Introduction
Colorectal cancer is one of the most frequently
diagnosed malignant diseases, with an estimated 1,023,000 new cases
and 529,000 associated mortalities each year worldwide (1). As a result of improved living standards
and changes in eating habits, the incidence of colorectal cancer in
China has increased, and it is now ranked as the fifth most lethal
malignancy. Despite improvements in treatment strategies, patients
with colorectal cancer have a relatively poor prognosis. Therefore,
the identification of optimal prognostic factors and the
development of personalized treatments is extremely important.
Previous studies have demonstrated that the mutational status of
the K-ras gene is important for the treatment of advanced
colorectal cancer with cetuximab, as it affects the tumor response
and has treatment-independent prognostic value (2,3).
Vascular endothelial growth factor (VEGF) is a
diffusible glycoprotein produced by normal and neoplastic cells,
which regulates physiological and pathological angiogenesis
(4,5).
Tumor development is a complex biological process that involves a
number of genes. Previous studies (6–10) have
demonstrated that angiogenesis is closely associated with the
formation, development and prognosis of malignant tumors, in which
VEGF and VEGF receptor-1 (VEFGR-1), also known as fms-like tyrosine
kinase-1 (FLT-1), are the core regulating factors. The prognostic
value of the tumor cell expression of VEGF and its receptor, FLT-1,
remains controversial. VEGF has been reported to be associated with
the clinical outcomes of a number of tumors, including head and
neck cancer, esophageal cancer and thyroid carcinoma (10–13). By
contrast, a similar correlation was not shown for pancreatic
adenocarcinoma, epithelial ovarian cancer or non-small cell lung
cancer by other studies (6,14,15).
Therefore, further investigation is required in order to better
define the predictive value of these two potential prognostic
factors in colorectal cancer. The present study evaluated the
expression of VEGF and FLT-1, and their correlation with
clinicopathological factors and clinical outcomes, in patients with
colorectal cancer.
Materials and methods
Materials
In total, 90 paraffin samples with complete clinical
data obtained from primary colorectal cancer patients who had
undergone surgery at the Suqian People's Hospital of Nanjing Drum
Tower Hospital Group (Suqian, Jiangsu, China) between January 2007
and June 2009 were eligible for use in the present study. The study
was approved by the Ethics Committee of Suqian People's Hospital of
Nanjing Drum Tower Hospital and written informed consent was
obtained from all patients. In total, 90 patients, including 55
males and 35 females, aged between 37 and 81 years old, with a
median age of 63.8 years, were retrospectively analyzed. The
additional patient characteristics are summarized in Table I. The primary tumor sites were as
follows: i) ileocecal back, 6 cases; ii) ascending colon, 20 cases;
iii) transverse colon, 7 cases; iv) descending colon, 13 cases; v)
sigmoid colon, 11 cases; and vi) rectum, 33 cases. Overall, lymph
node metastases were present in 39 cases, and absent in 51 cases.
Dukes' staging was recorded as follows: i) A, 8 cases; ii) B, 22
cases; iii) C, 49 cases; and iv) D, 11 cases. According to the
World Health Organization colorectal adenocarcinoma differentiation
standards, there were 38 highly-differentiated cases, 31
median-differentiated cases and 21 poorly-differentiated cases
(16).
 | Table I.Association between VEGF and FLT-1
expression and the clinicopathological characteristics of
colorectal cancer (n=90). |
Table I.
