Introduction
The incidence of acute myeloid leukemia (AML)
increases with age. Elderly patients have gradually become the main
individuals affected by AML due to an ageing population. AML
patients in developed countries are diagnosed at an average age of
71 years (1). Typically, the
threshold for leukemia in elderly patients is considered to be
between 55 and 65 years old (2). The
prognosis in patients <50 years old has been found to be
significantly different from that in patients between 50 and 65
years old (3). Compared with younger
patients, elderly patients with AML exhibit more karyotypes
associated with a poor prognosis (4,5), higher
expression rates of gene mutations associated with drug resistance
(6), and are more frequently
secondary to myelodysplastic syndrome (7,8).
Therefore, the prognosis of patients with AML declines with age.
The median overall survival (OS) time of AML patients aged 16–55,
56–65, 66–75 and ≥75 years has been recorded as 1,119, 359, 180 and
80 days, respectively (1).
In 2010, Guo et al (9) first reported that the 2-year OS rate in
elderly patients with AML increased from 11 to 39% with fractional
infusion of small amounts of allogeneic hematopoietic stem cells
(microtransplantation) following chemotherapy. Microtransplantation
was reported to have anti-leukemic effects and promoted
hematopoietic recovery (9,10). Although the prognosis in elderly
patients with AML has been significantly improved by
microtransplantation combined with chemotherapy, it remains
inferior compared with that in young patients. For AML patients
<65 years old, when microtransplantation was added to
chemotherapy, the 6-year OS rate for low- and medium-risk patients
reached 89.5 and 65.2%, respectively (10). These results indicate that there is
room for improvement in the efficacy of this treatment paradigm in
elderly patients with AML. To accomplish this, maintenance therapy
by low-dose chemotherapeutic treatment combined with
microtransplantation was performed in three elderly patients
(>55 years old) with leukemia, and the findings are reported in
the present study.
Patients and methods
Patients
Three elderly patients with acute leukemia,
consisting of two cases of AML and one case of acute mixed lineage
leukemia (AMLL), were admitted to the Department of Hematology of
the 303rd Hospital of the People's Liberation Army (PLA; Nanning,
Guangxi, China) between January 2011 and January 2014.
Microtransplantation is characterized by a smaller number of
transfused stem cells (~1/3–1/4 of the dosage compared with
allogeneic hematopoietic stem cell transplantation) in addition a
pre-conditioning regime is unecessary. Therefore, for elderly
patients, microtransplantation apears to be more tolerable than
allogeneic hematopoietic stem cell transplantation, which also has
a higher financial cost for treatment. Microtransplantation was
performed in all patients once informed consent had been obtained,
and under the approval of the Medical Ethics Committee of the 303rd
Hospital of the PLA.
Mobilization and collection of
peripheral blood stem cells
Healthy donors were selected, with preference for
haplo-identical human leukocyte antigen-matched donors. Recombinant
human granulocyte colony-stimulating factor (Kirin-Sankyo Co.,
Ltd., Tokyo, Japan) was subcutaneously injected at a dose of 5
µg/kg/day to mobilize the peripheral blood stem cells daily for 5
days. The peripheral blood stem cells were collected on days 5 and
6 with CS-3000 plus blood cell separators (Baxter Healthcare Ltd.,
Berkshire, UK).
Maintenance therapy regimes
As detailed in Table
I, all patients received maintenance treatment following
complete response (CR) induction and intensified consolidation
chemotherapy. For the patients who received chemotherapy, donor
stem cell infusion was performed at 36 h post-chemotherapy. In
total, 2–4×108/kg mononuclear cells were infused each
time. Single-dose 5 mg dexamethasone was used to prevent allergy.
Other immunosuppressants for the prevention of graft versus host
disease (GVHD) were not used.
 | Table I.General conditions and
microtransplantation efficacy in three patients treated by
microtransplantation. |
Table I.
