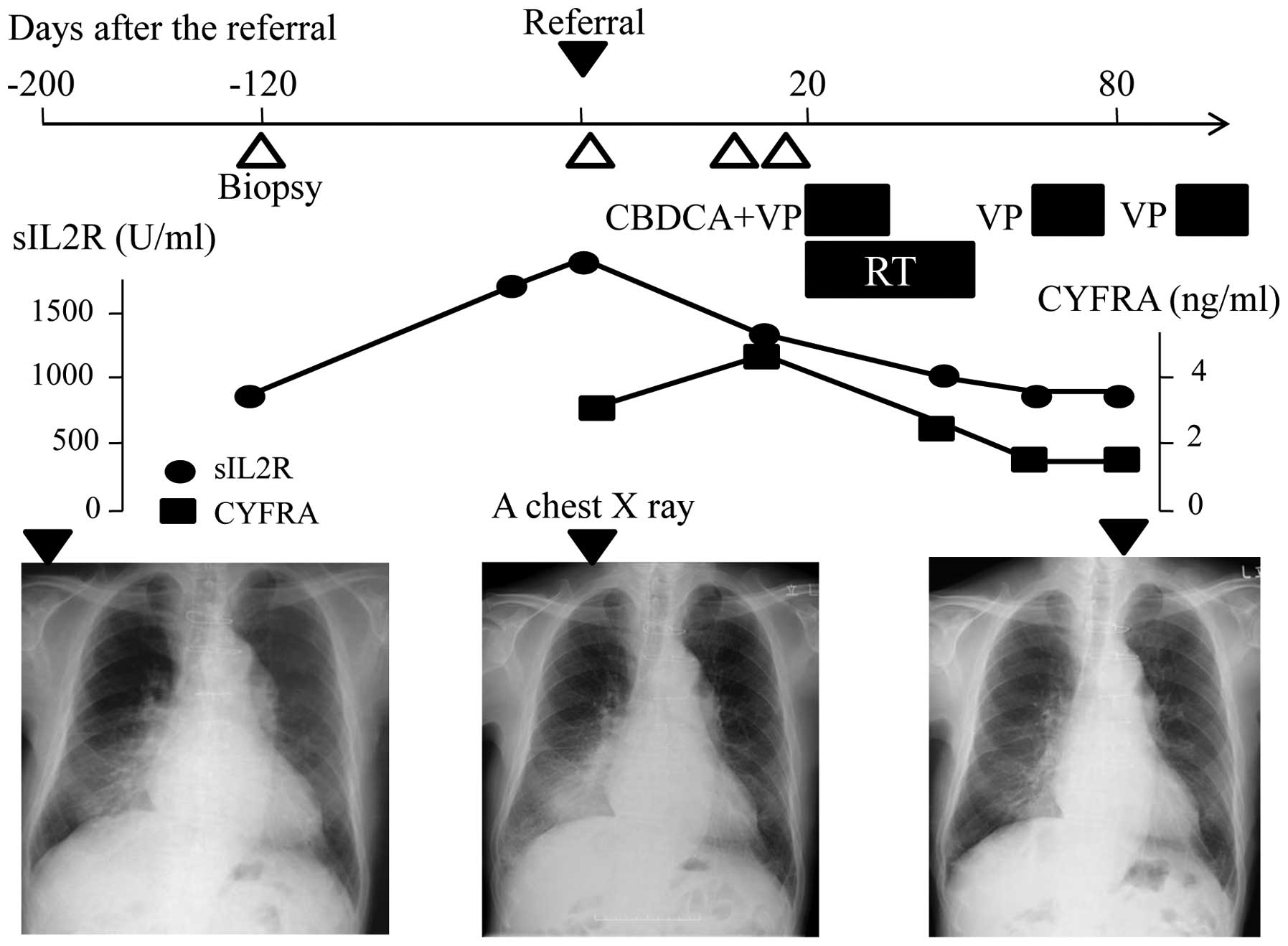
Chemotherapy, consisting of carboplatin (area under
the curve, 4; day 1) and etoposide (80 mg/m2; days 1–3),
combined with radiation therapy (45 Gy; 30 fractions) were
commenced, which markedly affected the mass and consolidation
(Fig. 4). Subsequent to four cycles
of chemotherapy, the mass was significantly reduced in size and the
consolidation was also reduced (Fig.