Association between VEGF and FLT-1
expression and the clinicopathological characteristics of
colorectal cancer (n=90).
|
|
| VEGF | FLT-1 |
|---|
|
|
|---|
| Groups | n | – | + | χ2 | P-value | – | + | χ2 | P-value |
|---|
| Age, years |
|
|
| 2.054 | 0.152 |
|
| 1.558 | 0.212 |
|
<60 | 51 | 16 | 35 |
|
| 29 | 22 |
|
|
| ≥60 | 39 | 18 | 21 |
|
| 17 | 22 |
|
|
| Gender |
|
|
| 0.010 | 0.921 |
|
| 1.800 | 0.178 |
| Male | 55 | 21 | 34 |
|
| 25 | 30 |
|
|
|
Female | 35 | 13 | 22 |
|
| 21 | 14 |
|
|
| Histological
grade |
|
|
| 14.546 | 0.001 |
|
| 3.824 | 0.148 |
| Well | 38 | 23 | 15 |
|
| 24 | 14 |
|
|
|
Moderately | 31 | 7 | 24 |
|
| 13 | 18 |
|
|
|
Poorly | 21 | 4 | 17 |
|
| 9 | 12 |
|
|
| Depth of
invasion |
|
|
| 4.618 | 0.032 |
|
| 4.211 | 0.040 |
| T1,
T2 | 23 | 13 | 10 |
|
| 16 | 7 |
|
|
| T3,
T4 | 67 | 21 | 46 |
|
| 30 | 37 |
|
|
| Lymph node
metastasis |
|
|
| 6.328 | 0.012 |
|
| 6.375 | 0.012 |
|
Negative | 51 | 25 | 26 |
|
| 32 | 19 |
|
|
|
Positive | 39 | 9 | 30 |
|
| 14 | 25 |
|
|
| Dukes' stage |
|
|
| 29.423 | 0.000 |
|
| 2.012 | 0.570 |
| A | 8 | 6 | 2 |
|
| 4 | 4 |
|
|
| B | 22 | 17 | 5 |
|
| 13 | 9 |
|
|
| C | 49 | 8 | 41 |
|
| 22 | 27 |
|
|
| D | 11 | 3 | 8 |
|
| 7 | 4 |
|
|
Immunohistochemistry examination
The archived paraffin-embedded tissues were used to
generate consecutive 4-µm thick sections. The streptavidin-biotin
complex (sABC) method with a known positive colorectal biopsy was
used as a positive control, and phosphate buffered saline was used
as a negative control. The mouse anti-human VEGF monoclonal
antibody (mAb; 1:100), mouse anti-human FLT-1 mAb (1:100), a
universal quick method secondary antibody, and the diaminobenzidine
(DAB) chromogenic kit were all purchased from Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
The staining procedure was as follows: The slices
were dewaxed, followed by application of 30 ml/l
H2O2 methanol solution to block endogenous
peroxidase activity and the addition of digestive juices to digest
the tissues. Next, the secondary antibody and sABC reagents were
applied, as well as 3-amino-9-ethylcarbazole color. The slides were
then stained with hematoxylin and dehydrated in a graded alcohol
series, followed by the addition of xylene and neutral gum
cementing. The slides were then assessed under a light microscope
using a double-blind format (AX70, Olympus Corporation, Tokyo,
Japan). Red staining in the nucleus or cytoplasm was used to
indicate a positive result.
VEGF and FLT-1 proteins
VEGF and FLT-1 staining was determined as follows:
i) Positive, ≥10% of the cancer cells stained; and ii) negative, no
positive staining or <10% of the cancer cells stained.
Statistical analysis
The associations between VEGF and FLT-1 expression
and the clinicopathological parameters were statistically analyzed
by χ2 test using SPSS version 13.0 software (SPSS, Inc.,
Chicago, IL, USA). The overall survival (OS) rates were determined
using the Kaplan-Meier method and compared by the log-rank test.
Multivariate analysis for survival was performed using a Cox
proportional hazards regression model. P<0.05 was used to
indicate a statistically significant difference.
Results
Expression of VEGF and FLT-1 in
colorectal cancer
VEGF expression was evident in the cytoplasm and
cell membranes. The overall positive expression rate was 62.2%
(56/90). FLT-1 was only observed in the cytoplasm of primarily
tumor vascular endothelial cells, with a positive expression rate
of 48.9% (44/90).
Association between VEGF and FLT-1
expression and clinicopathological factors
VEGF expression was associated with the histological
grade, depth of invasion, lymph node metastasis and Dukes' stage of
the colorectal cancer (P<0.05). FLT-1 expression was associated
with the depth of invasion and lymph node metastasis (P<0.05).