General conditions and
microtransplantation efficacy in three patients treated by
microtransplantation.
| Parameter | Patient 1 | Patient 2 | Patient 3 |
|---|
| Patient data |
|
|
|
|
Gender | Male | Female | Male |
| Age,
years | 59 | 58 | 62 |
|
Karyotype | 46(X,Y) | 46(X,X) | 46(X,Y) |
| Fused
genes | – | – | – |
|
Diagnosis | AMLL | AML-M5b | AML-M5b |
| Donor data |
|
|
|
|
Sources | Son | Son | Son |
| Age,
years | 33 | 31 | 29 |
| Blood
types of donors/recipients | O/O | O/O | B/B |
| HLA
matching of donors and recipients | 3/6 | 4/6 | 3/6 |
| Number of
stem cell collections | 1 | 2 | 1 |
| Date of
stem cell collection in donors | 2012.01 | 2012.11/2013.05 | 2013.04 |
| Cell counts |
|
|
|
| MNC
(108/kg recipient) | 1.3 | 15.5 | 2.9 |
|
CD34+ (106/kg
recipient) | 0.652 | 5.2 | 1.5 |
| Efficacy of
microtransplantation |
|
|
|
|
Chemotherapy prior to
maintenance | Medium-dose
Ara-C±MTX/VP-16d,
followed by auto-HSCT | Medium-dose
Ara-C±VP-16d | High-dose
Ara-Cc |
|
Chemotherapy prior to
microtransplantation during maintenance | Medium-dose | Standard-dose | Standard-dose |
|
| Ara-Ca | Ara-Cc | Ara-Cb |
| Courses
of microtransplantation, n | 5 | 7 | 5 |
|
Efficacy | CR | CR | CR |
|
Outcome | DFS, 27 months | DFS, 20 months | DFS, 16 months |
Evaluation of efficacy and adverse
reactions
CR was defined by the presence of <5% bone marrow
blasts, no extramedullary infiltration of leukemic cells and normal
results in the peripheral hemocyte analysis. The follow-up deadline
was April 30, 2014. Disease-free survival (DFS) was defined as the
duration from CR induction to the follow-up deadline.
Results
General data
The three patients were aged between 58 and 62 years
old and consisted of two males and one female. The detailed
diagnosis and treatment processes of the three cases are shown in
Table I.
Treatment efficacy
The detailed treatment process of the patient with
AMLL has previously been reported (11), and the patient was further followed in
the present study. In brief, subsequent to autologous stem cell
transplantation and sequential allogeneic hematopoietic stem cell
microtransplantation, the patient had achieved DFS for 27 months.
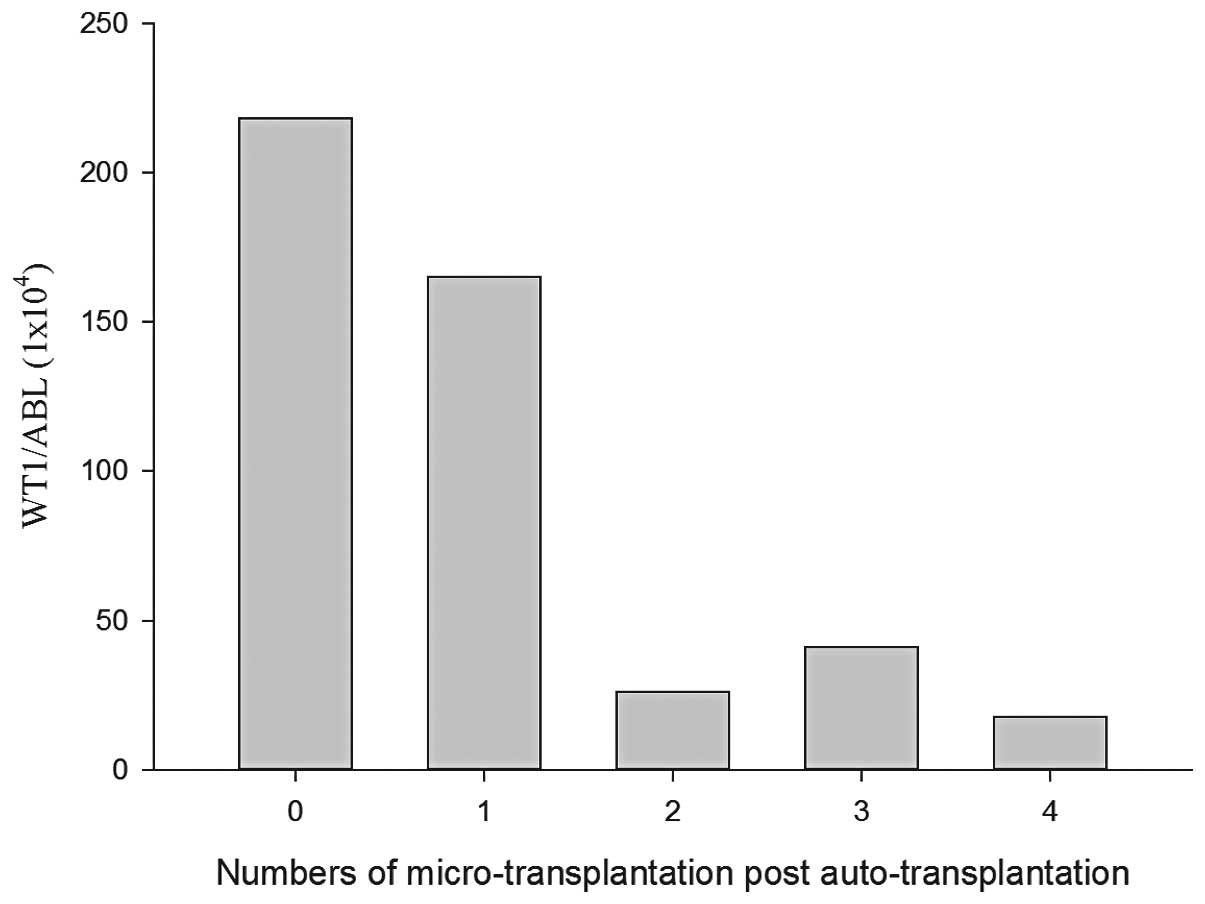
The bone marrow WT1 levels in the patient decreased following
microtransplantation and declined more significantly with an
increased number of courses of microtransplantation (Fig. 1). Upon reexamination in April 2014,
the patient maintained the CR state.
Cases 2 and 3 were AML patients with
