Introduction
The majority of cases of signet-ring cell
adenocarcinoma (SRCA) of the lung originate from the
gastrointestinal tract. Primary SRCA of the lung, originally
described in 1989 by Kish et al (1), is an extremely rare subtype of
adenocarcinoma, which confers a poor prognosis compared with other
subtypes of adenocarcinoma (2).
Previous studies have identified that a high proportion of
EML4-ALK-positive non-small cell lung cancer (NSCLC) tumors in
North America possess SRC components (3,4).
Therefore, the presence of an SRC component may be an important
clinicopathological feature of EML4-ALK-positive NSCLC. In 2007,
the discovery of EML4-ALK, a lung cancer-induced fusion gene, was
considered to be an advance in the understanding of NSCLC (5). Crizotinib, which was approved by the
Food and Drug Administration on August 26, 2011, is recommended for
patients who carry the EML4-ALK gene rearrangement (6). The development of an anaplastic lymphoma
kinase (ALK) protein inhibitor with approved clinical activity has
led to an understanding of the clinicopathological features and
survival rates of patients with EML4-ALK NSCLC (4,7). To the
best of our knowledge, the present study is the first to describe a
case of primary SRCA of the lung with an EML4-ALK gene
rearrangement. The clinical response to crizotinib was monitored
throughout the therapeutic process.
Case report
A 43-year-old male, who was a non-smoker, was
admitted to the Qingdao Municipal Hospital (Qingdao, China) on June
27, 2012, with a cough and dyspnea that had been apparent for one
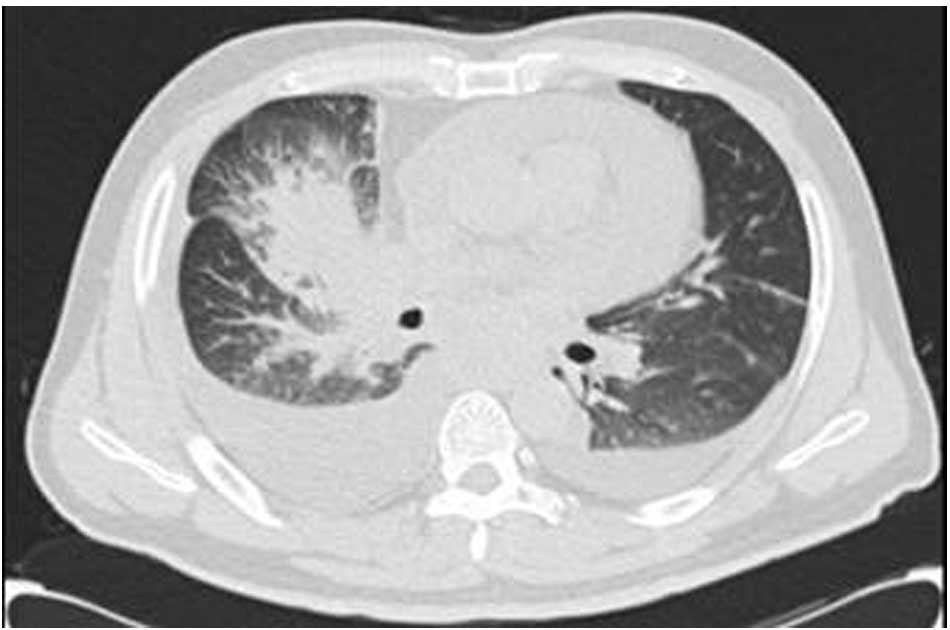
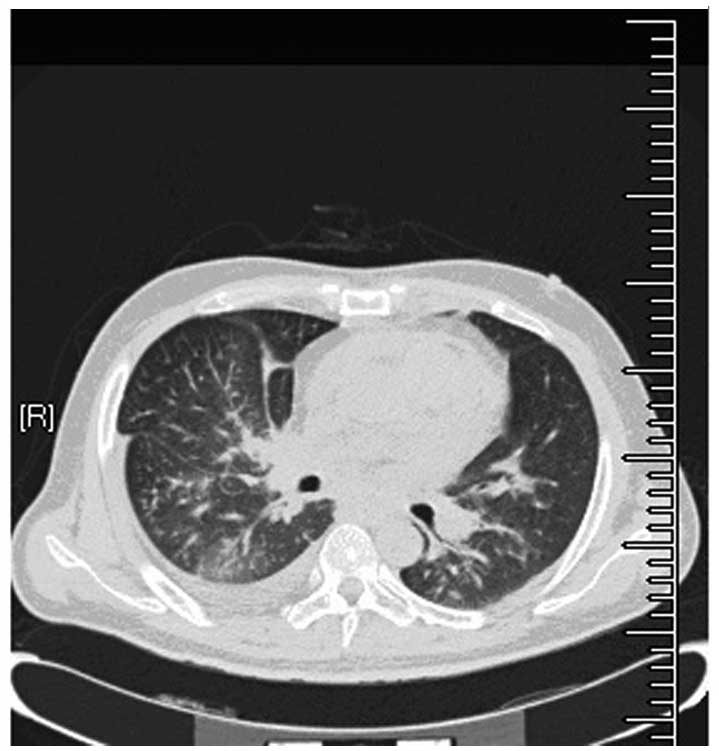
week. Chest computed tomography (CT) revealed an irregularly-shaped
mass in the middle lobe of the right lung. Bilateral pleural and
pericardial effusion was also observed (Fig. 1). The levels of the tumor markers,
cytokeratin (CK)-19 fragment 21-1, neuron-specific enolase,
carcinoembryonic antigen and cancer antigen series, were within the