Specific correlations between VEGF and FLT-1 and age, gender,
degree of differentiation, depth of invasion, lymph node metastasis
and Dukes' staging are shown in Table
I.
Prognostic value of VEGF and FLT-1
expression in colorectal cancer
In order to investigate the prognostic value of VEGF
and FLT-1 expression in colorectal cancer, cumulative survival
curves for the 90 patients with colorectal cancer were constructed
according to the Kaplan-Meier method. Differences in OS were then
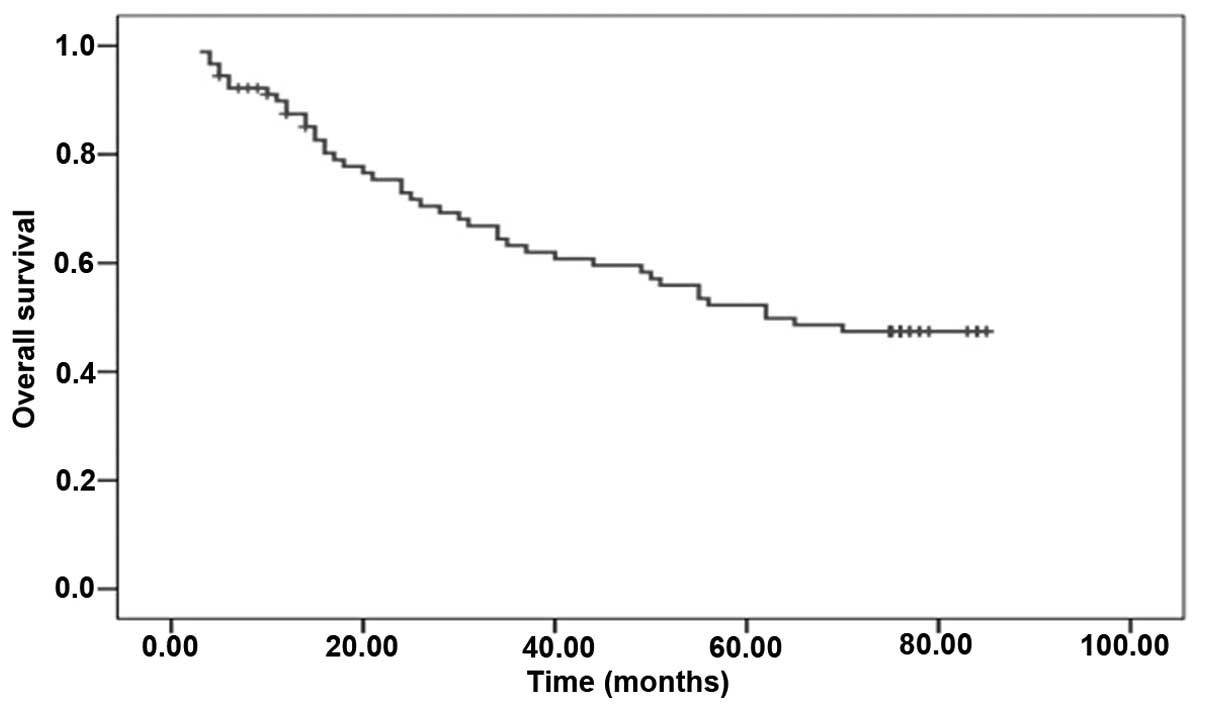
assessed using the log-rank test. The median OS time of the 90
patients was 62.00 months (Fig. 1).