intermediate-risk in chromosomal and molecular biological
evaluations. The two patients achieved CR induction using a
combined treatment paradigm of daunorubicin, arabinosylcytosine
(Ara-C) and etoposide (also known as the DAE regimen). Following
the DAE regimen treatment, medium-dose Ara-C (1.0–1.5
g/m2 every 12 h on days 1, 3 and 5) and combined Ara-C
(0.5–1.0 g/m2 every 12 h on days 1, 3 and 5) and
etoposide (50–70 mg/m2 every 12 h on days 1, 3 and 5)
was alternatively used for consolidation treatment (referred to as
AE treatment). The donor stem cells were infused at 36 h
post-chemotherapy. After two to four courses of consolidation
treatment by medium-dose Ara-C/AE, chemotherapy prior to
microtransplantation was changed to medium-dose Ara-C (0.5–1.0
g/m2 every 12 h on days 1, 3 and 5) or standard Ara-C
(0.4 g/m2 every 12 h on days 1, 3 and 5) with an
interval of 4–5 months. The last follow-up showed that the two
patients remained disease-free.
Adverse effects
Of the 17 times that microtransplantation treatment
was administered in the three patients, discomforts such as fever
and diarrhea were not observed. A transient rash and pruritus
occurred twice during transfusion and the patients recovered
without treatment.
Discussion
For elderly AML patients aged 55–65 years, even
intensive chemotherapy has been shown to only achieve an average OS
time of 530 days, which is considerably shorter than the 2,546 days
observed in patients <55 years old (1). For those patients with intermediate-risk
AML, the average OS time is only 12 months (12). The prognosis of patients with AMLL is
worse than that of elderly patients with AML, with a median
survival time of only 9 months if chemotherapy only was recommended
(13). Of the three patients in the
present study, one case with AMLL was treated by autologous
transplantation followed by microtransplantation as maintenance
therapy and two cases with intermediate-risk AML were treated and
maintained by microtransplantation combined with chemotherapy. All
three patients achieved a DFS time of >16 months (mean, 21
months), suggesting that maintenance with microtransplantation
combined with chemotherapy could improve the prognosis in elderly
patients with AML. These results were consistent with the results
from the study by Guo et al (9,10). The
study found that the 2 year OS and event free survival (EFS) rates
in elderly patients with AML treated by chemotherapy combined with
microtransplantation were increased significantly compared with
those of patients treated by chemotherapy alone.
