Introduction
Despite advancements in the prevention and treatment
through multimodal approaches, including targeted therapies,
gastric cancer is one of the most common types of malignant tumor
and remains the second leading cause of carcinoma-associated
mortality worldwide (1).
Few agents exist that are truly cancer cell specific
with regards to the induction of cell death. For example, tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) is an
agent that is preferentially cytotoxic to cancer cells over healthy
cells (2). However, the effectiveness
of TRAIL is significantly impeded by drug resistance, resulting in
poor survival outcomes of patients with cancer (3,4). Emerging
evidence provides support for the potential anticancer effect of
bioactive products derived from cruciferous vegetables, such as
brussel sprouts, broccoli, cabbage and cauliflower (5). Among these compounds,
3,3′-diindolylmethane (DIM) is generated in the acidic environment
of the stomach via dimerization of the indole-3-carbinol monomers
present in these vegetables (6).
A number of cellular stress conditions, including
nutrient deprivation, hypoxia and treatment with a variety of
pharmacological agents which inhibit glycosylation or deplete
endoplasmic reticulum (ER) calcium stores, may lead to the
accumulation and aggregation of unfolded and/or misfolded proteins
in the ER lumen, which is termed ER stress. Notably, ER-induced
apoptotic cell death has been identified as an important apoptotic
pathway (7). Various mechanisms have
been hypothesized to exhibit an important role in ER stress-induced
apoptosis; the C/enhancer binding protein homologous protein
(CHOP), glucose-regulated protein 78 (GRP78) and caspase-3, −9 and
−12 are all considered to be involved in the apoptotic signaling
pathway which occurs in response to ER stress (8). In addition, it has been demonstrated
that effective TRAIL-based combination therapy can be achieved by
upregulating death receptor 5 (DR5) expression (9). Furthermore, CHOP has been reported to
directly regulate DR5 expression in human carcinoma cells (10).
The aim of the present study was to explore whether
DIM potentiates TRAIL-induced apoptosis of gastric cancer cells and
investigate the possible mechanisms of this process.
Materials and methods
Cell culture
The human gastric cancer cell lines BGC-823 and
SGC-7901 were purchased from the Shanghai Institute of Biochemistry
and Cell Biology, Chinese Academy of Sciences (Shanghai, China).
Cells were cultured in Dulbecco's modified Eagle's medium (Gibco
Life Technologies, Grand Island, NY, USA) supplemented with 100
U/ml penicillin, 100 µg/ml streptomycin and 10% fetal bovine serum
(Tianhang Biological Technology Co., Ltd., Hangzhou, China). All
cells were maintained in a 5% CO2 atmosphere at a
temperature of 37°C.
Cell growth assay
To determine cell growth, a colorimetric
water-soluble tetrazolium salt assay (Cell Counting Kit 8; Dojindo
Laboratories, Kumamoto, Japan) was performed. This allowed the
number of viable cells to be evaluated following treatment with
various agent combinations.
Apoptosis assay
The detection of apoptotic cells was performed using
an Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection
kit (Beyotime Institute of Biotechnology, Nantong, China),
according to the manufacturer's instructions. Cells were plated in
6-well plate (5×105 cells/well) and allowed to grow to
75–80% confluence. Following treatment with DIM (10 µmol/l), TRAIL
(25 or 50 ng/ml) or DIM (10 µmol/l) + TRAIL (25 ng/ml) for 24 h,
the cells were collected, resuspended in 500 µl binding buffer, and
5 µl Annexin V-FITC and 5 µl propidium iodide were added. Untreated
cells were washed twice with phosphate-buffered saline (Beyotime
Institute of Biotechnology), collected, resuspended in 500 µl
binding buffer, and 5 µl Annexin V-FITC and 5 µl propidium iodide
were added, which served as the control. The data was quantified
and analyzed using a FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA).