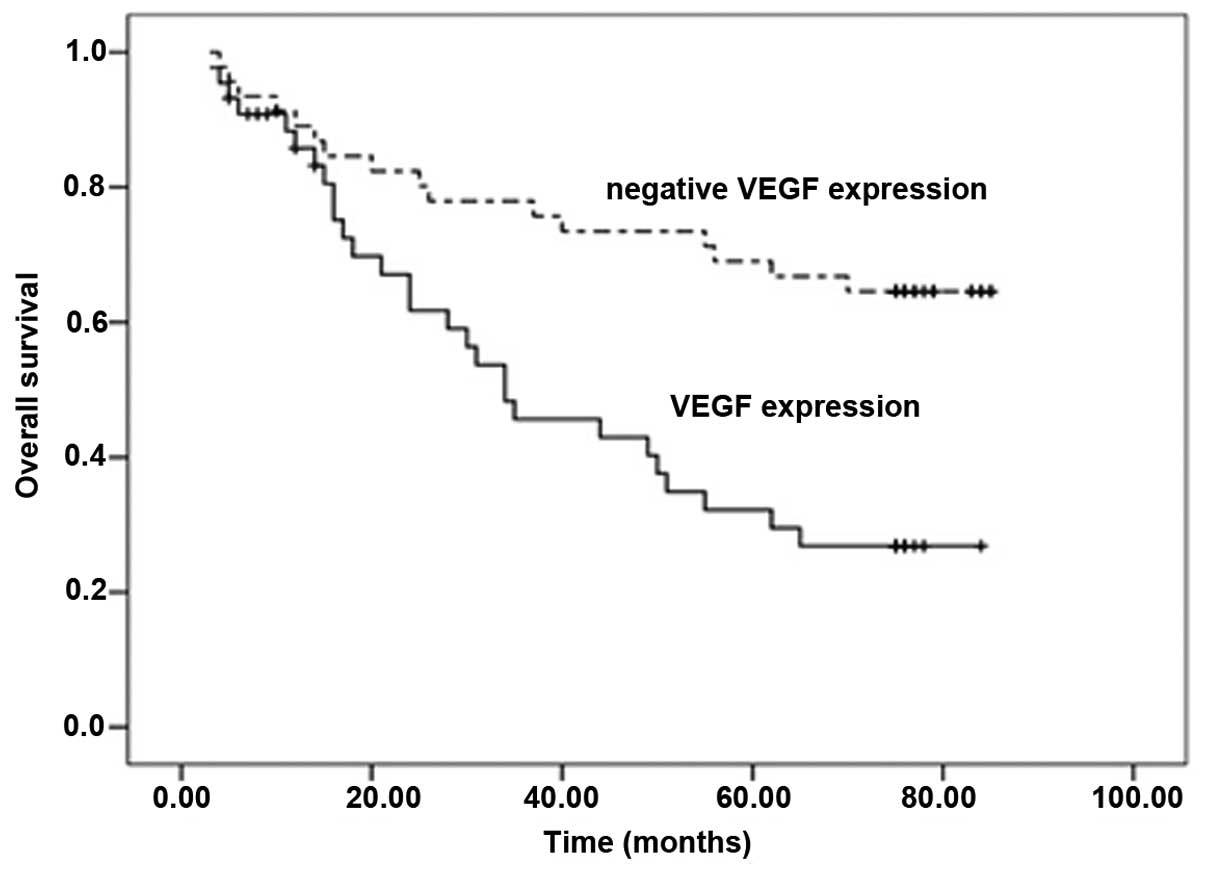
Upon univariate analysis, the OS rates of the colorectal cancer
patients with VEGF expression were significantly reduced compared
with the patients with no VEGF expression (P<0.0001; Fig. 2). Improved OS was significantly
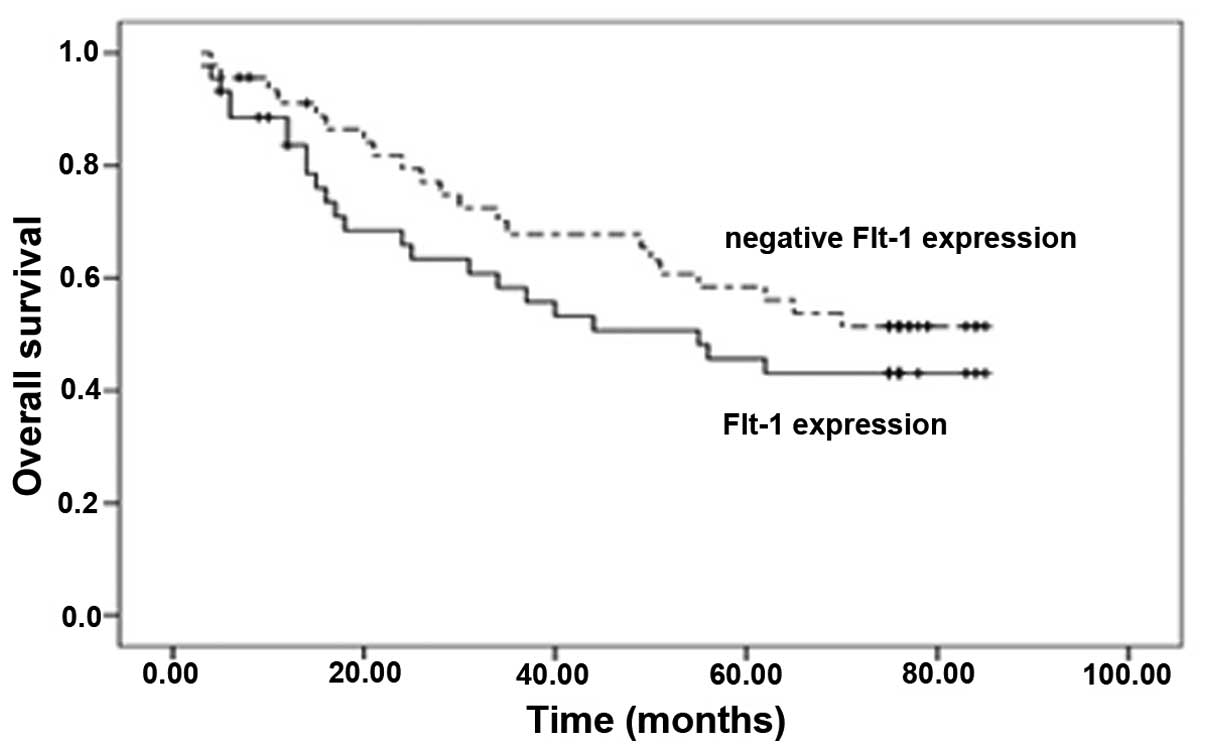
associated with negative VEGF expression. By contrast, a trend
toward improved OS was observed for negative FLT-1 expression
(P=0.289; Fig. 3).
Following multivariate Cox proportional hazards
regression analysis, VEGF expression and Dukes' stage were revealed
to be significant independent prognostic indicators of OS (P=0.038
and P=0.021, respectively; Table
II).
 | Table II.Multivariate Cox proportional hazards
regression analysis. |
Table II.
Multivariate Cox proportional hazards
regression analysis.
| Variable | Wald
χ2 | P-value |
|---|
| VEGF | 4.316 | 0.038 |
| FLT-1 | 3.655 | 0.056 |
| Dukes' stage | 5.314 | 0.021 |
| Lymph node
metastasis | 1.154 | 0.283 |
| Depth of
invasion | 2.461 | 0.117 |
| Histological
grade | 0.531 | 0.466 |
Discussion
Angiogenesis results in the formation of new blood
vessels from a pre-existing vascular network, and is therefore
required for tumor growth, invasion and metastasis. VEGF was first
purified from the culture medium of bovine pituitary follicular
stellate cells in 1989 by Bellamy et al (7), and was later identified to be a
homologous glycoprotein with heparin-binding activity. VEGF is an
endothelial cell-specific mitogen promoter (17). As a potent growth factor acting
directly on vascular endothelial cells, VEGF has an important role
in the division, proliferation and migration of vascular
endothelial cells. Synthesized and released by endothelial cells,
granulocytes and megakaryocytes, VEGF is widely present in body
tissues, and participates in the progression of a variety of
diseases (18). As a vascular
inducing factor in angiogenesis, VEGF can induce and enhance the
permeability of blood vessels. In addition, it aids in the
maintenance of the normal state and integrity of blood vessels.