Typically, maintenance therapy is not recommended
for patients with AML. In a study consisting of 789 AML patients
aged 15–64 years, patients post-CR induction were randomly divided
into a group administered four cycles of standard chemotherapy
consolidation and a group administered maintenance after three
cycles of standard chemotherapy consolidation. The results showed
that there was no significant difference in the DFS and
progression-free survival rates between the two groups, suggesting
that patients who completed intensive consolidation may not benefit
from maintenance therapy (14).
However, elderly patients with AML often cannot tolerate high-dose
chemotherapy owing to their declining physical health. In a study
of patients with AML aged 46–60 years, the increased intensity of
consolidation treatment following CR induction reduced the relapse
rate, but the benefits were counteracted by increased
treatment-related mortality and the overall survival rate was not
improved, compared with the conventional consolidation group
(15). Buchner et al (16) randomly divided 832 AML patients who
were >16 years of age into an intensive treatment group and a
maintenance treatment group. The Ara-c dose in the consolidation
program used in the patients >60 years of age was adjusted from
3 to 1 g/m2, and the results showed that the patients
>60 years of age benefited from the maintenance therapy. Ferrero
et al (17) also found that
maintenance therapy for patients with AML who were unsuitable for
chemotherapy could reduce the recurrence rate and improve the
long-term survival rate. Therefore, elderly patients with AML may
be more dependent on maintenance therapy to reduce the incidence of
relapses and improve the prognosis, as the low intensity of
chemotherapy during consolidation treatment may not be high enough
to eradicate residual leukemia.
Lowenberg et al (18) administered maintenance therapy by
low-dose Ara-C in elderly AML patients. The results showed that the
DFS rate of the patients was significantly improved, but that the
OS rate was not, as the non-relapse mortality rate in the
maintenance group was increased. Microtransplantation of allogeneic
hematopoietic stem cells may not only promote hematopoietic
recovery and improve the tolerance to chemotherapy, but it may also
activate specific anti-leukemia effects in elderly patients
(9,10). We hypothesized that
microtransplantation may be more feasible and effective in
maintenance therapy for elderly patients with AML.
Treatment-related mortality was not observed in the three patients
who received the maintenance therapy of microtransplantation
combined with low-dose chemotherapy following intensive therapy in
the present study. WT1 could be used as an indicator of residual
disease, and an increase in WT1 level may indicate early relapse
(19). In the present study, during
the maintenance therapy, dynamic changes in the WT1 level were
monitored in one patient. The results showed that the WT1 level
gradually declined with an increasing number of
microtransplantations, confirming that maintenance therapy with
microtransplantation could be effective in eradicating residual
disease. During the course of maintenance therapy using
microtransplantation, no patient suffered from GVHD, suggesting
that microtransplantation was safe to use as maintenance
therapy.
References
|
1
|
Juliusson G, Antunovic P, Derolf A, et al:
Age and acute myeloid leukemia: real world data on decision to
treat and outcomes from the Swedish Acute Leukemia Registry. Blood.
113:4179–4187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vey N: Targeting age-related changes in
the biology of acute myeloid leukemia: is the patient seeing the
progress? Interdiscip Top Gerontol. 38:73–84. 2013.PubMed/NCBI
|
|
3
|
Yanada M, Ohtake S, Miyawaki S, et al:
Japan Adult Leukemia Study Group: The demarcation between younger
and older acute myeloid leukemia patients: a pooled analysis of 3
prospective studies. Cancer. 119:3326–3333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimoni A, Kroger N, Zabelina T, et al:
Hematopoietic stem-cell transplantation from unrelated donors in
elderly patients (age >55 years) with hematologic malignancies:
older age is no longer a contraindication when using reduced
intensity conditioning. Leukemia. 19:7–12. 2005.PubMed/NCBI
|
|
5
|
Lazarevic V, Horstedt AS, Johansson B, et
al: Incidence and prognostic significance of karyotypic subgroups
in older patients with acute myeloid leukemia: the Swedish
population-based experience. Blood Cancer J. 4:e1882014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leith CP, Kopecky KJ, Chen IM, et al:
Frequency and clinical significance of the expression of the
multidrug resistance proteins MDR1/P-glycoprotein, MRP1 and LRP in
acute myeloid leukemia: a Southwest Oncology Group Study. Blood.