normal ranges. Metastatic tumors were detected in the bone by
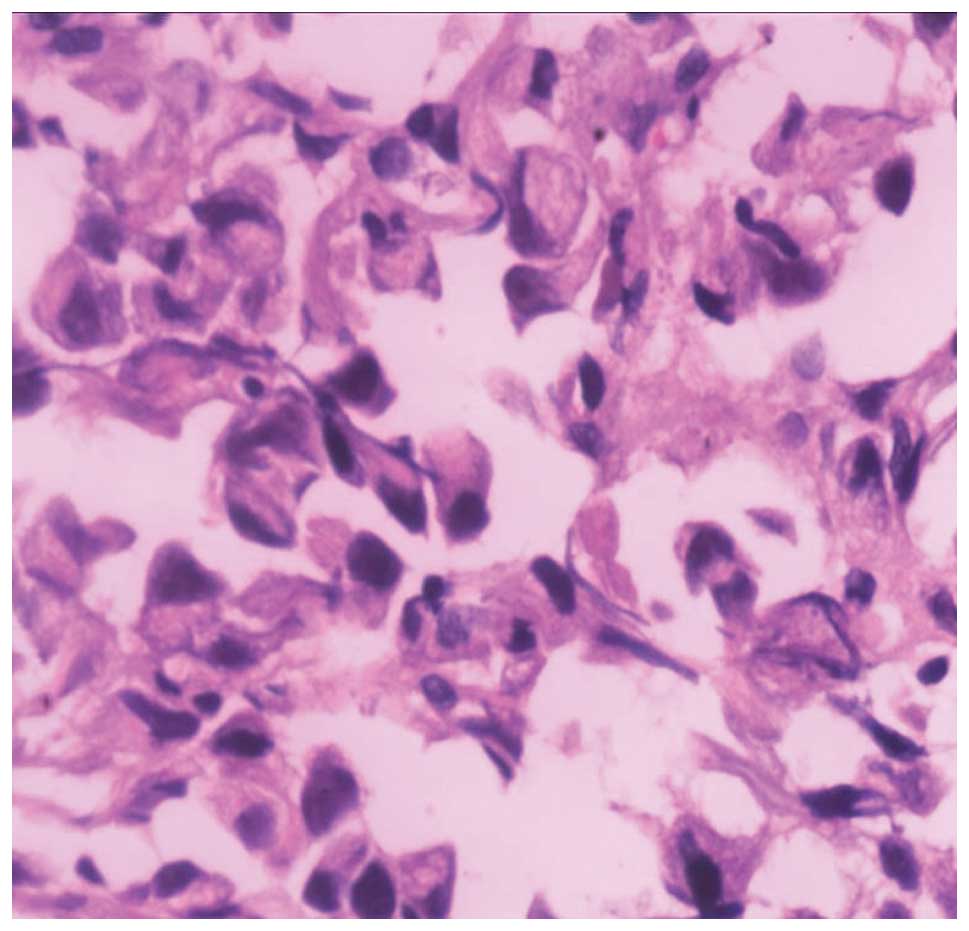
positron emission tomography/CT. The pathological findings of the
transbronchial lung biopsies revealed the presence of SRCA (70%)
mixed with poorly-differentiated adenocarcinoma cells (30%)
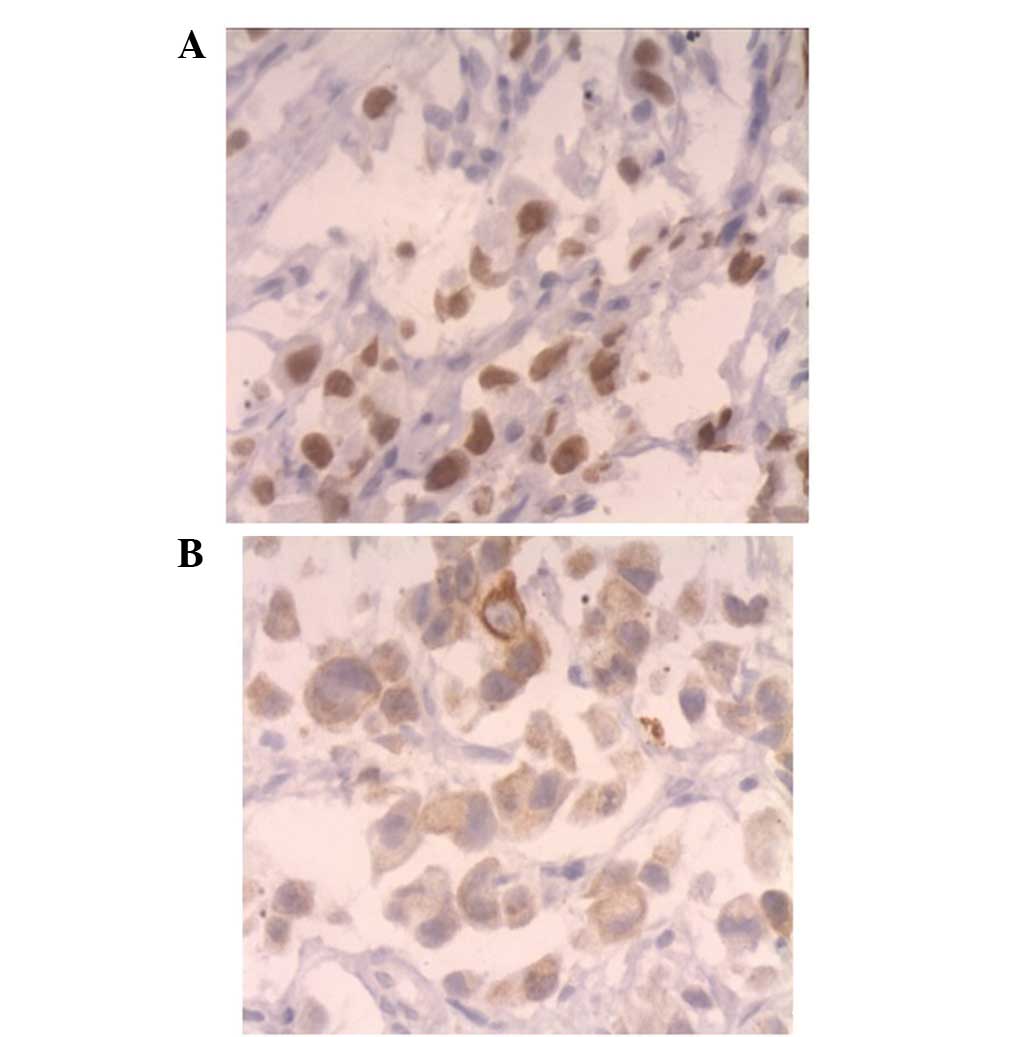
(Fig. 2). Immunohistochemical
analysis was performed in order to differentiate between primary
and metastatic SRCA. The results revealed that the SRCA cells were
positive for thyroid transcription factor-1 and CK7, but negative
for CK20 (Figs. 3 and 4). Upper and lower gastrointestinal
endoscopy revealed the absence of further tumors. Consequently, a
pathological diagnosis of stage IV SRCA of the lung was established
with a tumor-node-metastasis classification of T4N3M1. Upon
admission to the Qingdao Municipal Hospital, the patient
experienced rapid clinical deterioration. Massive pleural and
pericardial effusion was detected by type-B ultrasonic examination.