Western blot analysis
Western blot analysis was conducted as previously
described (11). Briefly, cells were
lysed by incubating in lysis buffer (50 mM Tris, 150 mM NaCl, 1%
Triton X-100, 1% sodium deoxycholate, 0.1% SDS; pH 7.4; Beyotime
Institute of Biotechnology) for 20 min at 4°C and the protein
concentration was determined using the Beyotime assay system
(Beyotime Institute of Biotechnology). Protein (60 µg) was run on
12.5% polyacrylamide gel and transferred to a nitrocellulose
membrane (Hybond-ECL; Amersham Pharmacia Biotech, Buckinghamshire,
UK). Next, the membrane was blocked with Tris-buffered saline
containing 0.1% Tween 20 (TBST) and 5% nonfat milk for 2 h at room
temperature and incubated overnight at 4°C with primary monoclonal
antibodies against DR5 (cat. no. sc-166624), DR4 (cat. no.
sc-8411), CCAAT/CHOP (cat. no. sc-7351), GRP78 (cat. no. sc-376768)
and GAPDH (cat. no. sc-365062) purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The membrane was washed
three times with TBST and incubated with horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (cat. no.
A0216, Beyotime Institute of Biotechnology) for 1 h at room
temperature. Immunoreactive bands were subsequently identified
using LumiGLO® chemiluminescent substrate (Cell Signaling
Technology, Inc.). The light emitted by destabilized LumiGLO®
reagent was subsequently captured on X-ray film, (Cell Signaling
Technology, Inc.), normalized to the corresponding GAPDH level and
analyzed with ImageJ software (version 1.44; National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
The SPSS software package (version 15.0; SPSS, Inc,
Chicago, IL, USA) was used for data analysis, with data presented
as the mean ± standard deviation (SD) and n indicating the number
of independent experiments performed. After determining equal
variance, comparisons among the means of multiple groups were