VEGFRs are extensively expressed in vascular endothelial cells.
VEGF binds with specific receptors in order to exert its biological
functions; in particular, increasing microvascular growth and
permeability (19). VEGFR-1, also
known as FLT-1, is primarily located on vascular endothelial cells,
and is expressed, albeit to a lesser degree, on mononuclear cells,
mesangial cells and trophoblast cells. VEGF exerts its functions by
binding to and activating FLT-1, stimulating the proliferation of
vascular endothelial cells, increasing vascular permeability and
promoting the formation of new blood vessels. Therefore, VEGF and
VEGFR-1 are considered to be the most promising anti-angiogenic
targets (20).
VEGF plays an important role in angiogenesis by
activating FLT-1 and KDR to stimulate the growth of tumor vascular
endothelial cells. Boiocchi et al (21) revealed that, in hepatocellular
carcinoma, angiogenesis is primarily mediated by the VEGF/FLT-1
system. Furthermore, in angiogenesis-rich regions, the incidence
and sensitivity of apoptosis is reduced. VEGFRs are involved in the
complex proliferation interaction between gastric cancer cells and
endothelial cells. In addition, it has been demonstrated that FLT-1
cannot reverse the tumor angiogenesis inhibition caused by negative
KDR mutations, which indicates that FLT-1 may mediate the
non-mitogenic effects caused by VEGF, and not the mitogenic
response (9,22,23).
However, despite these results, the prognostic value
of the tumor cell expression of VEGF and FLT-1 requires further
investigation, as available data are currently inconclusive.
Several studies (14,24,25) have
confirmed that VEGF expression is negatively associated with
patient prognosis in esophageal, and head and neck cancers. By
contrast, other studies have revealed no significant prognostic
association in patients with non-small cell lung and epithelial
ovarian cancer. Nevertheless, the majority of studies consistently
illustrate that VEGF expression is negatively corrected with
patient prognosis. With regard to the prognostic value of FLT-1
expression, available data in the literature are even more
heterogeneous than those for VEGF expression. Certain studies
(6,11,14) have
suggested a negative prognostic value of FLT-1 expression on the
clinical outcome of thyroid carcinomas or non-small cell lung
cancers, while others have demonstrated no significant correlation
between FLT-1 expression and the outcome of esophageal or ovarian
cancers (12,15). Furthermore, two previous studies
(6,26)
even established a positive impact of FLT-1 expression on survival
in pancreatic or locally advanced breast cancer.
The results of the present study revealed positive
expression rates of VEGF at 62.2% (56/90) and FLT-1 at 48.9%
(44/90) in 90 cases of colorectal cancer. The positive expression
rates of VEGF and FLT-1 in the present study were slightly lower
than those reported in previous studies, which may be due to the
earlier clinical stages and advanced age of the patients included
in the present study. It was demonstrated that VEGF expression was
significantly negatively associated with OS in colorectal cancer
patients, which is consistent with the results of previous studies
(20,27–29).
Seibord et al (29) reported
poorer survival rates in patients with VEGF-positive tumors in a
retrospective series of 117 patients. Furthermore, Kato et
al (12) demonstrated that VEGF
expression was associated with a poor prognosis in a retrospective
series of 64 patients (12). The
results of the present study indicate that VEGF has an important
role in the proliferation, invasion and metastasis of colorectal
cancer. However, the utility of FLT-1 as a prognostic indicator in
colorectal cancer remains unclear, as the present study failed to
demonstrate prognostic value. This result is similar to those of
previous retrospective studies that did not identify a correlation
between FLT-1 expression and the treatment outcomes of various
tumor types, including epithelial ovarian (15), tongue (13), nasopharyngeal (30) and non-small cell lung (31) cancer. However, two other retrospective
studies (6,26) suggested that FLT-1 expression was
associated with favorable outcomes. Therefore, according to the
present dilemma regarding FLT-1, it can be hypothesized that the
role of FLT-1 in tumor growth and spread is likely to be diverse
and complex.
