Introduction
Struma ovarii (SO) originates from ovarian primitive
germ cells, and is classified as a single layer, highly specific
mature ovarian teratoma (1). In cases
where the thyroid component of the tumor accounts for >50% of
all tumor tissue, or <50% but with symptoms of hyperthyroidism,
this may be diagnosed as SO (2). SO
is a rare condition, accounting for 0.3% of ovarian tumors and 2.7%
of ovarian teratomas; 5–10% of tumors become malignant (3). It predominantly occurs between 30–50
years of age, most often in the unilateral ovary, and occasionally
in combination with contralateral ovary mature teratoma and
cystadenoma (4). Frequently, a lack
of clinical characteristics are present; certain patients
experience peritoneal effusion and/or, pleural effusion [false
Meigs sign (5)], and elevated serum
CA125 is observed in a number of cases. However, the occurrence of
pleural effusion is not necessarily indicative of malignant
manifestation (5). In 5% patients
with thyroid tissue differentiation, mature thyroid hyperfunction
may be observed (6). Written informed
consent was obtained from the patient.
Case report
A 49 year-old female patient was admitted to The
Affiliated Hospital of Inner Mongolia Medical University (Hohhot,
China) with an adnexa uteri tumor, which was identified by magnetic
resonance imaging (MRI). Approximately two years previously, the