performed using one-way analysis of variance and Fisher's least
significant difference test. Additionally, two-tailed independent
samples t-tests were used when appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
DIM sensitizes gastric cancer cells to
TRAIL-induced cytotoxicity
The present study examined the role of TRAIL and DIM
treatment, alone or in combination, on the proliferation of human
gastric cancer cell lines BGC-823 and SGC-7901. The treatment of
gastric cancer cells with 10 µmol/l DIM for 24 h induced marginal
cytotoxicity (<20%). Similarly, limited cytotoxicity (20%) was
observed following treatment with TRAIL at concentrations of 25–50
ng/ml. However, compared to treatment with TRAIL or DIM alone,
significant cytotoxic effects were induced following co-treatment
of the two gastric cancer cell lines with TRAIL (25 ng/ml) and DIM
(10 µmol/l), compared with control (P=0.0001 in BGC-823, P=0.0001
in SGC-7901), TRAIL (P=0.0002 in BGC-823, P=0.0006 in SGC-7901) or
DIM alone (P=0.0002 in BGC-823, P=0.0006 in SGC-7901 (Fig. 1).
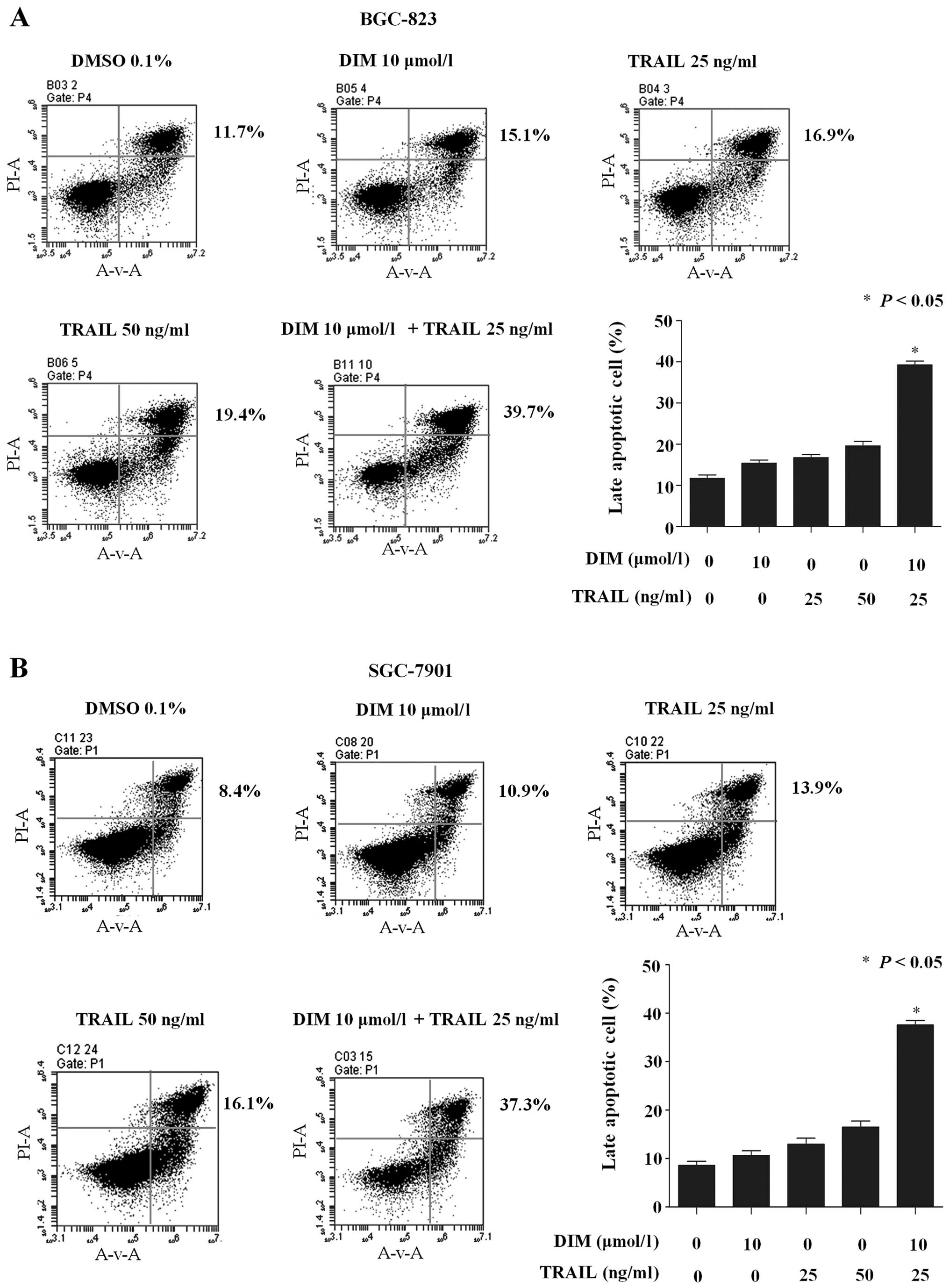
DIM enhances TRAIL-induced apoptosis
in gastric cancer cells
To investigate whether apoptosis is correlated with
the inhibition of cell proliferation following DIM and TRAIL
co-treatment, flow cytometric analysis was used to detect the
increase in hypodiploid cell populations. The mean (n=3)
proportions of late apoptotic BGC-823 cells were 15.1, 16.9, 19.4
and 39.7% for DIM (10 µmol/l), TRAIL (25 ng/ml), TRAIL (50 ng/ml),
and TRAIL (25 ng/ml) plus DIM (10 µmol/l), respectively (Fig. 2A). Similarly, in SGC-7901 cells, the
mean (n=3) proportions were 10.9, 13.9, 16.1 and 37.3% for DIM (10
µmol/l), TRAIL (25 ng/ml), TRAIL (50 ng/ml), and TRAIL (25 ng/ml)
plus DIM (10 µmol/l) (Fig. 2B). These
results indicate that DIM sensitizes gastric cancer cells to
TRAIL-induced apoptosis.