In conclusion, in the present study, VEGF expression
proved to be an independent negative predictor of OS in patients
with colorectal cancer, whereas FLT-1 expression appeared to have
no significant impact on clinical outcome. Owing to the limitations
of the retrospective study design, these results should be
confirmed by prospective studies.
References
|
1
|
Saltz LB, Cox JV, Blanke C, et al:
Irinotecan plus fluorouracil and leucovorin for metastatic
colorectal cancer. N Engl J Med. 343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Imamura Y, Lochhead P, Yamauchi M, et al:
Analyses of clinicopathological, molecular and prognostic
associations of KRAS codon 61 and codon 146 mutations in colorectal
cancer: cohort study and literature review. Mol Cancer. 13:1352014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buchler T, Pavlik T, Melichar B, et al:
Bevacizumab with 5-fluorouracil, leucovorin and oxaliplatin versus
bevacizumab with capecitabine and oxaliplatin for metastatic
colorectal carcinoma: results of a large registry-based cohort
analysis. BMC Cancer. 14::3232014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi S: Vascular endothelial growth
factor (VEGF), VEGF receptors and their inhibitors for
antiangiogenic tumor therapy. Biol Pharm Bull. 34:1785–1788. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saif MW: Antiangiogenesis therapy in
second line metastatic colorectal cancer: similar but different.
Expert Opin Biol Ther. 13:1489–1493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung GG, Yoon HH, Zerkowski MP, et al:
Vascular endothelial growth factor, FLT-1 and FLK-1 analysis in a
pancreatic cancer tissue microarray. Cancer. 106:1677–1684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bellamy WT, Richter L, Frutiger Y and
Grogan TM: Expression of vascular endothelial growth factor and its
receptors in hematopoietic malignancies. Cancer Res. 59:728–733.
1999.PubMed/NCBI
|
|
8
|
Daenen LG, Roodhart JM, van Amersfoort M,
et al: Chemotherapy enhances metastasis formation via
VEGFR-1-expressing endothelial cells. Cancer Res. 71:6976–6985.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee YJ, Karl DL, Maduekwe UN, et al:
Differential effects of VEGFR-1 and VEGFR-2 inhibition on tumor
metastases based on host organ environment. Cancer Res.
70:8357–8367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
11
|
Fenton C, Patel A, Dinauer C, Robie DK,
Tuttle RM and Francis GL: The expression of vascular endothelial
growth factor and the type 1 vascular endothelial growth factor
receptor correlate with the size of papillary thyroid carcinoma in
children and young adults. Thyroid. 10:349–357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kato H, Yoshikawa M, Miyazaki T, et al:
Expression of vascular endothelial growth factor (VEGF) and its
receptors (Flt-1 and Flk-1) in esophageal squamous cell carcinoma.
Anticancer Res. 22:3977–3984. 2002.PubMed/NCBI
|
|
13
|
Tanigaki Y, Nagashima Y, Kitamura Y,
Matsuda H, Mikami Y and Tsukuda M: The expression of vascular
endothelial growth factor-A and -C and receptors 1 and 3:
correlation with lymph node metastasis and prognosis in tongue
squamous cell carcinoma. Int J Mol Med. 14:389–395. 2004.PubMed/NCBI
|
|
14
|
Dales JP, Garcia S, Bonnier P, et al:
Prognostic significance of VEGF receptors, VEGFR-1 (Flt-1) and
VEGFR-2 (KDR/Flk-1) in breast carcinoma. Ann Pathol. 23:297–305.
2003.(In French). PubMed/NCBI
|
|
15
|
Secord AA, Darcy KM, Hutson A, et al:
Gynecologic Oncology Group study: Co-expression of angiogenic
markers and associations with prognosis in advanced epithelial
ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol.