patient incidentally identified a lump, which was∼30 mm in
diameter; no pain was experienced and the mass was palpable. After
a further year, an additional lump was identified in the right
lower abdomen. The bilateral masses increased gradually from this
time, and this was accompanied by intermittent abdominal pain over
the course of the disease. Gynecological examination revealed a
normal uterine size (diameter, ∼50 mm), a cystic and solid neoplasm
of ∼8 cm in diameter in the right adnexa uteri, and a cystic and
solid neoplasm of ∼7 cm in the left adnexa uteri. The two masses
were movable. The family history revealed no previous instances of
this condition. Physical examination revealed no abnormalities.
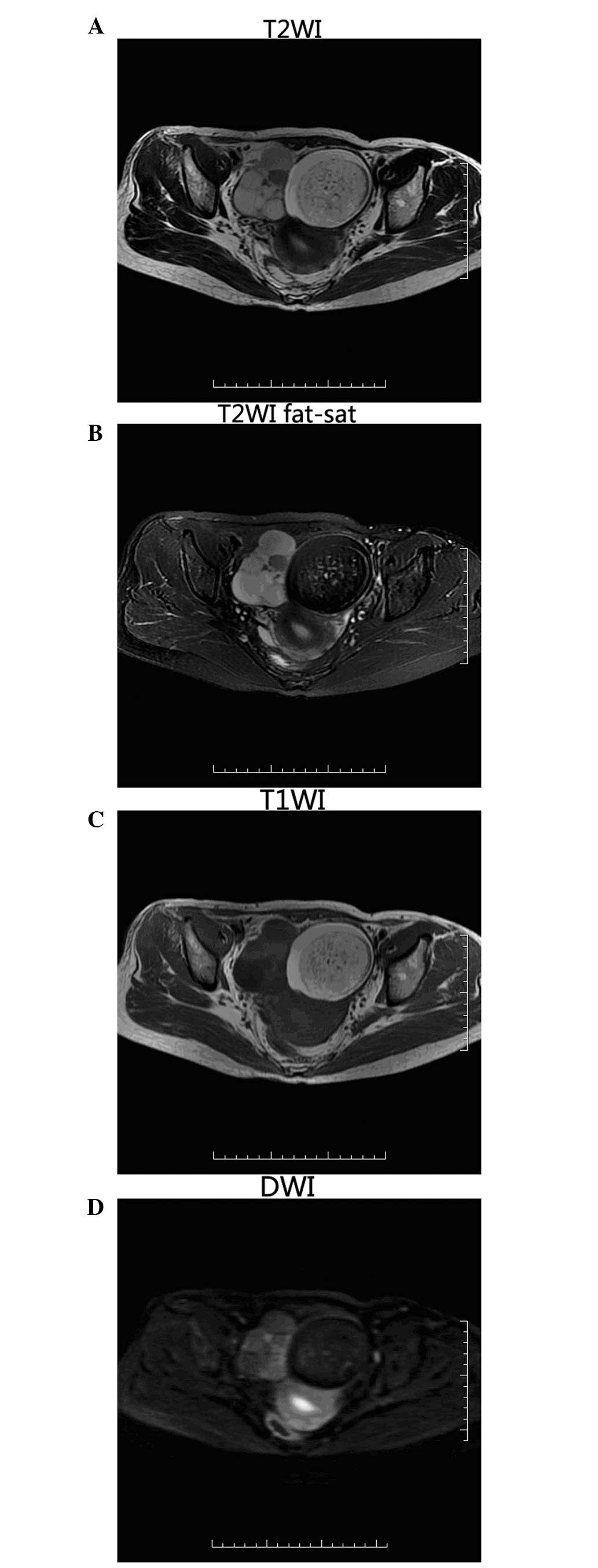
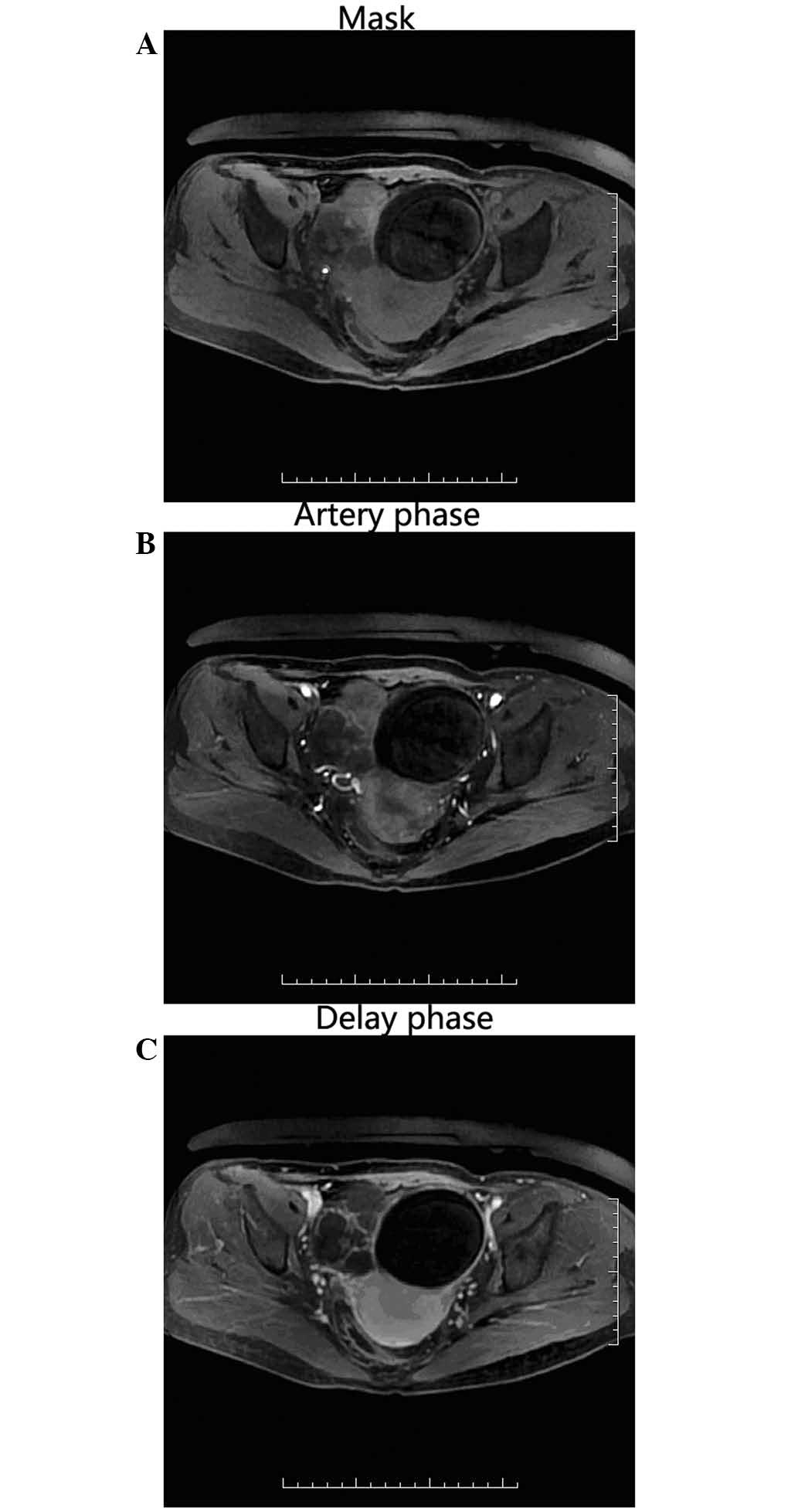
MRI showed abnormal signals in the bilateral adnexa
uteri (Figs. 1 and 2). During surgery to resect the bilateral
masses, the uterus was observed to be of normal size with a
diameter of 53 mm, the tumor on the right ovary was ∼73×58 mm in
diameter and was a complete capsule with a smooth surface,
containing a colorless liquid. The left ovarian mass was ∼75×87 mm
in diameter and was a complete capsule, with a surface scattered
with small blister-like nodules. Prior to surgery, the mass in the
right ovary was misdiagnosed as serous cystadenoma. However,
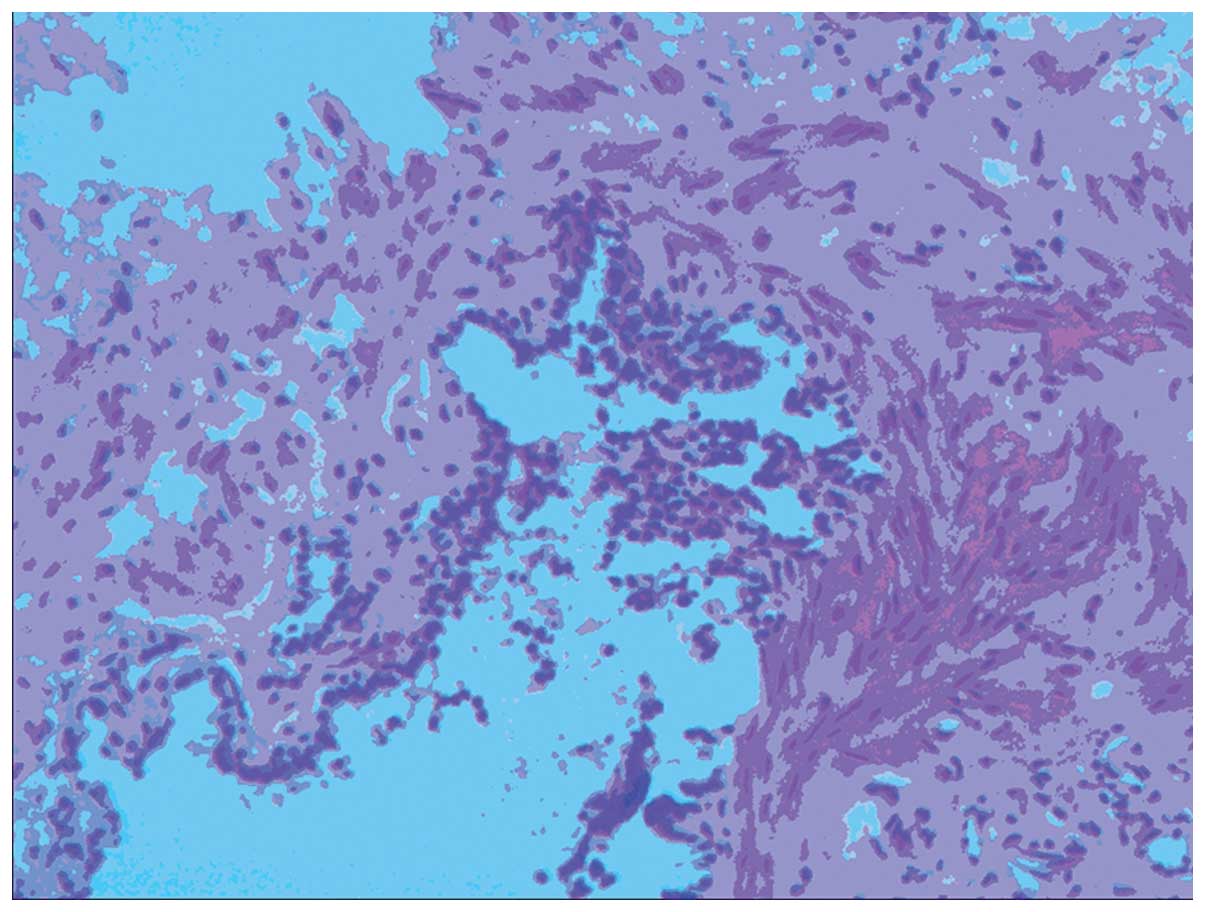
following resection of the two masses histological and
immunological analysis were performed, which revealed the
pathological diagnosis of struma ovarii in the right ovary and
mature cystic teratoma in the left ovary (Fig. 3). The tumor in the right ovary
consisted of thyroid tissue and stroma, with abundant blood vessels
and fibrous tissue. The tumor in the left ovary was a cystic solid
lesion, with thick regular borders and fatty components were
identified within the cyst. Follow-up examinations every four
months were planned. At the time of writing, the patient was well
and no recurrence had been identified.
Discussion
SO is a rare ovarian benign tumor, classified as
single layer ovarian teratoma by the WHO classification (7). SO is easily misdiagnosed prior to
surgery as the final diagnosis is dependent on pathological
analysis. Pathologically, all or the majority of the tumor tissue
was a particular type of mature teratoma, which was composed of
thyroid tissue (8). MRI may be used
to show the complex cystic and solid mass in cases of SO, with
variable signals, and the observable characteristics may be as
follows: i) A cystic and solid mass with clear boundaries, composed
of cystic intervals or where cystic components predominate
indicates the most common type of SO; ii) multiple cysts show
different signals from high to low on MRI, but are homogeneous.