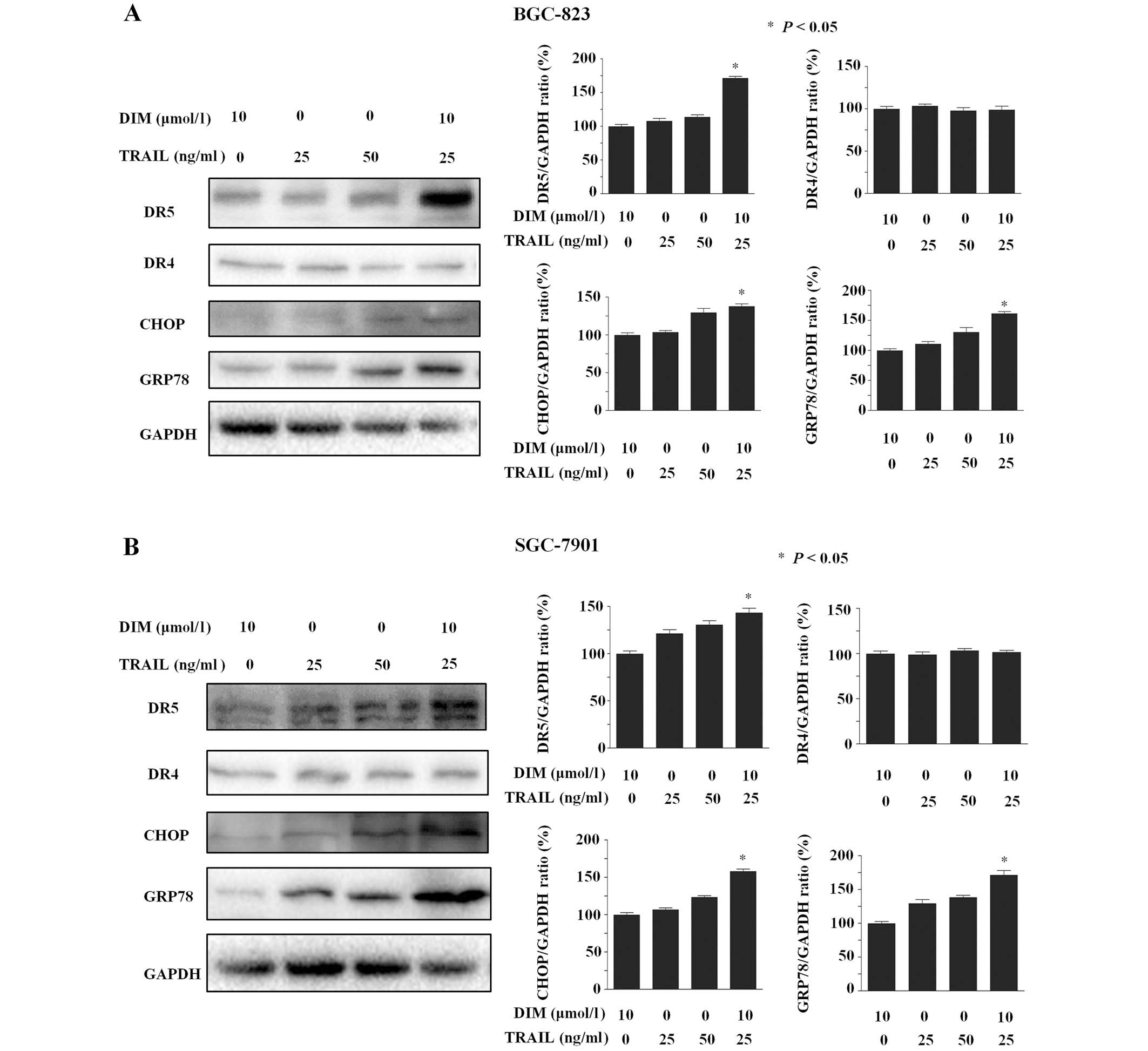
DIM plus TRAIL treatment induces the
expression of DR5, CHOP and GRP78 proteins in gastric cancer
cells
BGC-823 cells were treated with DIM for 24 h and
subsequently analyzed by western blot to investigate the expression
levels of various TRAIL receptor-associated proteins. The group of
cells treated with TRAIL plus DIM were demonstrated to
significantly induce the protein expression levels of DR5
(P<0.001) but exhibited no effect on DR4 expression. This data
indicates that DR5 may be involved in DIM enhancement of the
apoptotic effects of TRAIL in BGC-823 cells. Significant
upregulation of DR5 by DIM plus TRAIL was also identified in
SGC-7901 cells (P<0.05). Furthermore, DIM and TRAIL co-treatment
was observed to induce a significant increase in the protein
expression levels of CHOP and GRP78 (P>0.05; Fig. 3), two proteins that accumulate during
endoplasmic reticulum (ER) stress (12).
