Introduction
Osteosarcoma is a primary malignant tumor of the
skeleton, which frequently occurs in adolescents (1). Due to the infiltrating growth of
osteosarcomas, therapeutic approaches have failed to establish a
radical cure. However, treatments have improved in the last 30
years with the development of aggressive and efficient combination
chemotherapy regimens (1). Although
the use of neoadjuvant chemotherapies is effective in prolonging
patient survival, they are often associated with the acquisition of
drug resistance and the occurrence of adverse reactions, including
myelosuppression, hepatotoxicity, toxicity of the kidneys, heart
and nervous system, and gastrointestinal reactions (2,3).
Therefore, a requirement exists for a novel biomarker that could be
used to determine the characteristics and prognosis of
osteosarcoma, and that could be applied as a therapeutic target for
the gene therapy of osteosarcoma.
Glucose-regulated protein 78 (GRP78), also known as
immunoglobulin heavy chain binding protein, is primarily located in
the endoplasmic reticulum (ER). The functions of the protein
include the facilitation of protein folding, assembly and
transport, calcium homeostasis, and the regulation of ER stress
signaling (4–6). It has been suggested that the
overexpression of GRP78 during periods of cellular stress may be an
important defense mechanism, which has a protective effect on cells
to ensure cell survival in a variety of adverse conditions. Several
previous studies indicated that GRP78 was induced at high levels in
malignant tumors, and had an important role in the anti-apoptotic
processes of tumor cells. By contrast, GRP78 remained at basal
levels in normal tissues (5,7). Gazit et al (8) demonstrated that the level of GRP78
protein was 1.5–3 times higher in human breast cancer cell lines
compared with normal epithelial cells. Fernandez et al
(9) also confirmed that GRP78
expression in breast cancer specimens was significantly higher than
that in adjacent tissues.
In addition, an overexpression of GRP78 has been
detected in several cancers, including brain, breast, lung,
prostate, colorectal and gastric cancer, and in hepatocellular
carcinoma (HCC) and ureter tumors (10–17). These
studies also revealed that GRP78 had an important role in the
process of metastasis, and that the knockdown of GRP78 inhibited
the invasiveness of cancer cells in vitro, and inhibited the
growth and metastasis of a malignant tumor allograft model
(18,19). Li et al (20) demonstrated that the knockdown of GRP78
downregulated the expression and activity of matrix
metalloproteinase-2 and TIMP metallopeptidase inhibitor-2 in HCC
cells. In another study, anti-GRP78 autoantibodies were suggested
to be potential diagnostic markers for HCC (11). In view of its importance for the
survival of cancer cells, GRP78 could be used as an anticancer drug
target. Certain anticancer compounds, such as plant-derived
genistein, (-)-Epigallocatechin gallate, honokiol and salicylic
acid could be used to inhibit the expression or activity of GRP78
(21–23).
GRP78 overexpression is usually associated with
high-grade malignant tumors, recurrence and bad prognoses, which
have been reported in several malignant tumors. However, the
present study did not review the experimental literature concerning
patients with osteosarcoma. In addition, numerous studies have
demonstrated that GRP78 represents a concordant mechanism of drug
resistance in malignant tumors, and could therefore be applied as a
predictor for guiding the treatment for patients (11,20–23). The
present study aimed to investigate the expression of GRP78 in
patients with osteosarcoma, and to analyze the expressional
differences in tumor tissue and normal tissue, chemotherapy- and
non-chemotherapy-treated patients, and metastatic and
non-metastatic tumors. According to these results, the association
between the expression of GRP78 and tumor growth, metastasis and
chemotherapeutics could be determined. Furthermore, it was hoped
that the results of the present study could identify a novel
biomarker that could be used to determine the characteristics and
prognosis of osteosarcoma, and that could be applied as a
therapeutic target for osteosarcoma gene therapies.
Materials and methods
Specimen selection
Between 2007 and 2012, 60 patients were diagnosed
with osteosarcoma at the Affiliated Hospital of Shandong University
of Traditional Chinese Medicine (Jinan, China) were selected for
the present study. Of these patients, 20 presented with
non-metastatic tumors and 40 with metastatic tumors, and 20 had
been treated without chemotherapy and 40 with chemotherapy. In
addition, 60 specimens from adjacent normal tissues were collected
to form the control group. In total, 38 of the cases were male, and
22 were female. The mean age was 16.6 years (range, 6–53 years).
All patients had been previously diagnosed with osteosarcoma,
exclusive of any other malignant tumor on the locomotor system,
using the results from medical imaging, which consisted of
radiography, CT and MRI, followed by an open biopsy. All patients
had complete follow-up data.