5). The effect was considered to be a near-complete response,
which continued for 144 days. Subsequently, liver and bone
metastases appeared and the patient declined further chemotherapy.
Although the lung tumor and consolidation did not enlarge, the
metastatic lesions increased in size. Eight months subsequent to
referral, the patient succumbed to hepatic failure caused by liver
metastasis.
Discussion
The present study, to the best of our knowledge,
reports the first case of a patient with combined LCNEC and MALT
lymphoma of the lung that responded well to chemoradiotherapy and
was followed over six years.
MALT lymphoma is a low grade B cell lymphoma that
mostly occurs in the gastrointestinal tract, accounting for only
0.5% of lung tumors. Therefore, pulmonary MALT lymphoma is
extremely rare (2,3). LCNEC is a tumor that is regarded as a
variant of large cell carcinoma by the World Health Organization.
LCNEC is also rare, accounting for 2.4% of lung cancers. The
biological features of LCNEC are similar to those of small cell
carcinoma and the prognosis is extremely poor (8,9). Surgery
is performed to treat LCNEC, if possible, but the current results
are not satisfactory. Although a standard therapy for LCNEC has not
been established, chemoradiotherapy similar to that administered
for the treatment of small cell carcinoma is administered to
patients with inoperable LCNEC (11).
It has been speculated that MALT lymphoma of the
lung is associated with chronic respiratory infection, chronic
inflammatory diseases, autoimmune diseases or antigen exposure,
such as exposure to tobacco (12,13). The
median time to progression for pulmonary MALT lymphoma has been
reported to be 5.6 years, and the prognosis was the same regardless
of whether treatment consisted of chemotherapy or surgery (14,15). In
addition, Troch et al (6)
reported that pulmonary MALT lymphoma may not require immediate
treatment in asymptomatic patients and thus, a watch-and-wait
strategy may be adopted.
Cases of combined MALT lymphoma and gastric cancer
have been reported frequently. However, the combination of lung
cancer with MALT lymphoma is rare and few cases have been reported
at present (16–21). To the best of our knowledge, the
combination of LCNEC and MALT lymphoma has been reported in only
one other case (18), in which the
patient suffered from sarcoidosis. Surgery was performed for the
treatment of a mass shadow in the left lower lobe of the lung, and
the concurrent occurrence of LCNEC and MALT lymphoma was an
incidental finding. In the present case, the consolidation adjacent
to the pleura was thought to be MALT lymphoma and the novel mass
was LCNEC, since the consolidation appeared initially and increased
in size extremely slowly, whereas the mass appeared later and
rapidly became enlarged.
As the combination of lung cancer with MALT lymphoma
is rare, the mechanism is not well-elucidated. However, possible
causes of the formation of MALT lymphoma and lung cancer were
reported to be either associated with the API2-MALT1 fusion gene or
smoking (16,17). By contrast, when the rate of
combination of malignant lymphoma with other types of cancer was
statistically analyzed, the combination with lung cancer was
reported to be a coincidental occurrence (22). The present patient was followed for
six years, and this observation may indicate that stimulation from
the MALT lymphoma contributed to the formation of lung cancer.
However, there is insufficient data on the combination of lung
cancer with MALT lymphoma. This concept requires further
elucidation through the examination of additional cases.
In conclusion, the present study reported the case
of a patient with combined LCNEC and MALT lymphoma of the lung that
was followed over six years, including the onset of the
disease.
References
|
1
|
Isaacson P and Wright DH: Malignant
lymphoma of mucosa-associated lymphoid tissue. A distinctive type
of B-cell lymphoma. Cancer. 52:1410–1416. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Du MQ: MALT lymphoma: recent advances in
aetiology and molecular genetics. J Clin Exp Hematop. 47:31–42.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chilosi M, Zinzani PL and Poletti V:
Lymphoproliferative lung disorders. Semin Respir Crit Care Med.
26:490–501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cadranel J, Wislez M and Antoine M:
Primary pulmonary lymphoma. Eur Respir J. 20:750–762. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Addis BJ, Hyjek E and Isaacson PG: Primary
pulmonary lymphoma: a re-appraisal of its histogenesis and its
relationship to pseudolymphoma and lymphoid interstitial pneumonia.