Discussion
In the present study, the potential anticancer
effect of DIM, a bioactive product derived from cruciferous
vegetables, in regulating TRAIL signaling was explored in two
gastric cancer cell lines. DIM was demonstrated to potentiate
TRAIL-induced apoptosis in the gastric cancer cell lines BGC-823
and SGC-7901, as well as inducing increased DR5, CHOP and GRP78
protein expression levels.
Cytotoxic and apoptotic effects were observed when
gastric cancer cells were treated with DIM or TRAIL alone; these
results are consistent with a number of previous studies on various
types of cancer, including gastric cancer (13–15).
However, to the best of our knowledge, the present study is the
first to demonstrate that DIM sensitizes TRAIL-induced cytotoxicity
and apoptosis in gastric cancer cells. As the widespread
application of TRAIL has been limited due to increasing drug
resistance and cost (3,4), the results of the current study indicate
that the use of low-cost DIM may enhance the clinical application
of TRAIL-based therapy for various types of cancer in the
future.
El-Deiry (9)
previously reported that effective TRAIL-based combination therapy
may be achieved by the upregulation of DR4 and DR5. Furthermore,
DIM appears to induce apoptosis in pancreatic cancer cells via ER
stress-dependent upregulation of DR5 (16). Based on this data, we hypothesized
that DIM potentiates TRAIL-induced apoptosis of gastric cancer
cells by upregulating DR5 expression. In the current study, DIM and
TRAIL co-treatment appeared to induce DR5 expression, confirming
this hypothesis.
CHOP has been reported to directly regulate DR5
expression in human carcinoma cells (10,17). For
example, specific agents, such as dimethyl-celecoxib and
5,7-dimethoxyflavone, have been shown to induce the expression of
DR5 via CHOP-dependent DR5 gene transactivation (18,19).
Furthermore, previous studies have identified a close association
between DR5 expression and ER stress (10,17). In
the ER lumen, ER stress is activated when unfolded proteins
accumulate (20). Through this
response, specific apoptotic pathways may be activated to clear
severely damaged cells in which the defects of protein folding
cannot be resolved (12,21). Although the molecular mechanisms by
which ER stress inducers regulate DR5 expression may vary between
cell types, CHOP, an ER stress-inducible transcription factor,
provides a common association between ER stress and DR5 expression.
In the present study, treatment of gastric cancer cells with DIM
plus TRAIL was demonstrated to induce the expression of CHOP and
GRP78, two proteins that also accumulate during ER stress (12). The identification of CHOP induction by
DIM indicates that DIM may trigger ER stress and increase the
expression levels of GRP78, although this mechanism has yet to be
fully clarified.
In conclusion, to the best of knowledge, the present
study demonstrates for the first time that DIM sensitizes
TRAIL-induced cytotoxicity and apoptosis in gastric cancer cells,
accompanied by the upregulation of DR5, CHOP and GRP78 protein
expression levels. We propose that this process of sensitization
may involve ER stress mechanisms. The results of the current study
highlight the possibility of producing less costly anticancer
agents in the future, and the use of low-cost DIM may enhance the
clinical application of TRAIL-based therapy for various types of
cancer. Thus, the results of this study indicate that future
studies, which investigate the anticancer effects of other active
components derived from the diet or Traditional Chinese medicine
may be of value.
Acknowledgements
The present study was supported by the Research
Foundation for Advanced Talents of Jiangsu University (grant nos.