The osteotomy plane was confirmed for all patients
at 30 mm distal from the primary tumor using T1-weighted MRI
(24). Primary tumor specimens were
obtained for the experimental group to detect every indicatrix.
Normal tissue around the primary tumors was also collected for the
control group. In addition, the experimental group was divided into
a pre-and post-chemotherapy group, and a metastasis and
non-metastasis group. The protocol of the present study was
prepared according to the Declaration of Helsinki, and was approved
by the Ethics Committee of the Affiliated Hospital of Shandong
University of Traditional Chinese Medicine (Jinan, Shandong,
China). Written informed consent was obtained from all
patients.
Reagents
The rabbit anti-human monoclonal GRP78 antibody was
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit
immunoglobulin G [IgG; heavy and light (H+L) chain] was obtained
from EarthOx Life Sciences. (Millbrae, CA, USA). The total RNA
extraction kit was purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). The reverse transcription polymerase chain
reaction (RT-PCR) kit was obtained from Thermo Fisher Scientific
(Pittsburgh, PA, USA) and the Bradford protein assay kit was
purchased from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). The
sequences of the GRP78 gene primer were as follows: Upstream
primer, 5′-CGTCCTATGTCGCTT CACT-3′; and downstream primer,
5′-TGTCTTTGTTTGCCCACCTC-3′.
Immunofluorescence staining
The tissue samples, measuring ∼1.5×1.5×0.2 cm, were
embedded in paraffin following fixation in 4% paraformaldehyde for
72 h, according to standard laboratory procedures. The paraffin
blocks which had been stained with hematoxylin and eosin in order
to establish a diagnosis, were used in the subsequent
immunofluoresence analysis. First, the paraffin blocks were cut
into 7-µm sections and open-air dried at room temperature. Next,
the tissue sections were fixed in acetone at 4°C for 15 min, and
washed with phosphate-buffered saline (PBS). The sections were then
incubated in 3% hydrogen peroxide for 5–10 min in order to quench
the endogenous peroxidase activity, and then washed again with PBS.
Next, the sections were blocked with 50 µl 5% normal goat serum
(diluted with PBS) and incubated for 20–30 min in a moist chamber.
The sections were first incubated with the primary anti-GRP78
antibody (dilution, 1:50) in a moist chamber at 4°C for 36 h, prior
to washing three times with PBS. Next, the sections were incubated
with a goat anti-rabbit IgG/FITC antibody (dilution, 1:200) in a
moist chamber at 37°C for 30 min, prior to washing three times with
PBS. The sections were then blocked with glycerol following
incubation with DAPI (dilution, 1:200) for 5 min at room
temperature.
Sections of normal tissue were similarly prepared,
using each assay as a positive control. Subsequent to performing
all steps, except for the addition of the primary antibody, each
case had a negative control specimen.
The sections were analyzed using a Leica DM4000B
microscope (Leica Microsystems GmbH, Wetzlar, Germany), and images
were captured using the Image Pro Plus image analysis system 7.0
(Media Cybernetics, Inc., Rockville, MD, USA) in order to detect
the expression level. Histiocytes in which the cytoplasm was
stained green were considered to be GRP78-positive cells.
RT-PCR
The total RNA was extracted using a total RNA
extraction kit, according to the manufacturer's instructions.
Overall, 3 µg total RNA was subjected to RT-PCR using M-MuLV
reverse transcriptase (Thermo Fisher Scientific) and
oligo(dT)18 primers. PCR was performed in the presence
of 25 mM Mg2+, using equal amounts of cDNA, 1 unit of
Taq polymerase (Promega Corporation, Madison, WI, USA) and 20 mM of
the following primers: Forward, 5′-CGTCCTATGTCGCCTTCACT-3′; and
reverse, 5′-TGTCTTTGTTTGCCCACCTC-3′. The cycling parameters (30
cycles) were as follows: Denaturation at 94°C for 1 min, annealing
at 58°C for 1 min and extension at 72°C for 1 min. All experiments
were performed in triplicate. Next, 3 µl PCR product with 0.5 µl 5X
loading buffer was transferred to a 2% agarose gel electrophoresis
system with conditions of 120 V and 100 mA for 30 min. The
electropherogram was transferred to the electrophoresis image
analysis system to measure the expression intensity of GRP78 mRNA.
The formula of the relative level of GRP78 mRNA expression was as
follows: Relative level of GRP78 mRNA = value of GRP78 mRNA in
samples / value of β-actin in samples.