A clinical cytological examination revealed the presence of
malignant cells in the pleural and pericardial effusion. Immediate
pericardiocentesis and pleurocentesis were required due to the
onset of cardiac tamponade. However, as the effusion developed
rapidly, a pericardiotomy was performed for continued drainage with
a catheter. On July 13, 2012, the patient underwent tracheal
intubation and mechanical ventilation for respiratory support, and
was transferred to the intensive care unit (ICU). During this
period, the tumor was analyzed for mutations in the epidermal
growth factor receptor, but the result was negative. Inversion of
the EML4-ALK gene was then detected by fluorescence in situ
hybridization from a sample sent to the Peking Union Medical
College Hospital (Beijing, China). On July 20, 2012, it was
established that the tumor was ALK-positive. By that time, the
patient had been transferred to the ICU and had received mechanical
ventilation for seven days. Crizotinib (250 mg) was therefore
administered twice daily via a nasogastric tube for three months.
The following month, the condition of the patient had improved. The
symptom of dyspnea was relieved and the volume of pericardial and
pleural effusion was gradually reduced. A CT scan revealed lung
tumor regression (Fig. 5). As a
result, the pericardial catheter and nasogastric and tracheal tubes
were successfully removed. However, one month after removal, the
patient began to deteriorate, following three months the patient
succumbed to the disease.
Written informed consent was obtained from the
patient for publication of this case study and any accompanying
images.
Discussion
The identification of the EML4-ALK gene
translocation in cases of NSCLC, and the development of an approved
ALK inhibitor has led to an understanding of the
clinicopathological characteristics and prognosis of EML4-ALK NSCLC
patients. EML4-ALK has become a novel therapeutic target for the
treatment of NSCLC. ALK inhibitors have presented an opportunity
for the individualized treatment of lung cancers (8) and provided an alternative therapeutic
approach for those patients intolerant to chemotherapy and
radiation therapy. In the last two decades, cases of primary SRCA
have failed to respond to traditional chemotherapy and radiation
therapy, and therefore, the condition was considered to have a
generally poor prognosis. The significance of SRCA was not truly
appreciated until the publication of recent study results, which
linked SRC to EML4-ALK NSCLC (9,10). In
early clinical trials, the ALK inhibitor, crizotinib, demonstrated
a high response rate in patients with ALK-positive NSCLC (11,12).
Despite this, a number of patients enrolled in the trials developed
resistance to the drug (13). In
fact, acquired drug resistance is a problem encountered for a
considerable number of targeted cancer drugs. In the present study,
the patient was, at first, highly responsive to crizotinib.
However, subsequent to just one month, resistance to crizotinib
developed and the condition of the patient began to deteriorate.
After three months, the patient succumbed to the disease. Cases of
acquired drug resistance to targeted treatments following such a
short duration are generally rare. It remains unclear as to whether
such resistance is specific to primary SRCA of the lung as a rare
subtype, or whether crizotinib is responsible for generating such
extensive drug resistance. Therefore, it remains important to
continue to investigate the survival outcomes of NSCLC patients
with an EML4-ALK translocation who are treated with crizotinib,
particularly those with SRCA.