106:221–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamilton SR and Aaltonen LA: World Health
Organization Classification of TumoursPathology and Genetics of
Tumors of the Digestive System. IARC Press; Lyon, France: 2000
|
|
17
|
Itakura J, Ishiwata T, Shen B, Kornmann M
and Korc M: Concomitant over-expression of vascular endothelial
growth factor and its receptors in pancreatic cancer. Int J Cancer.
85:27–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Delmotte P, Martin B, Paesmans M, et al:
VEGF and survival of patients with lung cancer: a systematic
literature review and meta-analysis. Rev Mal Respir. 19:577–584.
2002.(In French). PubMed/NCBI
|
|
19
|
Bando H, Weich HA, Brokelmann M, et al:
Association between intratumoral free and total VEGF, soluble
VEGFR-1, VEGFR-2 and prognosis in breast cancer. Br J Cancer.
92:553–561. 2005.PubMed/NCBI
|
|
20
|
Wang L, Liu X, Wang H and Wang S:
Correlation of the expression of vascular endothelial growth factor
and its receptors with microvessel density in ovarian cancer. Oncol
Lett. 6:175–180. 2013.PubMed/NCBI
|
|
21
|
Boiocchi L, Vener C, Savi F, et al:
Increased expression of vascular endothelial growth factor receptor
1 correlates with VEGF and microvessel density in Philadelphia
chromosome-negative myeloproliferative neoplasms. J Clin Pathol.
64:226–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okita NT, Yamada Y, Takahari D, et al:
Vascular endothelial growth factor receptor expression as a
prognostic marker for survival in colorectal cancer. Jpn J Clin
Oncol. 39:595–600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhan P, Wang J, Lv XJ, et al: Prognostic
value of vascular endothelial growth factor expression in patients
with lung cancer: a systematic review with meta-analysis. J Thorac
Oncol. 4:1094–1103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen G, Ding J, Luo L, et al: Expression
of vascular endothelial growth factor and its receptors in
laryngeal carcinoma cell and its significance. Lin Chuang Er Bi Yan
Hou Ke Za Zhi. 19:842–844. 2005.(In Chinese). PubMed/NCBI
|
|
25
|
Fan F, Wey JS, McCarty MF, et al:
Expression and function of vascular endothelial growth factor
receptor-1 on human colorectal cancer cells. Oncogene.
24:2647–2653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhukova LG, Zhukov NV and Lichinitser MR:
Expression of Flt-1 and Flk-1 receptors for vascular endothelial
growth factor on tumor cells as a new prognostic criterion for
locally advanced breast cancer. Bull Exp Biol Med. 135:478–481.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhakal HP, Naume B, Synnestvedt M, et al:
Expression of vascular endothelial growth factor and vascular
endothelial growth factor receptors 1 and 2 in invasive breast
carcinoma: prognostic significance and relationship with markers
for aggressiveness. Histopathology. 61:350–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kopparapu PK, Boorjian SA, Robinson BD, et
al: Expression of VEGF and its receptors VEGFR1/VEGFR2 is
associated with invasiveness of bladder cancer. Anticancer Res.
33:2381–2390. 2013.PubMed/NCBI
|
|
29
|
Seibold ND, Schild SE, Bruchhage KL,
Gebhard MP, Noack F and Rades D: Prognostic impact of VEGF and
FLT-1 receptor expression in patients with locally advanced
squamous cell carcinoma of the head and neck. Strahlenther Onkol.
189:639–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sha D and He YJ: Expression and clinical
significance of VEGF and its receptors Flt-1 and KDR in
nasopharyngeal carcinoma. Ai Zheng. 25:229–234. 2006.(In Chinese).
PubMed/NCBI
|
|
31
|
Rades D, Setter C, Dunst J, Dahl O, Schild
SE and Noack F: Prognostic impact of VEGF and VEGF receptor 1
(FLT1) expression in patients irradiated for stage II/III non-small
cell lung cancer (NSCLC). Strahlenther Onkol. 186:307–314. 2010.
View Article : Google Scholar : PubMed/NCBI
|