Western blot analysis
In total, ∼100 mg of frozen tissue samples were
homogenized in 400 µl ice-cold RIPA buffer [containing
phenylmethylsulfonyl fluoride]. Following 30 min of schizolysis on
ice, the samples were spun at 12,000 × g for 5 min at 4°C and the
supernatants were collected. Next, two additional centrifugations
at 2,500 × g were performed in order to produce clarified lysates.
The protein concentrations of the resulting lysates were determined
using the Bradford Protein Assay kit. Sample volumes equivalent to
30 µg of protein were aliquotted, normalized to equivalent volumes
of RIPA buffer, and then lyophilized in vacuo at a low heat.
The samples were then rehydrated with 10 ml of deionized water
followed by an equivalent volume of electrophoresis sample buffer
(1.0 ml glycerol, 0.5 ml β-mercaptoethanol, 3.0 ml 10% SDS, 1.25 ml
1.0M Tris-HCl pH 6.7 and 1–2 mg bromophenol blue). Next, the
samples were denatured at 90°C for 5 min, loaded onto an 8%
SDS-polyacrylamide gel containing a 4% stacking gel and
electrophoresed at 100 V for 1.5 h in Tris-glycine running buffer
(25 mM Tris-base, 250 mM glycine and 0.1% SDS). In addition to the
pairs of tumor and normal tissue samples, each gel was also loaded
with a molecular weight standard. The proteins were electroblotted
onto a nitrocellulose membrane (Gelman Sciences, Ann Arbor, MI,
USA) at 60 V for 3 h. Next, the membrane was blocked for 1 h with
1% bovine serum albumin (BSA) in Tris-buffered saline with Tween-20
[TBST; 10 mM Tris-HCl (pH 8.0), 150 mM NaCl and 0.05% Tween-20]