Crizotinib is an attractive option for the treatment
of SRCA. The present study described a radiological and symptomatic
partial response to first-line crizotinib therapy in a patient with
advanced primary SRCA of the lung. However, as acquired drug
resistance developed after just one month of treatment, it would be
valuable to investigate the mechanisms that underlie crizotinib
resistance in future studies, in order to establish methods to
improve the treatment.
References
|
1
|
Kish JK, Ro JY, Ayala AG and McMurtrey MJ:
Primary mucinous adenocarcinoma of the lung with signet-ring cells:
a histochemical comparison with signet-ring cell carcinomas of
other sites. Hum Pathol. 20:1097–1102. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iwasaki T, Ohta M, Lefor AT and Kawahara
K: Signet-ring cell carcinoma component in primary lung
adenocarcinoma: potential prognostic factor. Histopathology.
52:639–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaw AT, Yeap BY, Mino-Kenudson M,
Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S,
McDermott U, Settleman J, Kobayashi S, Mark EJ, Rodig SJ, Chirieac
LR, Kwak EL, Lynch TJ and Iafrate AJ: Clinical features and outcome
of patients with non-small-cell lung cancer who harbor EML4-ALK. J
Clin Oncol. 27:4247–4253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rodig SJ, Mino-Kenudson M, Dacic S, Yeap
BY, Shaw A, Barletta JA, Stubbs H, Law K, Lindeman N, Mark E, Janne
PA, Lynch T, Johnson BE, Iafrate AJ and Chirieac LR: Unique
clinicopathologic features characterize ALK-rearranged lung
adenocarcinoma in the western population. Clin Cancer Res.
15:5216–5223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soda M, Choi YL, Enomoto M, et al:
Identification of the transforming EML4-ALK fusion gene in
non-small-cell lung cancer. Nature. 448:561–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doebele RC, Pilling AB, Aisner DL, et al:
Mechanisms of resistance to crizotinib in patients with ALK gene
rearranged non-small cell lung cancer. Clin Cancer Res.
18:1472–1482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kwak EL, Camidge DR, Clark J, et al:
Clinical activity observed in a phase I dose escalation trial of an
oral c-met and ALK inhibitor, PF-02341066. J Clin Oncol.
27:(Suppl). 35092009.
|
|
8
|
Kwak EL, Bang YJ, Camidge DR, Shaw AT,
Solomon B, Maki RG, Ou SH, Dezube BJ, Jӓnne PA, Costa DB,
Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman
JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve
SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K,
Salgia R, Shapiro GI, Clark JW and Iafrate AJ: Anaplastic lymphoma
kinase inhibition in non-small-cell lung cancer. N Engl J Med.
363:1693–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuta K, Ishii G, Yoh K, Nitadori J,
Hasebe T, Nishiwaki Y, Endoh Y, Kodama T, Nagai K and Ochiai A:
Primary lung carcinoma with signet-ring cell carcinoma components:
clinicopathological analysis of 39 cases. Am J Surg Pathol.
28:868–874. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ou SH, Ziogas A and Zell JA: Primary
signet-ring carcinoma (SRC) of the lung: a population-based
epidemiologic study of 262 cases with comparison to adenocarcinoma
of the lung. J Thorac Oncol. 5:420–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Girard N: Crizotinib in ALK-positive lung
cancer. Lancet Oncol. 13:962–963. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bang YJ: Treatment of ALK-positive
non-small cell lung cancer. Arch Pathol Lab Med. 136:1201–1204.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanizaki J, Okamoto I, Okabe T, Sakai K,
Tanaka K, Hayashi H, Kaneda H, Takezawa K, Kuwata K, Yamaguchi H,
et al: Activation of HER family signaling as a mechanism of
acquired resistance to ALK inhibitors in EML4-ALK-positive
non-small cell lung cancer. Clin Cancer Res. 18:6219–6226. 2012.
View Article : Google Scholar : PubMed/NCBI
|