Histopathology. 13:1–17. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Troch M, Streubel B, Petkov V, Turetschek
K, Chott A and Raderer M: Does MALT lymphoma of the lung require
immediate treatment? An analysis of 11 untreated cases with
long-term follow-up. Anticancer Res. 27:3633–3637. 2007.PubMed/NCBI
|
|
7
|
Travis WD, Linnoila RI, Tsokos MG, et al:
Neuroendocrine tumors of the lung with proposed criteria for
large-cell neuroendocrine carcinoma. An ultrastructural,
immunohistochemical and flow cytometric study of 35 cases. Am J
Surg Pathol. 15:529–553. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iyoda A, Hiroshima K, Toyozaki T, Haga Y,
Fujisawa T and Ohwada H: Clinical characterization of pulmonary
large cell neuroendocrine carcinoma and large cell carcinoma with
neuroendocrine morphology. Cancer. 91:1992–2000. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iyoda A, Hiroshima K, Nakatani Y and
Fujisawa T: Pulmonary large cell neuroendocrine carcinoma: its
place in the spectrum of pulmonary carcinoma. Ann Thorac Surg.
84:702–707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iyoda A, Hiroshima K, Moriya Y, Sekine Y,
Shibuya K, Iizasa T, Nakatani Y and Fujisawa T: Prognostic impact
of large cell neuroendocrine hisotology in patients with pathologic
stage Ia pulmonary non-small cell carcinoma. J Thorac Cardiovasc
Surg. 132:312–315. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujiwara Y, Sekine I, Tsuta K, et al:
Effect of platinum combined with irinotecan or paclitaxel against
large cell neuroendocrine carcinoma of the lung. Jpn J Clin Oncol.
37:482–486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thieblemont C, Bastion Y, Berger F, et al:
Mucosa-associated lymphoid tissue gastrointestinal and
nongastrointestinal lymphoma behavior: analysis of 108 patients. J
Clin Oncol. 15:1624–1630. 1997.PubMed/NCBI
|
|
13
|
Stagnaro E, Tumino R, Parodi S, et al:
Non-Hodgkin's lymphoma and type of tobacco smoke. Cancer Epidemiol
Biomarkers Prev. 13:431–437. 2004.PubMed/NCBI
|
|
14
|
Oh SY, Kim WS, Kim JS, et al: Pulmonary
marginal zone B-cell lymphoma of MALT type - what is a prognostic
factor and which is the optimal treatment, operation, or
chemotherapy?: Consortium for Improving Survival of Lymphoma (CISL)
study. Ann Hematol. 89:563–568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thieblemont C, Berger F, Dumontet C, et
al: Mucosa-associated lymphoid tissue lymphoma is a disseminated
disease in one third of 158 patients analyzed. Blood. 95:802–806.
2000.PubMed/NCBI
|
|
16
|
Ichihara E, Tabata M, Takigawa N, et al:
Synchronous pulmonary MALT lymphoma and pulmonary adenocarcinoma
after metachronous gastric MALT lymphoma and gastric
adenocarcinoma. J Thorac Oncol. 3:1362–1363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki T, Akizawa T, Suzuki H, Kitazume K,
Omine M and Mitsuya T: Primary tracheal mucosa-associated lymphoid
tissue lymphoma accompanying lung cancer. Common tumorigenesis or
coincidental coexistence? Jpn J Thorac Cardiovasc Surg. 48:817–819.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Itoh T, Kobayashi D, Shiratuchi N, Rensha
K and Minami K: Case of overlapping cancers complicated with
sarcoidosis. Nihon Kokyuki Gakkai Zasshi. 47:410–414. 2009.(In
Japanese). PubMed/NCBI
|
|
19
|
Chanel S, Burke L, Fiche M, et al:
Synchronous pulmonary adenocarcinoma and extranodal marginal
zone/low-grade B-cell lymphoma of MALT type. Hum Pathol.
32:129–132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung CY and Kwon KY: A case of synchronous
lung adenocarcinoma and extranodal marginal zone b-cell lymphoma of
mucosa-associated lymphoid tissue (MALT) type. Tuberc Respir Dis
(Seoul). 73:61–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adrish M, Venkatram S, Niazi M and
Diaz-Fuentes G: Concurrent lung squamous cell carcinoma and
extranodal marginal zone B-cell lymphoma of mucosa-associated
lymphoid tissue type. J Bronchology Interv Pulmonol. 21:96–99.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tihan T and Filippa DA: Coexistence of
renal cell carcinoma and malignant lymphoma. A causal relationship
or coincidental occurrence? Cancer. 77:2325–2331. 1996. View Article : Google Scholar : PubMed/NCBI
|