with gentle shaking at room temperature, and then incubated
overnight at 4°C without agitation in 1% BSA/TBST containing a
GRP78 monoclonal antibody (dilution, 1:1,000), and an actin
monoclonal antibody (dilution, 1:500). Following two rinses with
TBST, the membrane was washed in TBST with gentle shaking for 1 h,
with buffer changes every 10 min. The membrane was then incubated
in 1% BSA/TBST containing an FITC goat anti-rabbit IgG secondary
antibody (1:5,000 dilution) for 1 h with gentle shaking. Following
two rinses with TBST, the membrane was washed in TBST with gentle
shaking for 1 h, with buffer changes every 5 min.
GRP78 and β-actin protein expression was
simultaneously detected using an enhanced chemiluminescence
detection system (DuPont NEN, Boston, CA, USA). The signals were
visualized by autoradiography and quantified with densitometry.
GRP78 protein expression was normalized to β-actin for the loading
control. The expression level of GRP78 protein was indicated by the
optical density value.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using a one-way analysis of
variance and Tamhane's T2 test to determine significant differences
among the groups. P<0.05 was used to indicate a statistically
significant difference.
Results
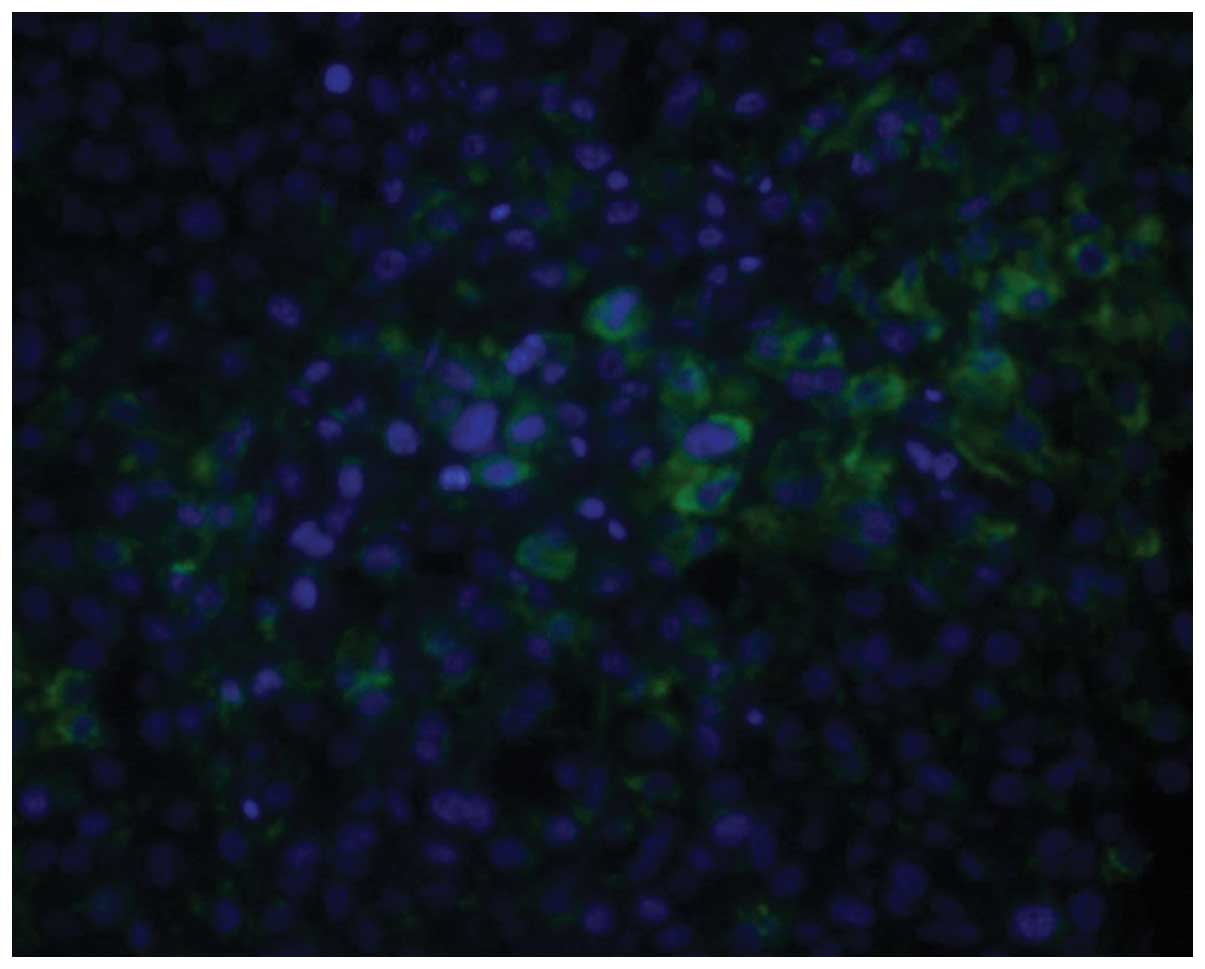
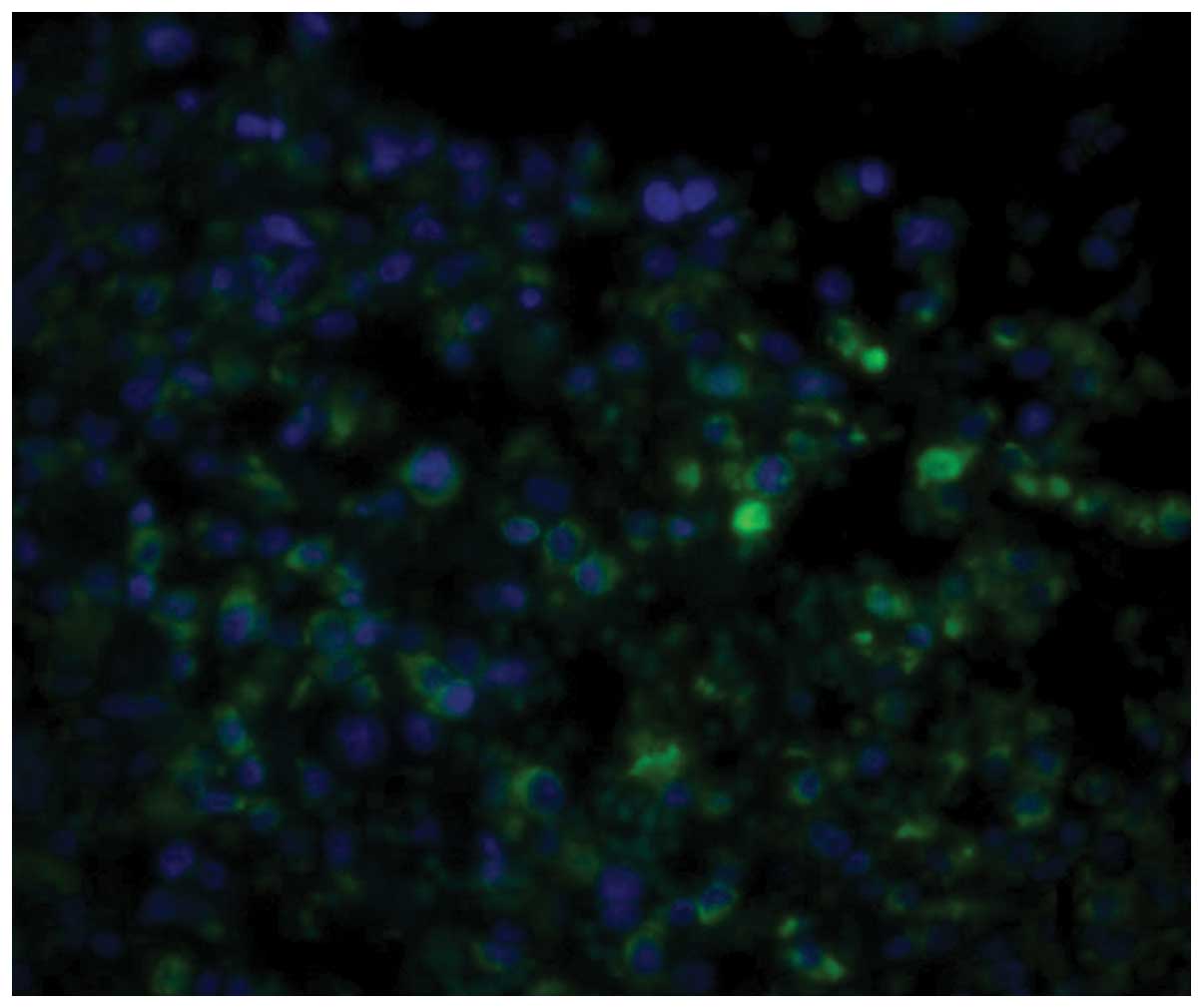
Immunofluorescence staining
GRP78 was mainly located in the ER. Histiocytes in
which the cytoplasm was stained green were considered to be
GRP78-positive cells. The expression level of GRP78 in the tumor