Tumor hemorrhage may be observed on short T1 and short T2, or short
T1 and long T2 signals. On T2 weighted imaging (WI), no chemical
shift artifact is present. Okada et al (9) demonstrated that the low signal observed
for the mass on T2WI, occasionally referred to as the vacuum
phenomenon, is associated with the viscous fluid within cysts,
which is a specific indicator of SO; iii) the wall and interval of
cyst may be thick (∼5 mm); nodules are rare, and where present, are
significantly enhanced in the contrast MRI scan; and iv) in
instances where the lump is solid, it may also be enhanced.
The MRI revealed mixed T1 and T2 signals; the SO had
short T2 signals, uneven thickness, and was enhanced in the
contrast MRI. A region of the cyst exhibited short T2 and
marginally long T1 signals, and the signal was similar in the
images acquired using fat-suppressed and non-fat-suppressed
sequences, and was not enhanced on the contrast MRI scan. This was
proposed to be the vacuum phenomenon, as discussed above. The
contralateral ovarian lesion was a typical cystic teratoma, which
exhibited high T1, T2 based mixed signals; the high signals were
suppressed and lower than the signals of the tissues on
fat-suppressed sequence, and were not enhanced throughout the
dynamic enhanced MRI scan.
In the differential diagnosis, SO must be
differentiated from other cysts and cystic masses of the adnexa
uteri including ovarian serous cystadenoma, mucinous cystadenoma
and cystadenocarcinoma. Ovarian serous cystadenomas are
predominantly single cysts with abnormal long T1 and long T2
signals inside. They differ from SO in that the wall and interval
of the cyst is thicker and only certain SOs exhibit extremely low
signals on T2WI. The present case was initially misdiagnosed as
serous cystadenoma prior to surgery. Mucinous cystadenoma is
predominantly multicystic, with a variable signal; high signals are
usually observed on T2WI. The cyst wall and interval are
consistently thinner than that of SO. No obvious enhancement is
observed on the contrast MRI scan. Cystadenocarcinoma contains more
solid constituents than SO, the cystic wall is uneven and is
accompanied by nodules in the wall. Therefore, MRI may aid in
distinguishing SO from other carcinomas.
In conclusion, a diagnosis of SO must be considered
in females of child-bearing age, where a single solid and cystic
mass is observed in adnexa uteri, with a lobulated appearance,
clear boundaries and a thick cystic wall. Certain cases of SO
exhibit calcification, with a cystic region showing long T1 and
long T2 signals on plain MRI; additionally, a significantly
enhanced solid component, cystic wall and septum may be observed on
contrast MRI. Therefore, the MRI features of SO may aid with
pathological diagnosis prior to surgery, in patients with ovarian
tumors that exhibit atypical features.
References
|
1
|
Raina A, Stasi G, Monzio Compagnoni B, et
al: Struma ovarii - a rare gynecological tumor. Acta Oncol.
36:533–534. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krishnamurthy A, Ramshankar V,
Vaidyalingam V and Majhi U: Synchronous papillary carcinoma thyroid
with malignant struma ovarii: A management dilemma. Indian J Nucl
Med. 28:243–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Outwater EK, Siegelman ES and Hunt JL:
Ovarian teratomas: tumortypes and imaging characteristics.
Radiographics. 21:475–490. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nurliza Binti Md Nor, Kusumoto T, Inoue S,
et al: Three cases of struma ovarii underwent laparoscopic surgery
with definite prepperative diagnosis. Acta Med Okayama. 67:191–195.
2013.PubMed/NCBI
|
|
5
|
Mostaghel N, Enzevaei A, Zare K and
Fallahian M: Struma ovarii associated with Pseudo-Meig'ssyndrome
and high serum level of CA125; a case report. J Ovarian Res.
21:102012. View Article : Google Scholar
|
|
6
|
Teale E, Gouldesbrough DR and Peacey SR:
Gravesdisease and coexisting struma ovarii:struma expression of
thyrotropin receptors stimulating antibodies. Thyroid. 16:791–793.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Antonio A, Caleo A, Caleo O, et al:
Hashimoto thyroiditis as a manifestation of struma ovarii.
Endocrinologist. 20:220–221. 2010. View Article : Google Scholar
|
|
8
|
Matsuda K, Maehama T and Kanazawa K:
Malignant struma ovarii with thyrotoxicosis. Gynecol Oncol.
82:575–577. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okada S, Ohaki Y, Kawamura T, et al:
Cystic struma ovarii: imaging findings. J Comput Assist Tomogr.
24:413–415. 2000. View Article : Google Scholar : PubMed/NCBI
|