94:1086–1099. 1999.PubMed/NCBI
|
|
7
|
Leone G, Mele L, Pulsoni A, Equitani F and
Pagano L: The incidence of secondary leukemias. Haematologica.
84:937–945. 1999.PubMed/NCBI
|
|
8
|
Ostgård LS, Kjeldsen E, Holm MS, et al:
Reasons for treating secondary AML as de novo AML. Eur J Haematol.
85:217–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo M, Hu KX, Yu CL, et al: Infusion of
HLA-mismatched peripheral blood stem cells improves the outcome of
chemotherapy for acute myeloid leukemia in elderly patients. Blood.
117:936–941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo M, Hu KX, Liu GX, et al:
HLA-mismatched stem-cell microtransplantation as postremission
therapy for acute myeloid leukemia: long-term follow-up. J Clin
Oncol. 30:4084–4090. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YS, Kong XJ, Zhang XH and Yin XL:
Treatment of acute mixed-cell leukemia with autologous
hematopoietic SCT followed by allogeneic hematopoietic stem cell
micro-transplantation. Bone Marrow Transplant. 49:984–985. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Appelbaum FR, Gundacker H, Head DR, et al:
Age and acute myeloid leukemia. Blood. 107:3481–3485. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Seraihy AS, Owaidah TM, Ayas M, El-Solh
H, Al-Mahr M, Al-Ahmari A and Belgaumi AF: Clinical characteristics
and outcome of children with biphenotypic acute leukemia.
Haematologica. 94:1682–1690. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyawaki S, Sakamaki H, Ohtake S, et al:
Japan Adult Leukemia Study Group AML 97 Study: A randomized,
postremission comparison of four courses of standard-dose
consolidation therapy without maintenance therapy versus three
courses of standard-dose consolidation with maintenance therapy in
adults with acute myeloid leukemia: the Japan Adult Leukemia Study
Group AML 97 Study. Cancer. 104:2726–2734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hengeveld M, Suciu S, Karrasch M, et al:
Intensive consolidation therapy compared with standard
consolidation and maintenance therapy for adults with acute myeloid
leukaemia aged between 46 and 60 years: final results of the
randomized phase III study (AML 8B) of the European Organization
for Research and Treatment of Cancer (EORTC) and the Gruppo
Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA)
Leukemia Cooperative Groups. Ann Hematol. 91:825–835. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Büchner T, Hiddemann W, Berdel WE, et al:
German AML Cooperative Group: 6-Thioguanine, cytarabine and
daunorubicin (TAD) and high-dose cytarabine and mitoxantrone (HAM)
for induction, TAD for consolidation and either prolonged
maintenance by reduced monthly TAD or TAD-HAM-TAD and one course of
intensive consolidation by sequential HAM in adult patients at all
ages with de novo acute myeloid leukemia (AML): a randomized trial
of the German AML Cooperative Group. J Clin Oncol. 21:4496–4504.
2003.PubMed/NCBI
|
|
17
|
Ferrero D, Crisà E, Marmont F, et al:
Survival improvement of poor-prognosis AML/MDS patients by
maintenance treatment with low-dose chemotherapy and
differentiating agents. Ann Hematol. 93:1391–1400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lö Wenberg B, Suciu S, Archimbaud E, et
al: Mitoxantrone versus daunorubicin in induction-consolidation
chemotherapy - the value of low-dose cytarabine for maintenance of
remission, and an assessment of prognostic factors in acute myeloid
leukemia in the elderly: final report. European Organization for
the Research and Treatment of Cancer and the Dutch-Belgian
Hemato-Oncology Cooperative Hovon Group. J Clin Oncol. 16:872–881.
1998.PubMed/NCBI
|
|
19
|
Polák J, Hájková H, Maalaufová-Soukupová
J, et al: Estimation of molecular upper remission limit for
monitoring minimal residual disease in peripheral blood of acute
myeloid leukemia patients by WT1 expression. Exp Ther Med.
3:129–133. 2012.PubMed/NCBI
|