tissue was significantly higher than that in the normal tissue
surrounding the tumor (P<0.01). Furthermore, the expression
level of GRP78 was correlated to metastasis and chemotherapy status
(Figs. 1–5). As shown in Table I, there was a significant difference
between the non-chemotherapy and chemotherapy groups (P<0.01).
In addition, the expression level of GRP78 in the metastasis group
was higher than that in the non-metastasis group (P<0.05).
 | Table I.Immunofluorescence staining results of
GRP78 in normal and tumor tissues (n=60). |
Table I.
Immunofluorescence staining results of
GRP78 in normal and tumor tissues (n=60).
|
|
| Tumor tissue |
|---|
|
|
|
|
|---|
|
|
| Chemotherapy | Non-chemotherapy |
|---|
|
|
|
|
|
|---|
| Variable | Normal tissue | Non-metastasis | Metastasis | Non-metastasis | Metastasis |
|---|
| n | 60 | 10 | 30 | 10 | 10 |
| Fluorescence
intensity | 0.57±0.13 |
1.51±0.22a |
1.89±0.35a,b |
2.15±0.44a,c |
2.91±0.57a,b,c |
RT-PCR
GRP78 mRNA was identified in all specimens. As shown
in Table II, the expression level of
GRP78 mRNA was 0.24±0.02 in normal tissue, which was significantly
lower than that in the tumor tissue (P<0.01). Furthermore, the
expression level of GRP78 mRNA in the tumor tissues was correlated
to metastasis and chemotherapy status (Fig. 6). The expression level of GRP78 mRNA
in the metastasis group was higher than in the non-metastasis group
(P<0.05). The expression level was also higher in the
non-chemotherapy group than in the chemotherapy group
(P<0.05).