14JDG044, 1291270021) and the Natural Science Foundation of Jiangsu
Province (grant nos. BK20130502 and BK20140576), the open project
of Key Lab of Modern Toxicology (grant no. NJMU), the Ministry of
Education (grant no. NMUMT201410) and Jiangsu Planned Projects for
Postdoctoral Research Funds (grant no. 1402169C).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Belkhiri A, Zhu S, Chen Z, et al:
Resistance to TRAIL is mediated by DARPP-32 in gastric cancer. Clin
Cancer Res. 18:3889–3900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lane D, Côté M, Grondin R, et al: Acquired
resistance to TRAIL-induced apoptosis in human ovarian cancer cells
is conferred by increased turnover of mature caspase-3. Mol Cancer
Ther. 5:509–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scott EN, Gescher AJ, Steward WP and Brown
K: Development of dietary phytochemical chemopreventive agents:
biomarkers and choice of dose for early clinical trials. Cancer
Prev Res (Phila). 2:525–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujioka N, Ainslie-Waldman CE, Upadhyaya
P, et al: Urinary 3,3′-diindolylmethane: a biomarker of
glucobrassicin exposure and indole-3-carbinol uptake in humans.
Cancer Epidemiol Biomarkers Prev. 23:282–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schröder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rutkowski DT and Kaufman RJ: A trip to the
ER: coping with stress. Trends Cell Biol. 14:20–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El-Deiry WS: Insights into cancer
therapeutic design based on p53 and TRAIL receptor signaling. Cell
Death Differ. 8:1066–1075. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamaguchi H and Wang HG: CHOP is involved
in endoplasmic reticulum stress-induced apoptosis by enhancing DR5
expression in human carcinoma cells. J Biol Chem. 279:45495–45502.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Wu X, Chen Y, et al: Prognostic
and predictive role of JWA and XRCC1 expressions in gastric cancer.
Clin Cancer Res. 18:2987–2996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SH, Min KW, Zhang X and Baek SJ:
3,3′-diindolylmethane induces activating transcription factor 3
(ATF3) via ATF4 in human colorectal cancer cells. J Nutr Biochem.
24:664–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin XF, Chen J, Mao W, et al: A selective
aryl hydrocarbon receptor modulator 3,3′-diindolylmethane inhibits
gastric cancer cell growth. J Exp Clin Cancer Res. 31:462012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jalving M, Heijink DM, Koornstra JJ, et
al: Regulation of TRAIL receptor expression by β-catenin in
colorectal tumours. Carcinogenesis. 35:1092–1099. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abdelrahim M, Newman K, Vanderlaag K, et
al: 3,3′-diindolylmethane (DIM) and its derivatives induce
apoptosis in pancreatic cancer cells through endoplasmic reticulum
stress-dependent upregulation of DR5. Carcinogenesis. 27:717–728.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshida T, Shiraishi T, Nakata S, et al:
Proteasome inhibitor MG132 induces death receptor 5 through
CCAAT/enhancer-binding protein homologous protein. Cancer Res.
65:5662–5667. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen S, Liu X, Yue P, et al:
CCAAT/enhancer binding protein homologous protein-dependent death
receptor 5 induction and ubiquitin/proteasome-mediated cellular
FLICE-inhibitory protein down-regulation contribute to enhancement
of tumor necrosis factor-related apoptosis-inducing ligand-induced
apoptosis by dimethyl-celecoxib in human non small-cell lung cancer
cells. Mol Pharmacol. 72:1269–1279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang JF, Cao JG, Tian L and Liu F:
5,7-Dimethoxyflavone sensitizes TRAIL-induced apoptosis through DR5
upregulation in hepatocellular carcinoma cells. Cancer Chemother
Pharmacol. 69:195–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Görlach A, Klappa P and Kietzmann T: The
endoplasmic reticulum: folding, calcium homeostasis, signaling, and
redox control. Antioxid Redox Signal. 8:1391–1418. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Malhotra JD and Kaufman RJ: The
endoplasmic reticulum and the unfolded protein response. Semin Cell
Dev Biol. 18:716–731. 2007. View Article : Google Scholar : PubMed/NCBI
|