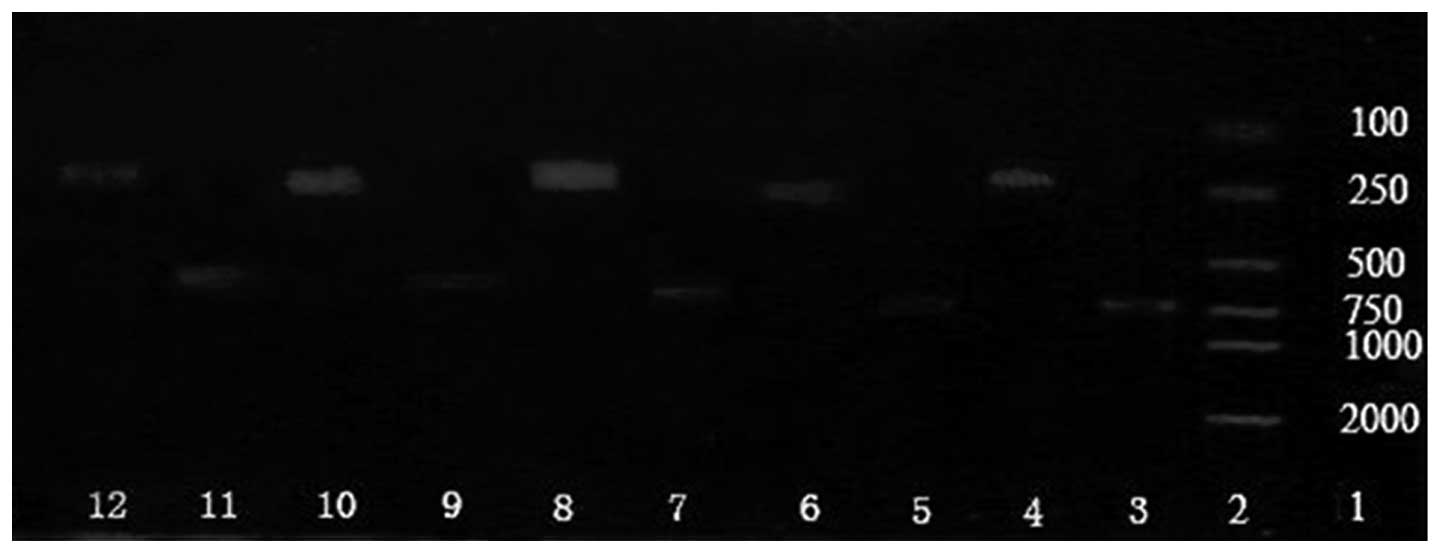 | Figure 6.Expression of GRP78 mRNA in normal and
tumor tissues. Lane 1, marker DL20006; lane 2, prestained protein
molecular weight marker; lane 3, β-actin in metastatic tumor tissue
treated with chemotherapy; lane 4, GRP78 in metastatic tumor tissue
treated with chemotherapy; lane 5, β-actin in non-metastatic tumor
tissue treated with chemotherapy; lane 6, GRP78 in non-metastatic
tumor tissue treated with chemotherapy; lane 7, β-actin in
metastatic tumor tissue treated without chemotherapy; lane 8, GRP78
in metastatic tumor tissue treated without chemotherapy; lane 9,
β-actin in non-metastatic tumor tissue treated without
chemotherapy; lane 10, GRP78 in non-metastatic tumor tissue treated
without chemotherapy; lane 11, β-actin in normal tissue; lane 12,
GRP78 in normal tissue. GRP78, glucose-regulated protein 78. |
 | Table II.Immunofluorescence staining results of
GRP78 in normal tissue and tumor tissue (n=60). |
Table II.
Immunofluorescence staining results of
GRP78 in normal tissue and tumor tissue (n=60).
|
|
| Tumor tissue |
|---|
|
|
|
|
|---|
|
|
| Chemotherapy |
Non-chemotherapy |
|---|
|
|
|
|
|
|---|
| Variable | Normal tissue | Non-metastasis | Metastasis | Non-metastasis | Metastasis |
|---|
| n | 60 | 10 | 30 | 10 | 10 |
| Relative
expression | 0.24±0.02 |
0.70±0.05a |
1.21±0.04a,b |
1.54±0.06a,c |
1.87±0.05a,b,c |
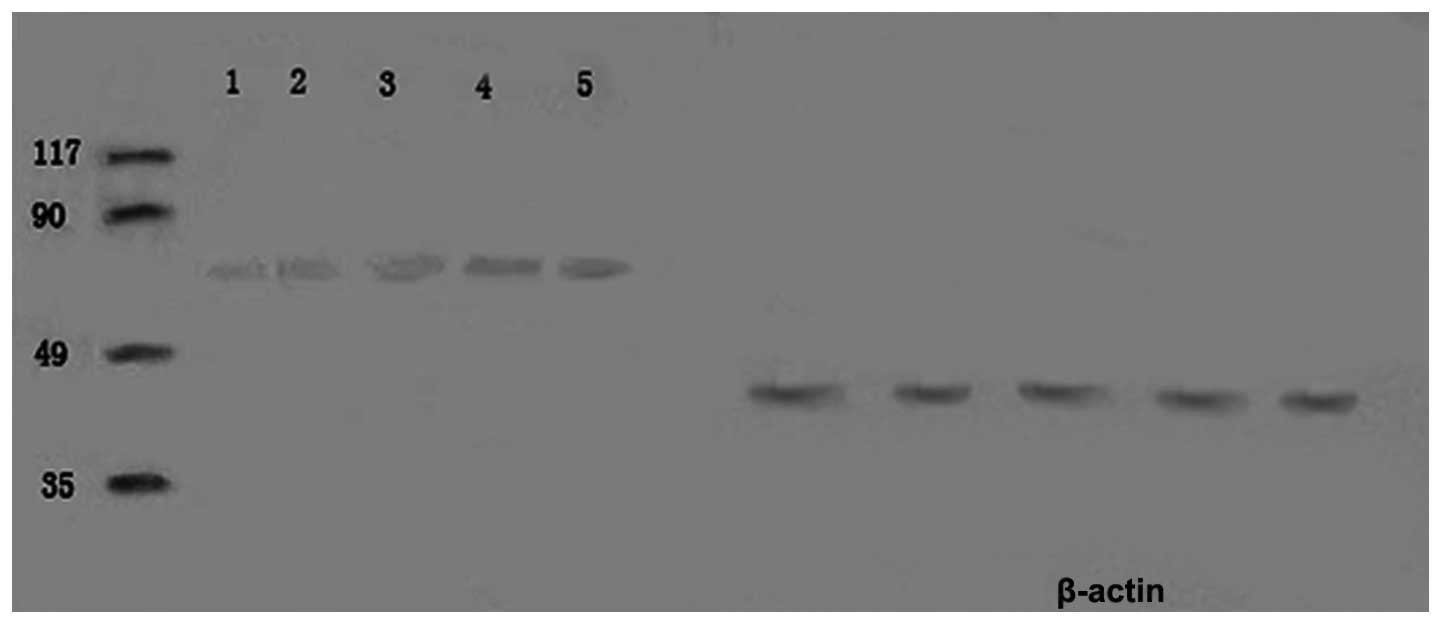
Western blot analysis
GRP78 protein was expressed in all specimens. The
expression level of GRP78 protein in the tumor tissue was
significantly higher than in the normal tissue surrounding the
tumor (P<0.01), which was consistent with the results of the
immunofluorescence staining (Fig. 7).
Furthermore, the expression level of GRP78 protein in the tumor
tissues was correlated with metastasis and chemotherapy status. The
expression level of GRP78 protein in the metastasis group was
higher than in the non-metastasis group (P<0.05). The expression
level was also higher in the non-chemotherapy group than in the
chemotherapy group (P<0.01; Table
III).
 | Table III.Expression level of GRP78 protein in
normal tissue and tumor tissue (n=60). |
Table III.
Expression level of GRP78 protein in
normal tissue and tumor tissue (n=60).
|
|
| Tumor tissue |
|---|
|
|
|
|
|---|
|
|
| Chemotherapy |
Non-chemotherapy |
|---|
|
|
|
|
|
|---|
| Variable | Normal tissue | Non-metastasis | Metastasis | Non-metastasis | Metastasis |
|---|
| n | 60 | 10 | 30 | 10 | 10 |
| OD value | 210.53±4.74 |
271.63±4.97a |
315.29±6.46a,b |
366.78±5.82a,c |
454.31±7.35a,b,c |
Discussion
GRP78 belongs to the Hsp70 family of proteins and
was identified as a glucose-deficiency protein alongside GRP94 and
GRP58 in the late 1970s. The protein is recognized as a major
molecular chaperone and signal-regulated factor in the ER stress
signaling pathway (4,5). The results of previous studies have
suggested that the overexpression of GRP78 in periods of cellular
stress may be an important defense mechanism, which has a
protective effect on cells and ensures cell survival in the
presence of a variety of adverse factors (6). GRP78 is primarily located at the ER,
which is consistent with the results of the immunofluorescence
staining analysis in the present study. In the present study,
histiocytes in which the cytoplasm was stained green were
considered to be GRP78-positive cells.
A previous study revealed that GRP78 decreased the
cytotoxic T cell-mediated destruction of tumor cells, promoted
tumor formation and resistance to chemotherapy, and prevented
apoptosis (25). Furthermore, several
studies have indicated that GRP78 is induced at high levels in
malignant tumors, despite GRP78 remaining at low levels in the main
organs. Gazit et al (8)
demonstrated that the protein level of GRP78 was 1.5–3 times higher
in a human breast cancer cell line compared with normal epithelial
cells. In addition, Fernandez et al (9) confirmed that GRP78 expression in breast
cancer specimens was significantly higher than in adjacent tissues.
A study by Koomägi et al (18)
indicated that GRP78 was overexpressed in human non-small cell lung
cancer. Furthermore, a number of previous studies demonstrated that
GRP78 was induced and expressed at a high level in brain, prostate,
colorectal and gastric cancers, and in HCCs and ureter tumors
(10–12,14,15,17).
Using immunohistological staining, RT-PCR and western blotting, the
present study revealed that the expression level of GRP78 in human
osteosarcoma tissues was higher than that in the normal tissues
surrounding the tumors. To the best of our knowledge, this is the
first study to demonstrate the overexpression of GRP78 in human
osteosarcoma.
In recent years, studies have attempted to
investigate the association between GRP78 expression and tumor
stage and patient survival. In a retrospective cohort study, it was
revealed that high GRP78 expression in visceral adipocytes from
endometrial cancer patients was positively correlated with
advanced-stage disease, deep myometrial invasion and decreased
disease-free survival time (26).
Park et al (17) also
demonstrated that the overexpression of GRP78 in patients with
ureter tumors was associated with a high tumor (T) stage and
nuclear grade, a high bladder cancer recurrence rate and a low
survival rate.
Another previous study, which analyzed 137 renal
cell carcinoma specimens, established that there was a significant
association between GRP78 positivity and a higher tumor grade, an
advanced T stage, lymphovascular invasion, regional nodal
involvement and distant metastases (27). In the present study, despite the
association between GRP78 and osteosarcoma grade, a correlation
with the T stage was not detected. However, it was identified that
the expression level of GRP78 in the metastasis group was higher
than that in the non-metastasis group. However, certain studies
contradict the findings of the present study. For example, Hardy
et al (28) demonstrated that
GRP78 negativity was correlated with a high colon cancer cell
proliferation rate and the presence of liver metastasis in nude
mice. By contrast, GRP78-positive cells exhibited reduced
proliferation, tumor growth and liver metastasis. Therefore,
whether or not the expression level of GRP78 and its role in tumor
cells is associated with the origin of the tumor cells requires
further investigation.
Due to its protective effect upon tumor cells, GRP78
could be used as a target of chemotherapy. The suppression of GRP78
could increase the apoptosis of tumor cells, slow down tumor growth
and improve patient survival. Furthermore, recent studies verified
that targeting GRP78 promoted apoptosis and overcame resistance to
drug-induced cell death in several cancer cells (29–31). Kuo
et al (32) demonstrated that
silencing GRP78 not only inhibited the formation of colon cancer
tumors, but also decreased the expression of vascular endothelial
growth factor (VEGF) and VEGF receptor 2. Furthermore, certain
studies have used overexpressed GRP78 as a target protein for
guiding drugs to gastric cancer tumor cells, which may lead to the
precise targeting of tumor cells and less side-effects (33). The present study established that the
expression level of GRP78 in the non-chemotherapy group was higher
than that in the chemotherapy group. This result demonstrated that
chemotherapeutics are able to decrease the expression level of
GRP78 in human osteosarcoma cells. However, whether or not GRP78 is
the primary molecular target involved in this process should be
investigated further.
Using immunohistological staining, RT-PCR and
western blot analysis, the present study revealed that the
expression level of GRP78 in human osteosarcoma tissues was higher
than that in the normal tissues surrounding the tumor. The
expression level was also higher in the metastasis group compared
with the non-metastasis group, and in the non-chemotherapy group
compared with the chemotherapy group. The results indicated that
there was a direct association between GRP78 expression and tumor
growth, metastasis and chemotherapy. The results of the present
study not only verified the expression of GRP78 in human
osteosarcoma cells, but also provided experimental and theoretical
evidence for the evaluation of therapy and prognosis. Therefore, it
can be concluded that GRP78 should be applied as a molecular target
in order to analyze tumor behaviors and therapeutic reactions. In a
clinical setting, GRP78 may represent a novel biomarker that could
be used to determine the development and prognosis of
osteosarcomas. Combination therapies for suppressing GRP78
expression could inhibit tumor growth, increase sensitivity to
chemotherapy, suppress metastasis and improve the prognosis. Such
treatments could therefore be applied as a novel form of gene
therapy for osteosarcoma. However, further studies are required in
order to determine whether the combined application of GRP78
inhibitors and conventional chemotherapies could enhance the
efficacy of drugs and cure primary tumors, and whether GRP78 could
be applied as a serological diagnostic biomarker for
osteosarcoma.
Acknowledgements
This study was supported by the National Science
Foundation of Shandong Province (grant. no. ZR2014HQ034).
References
|
1
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. 2:62007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uchida A: Recent advances in management of
musculoskeletal tumors. Gan To Kagaku Ryoho. 26:185–190. 1999.[In
Japanese]. PubMed/NCBI
|
|
3
|
Bacci G and Lari S: Current treatment of
high grade osteosarcoma of the extremity: review. J Chemother.
13:235–243. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee AS: The glucose-regulated proteins:
stressinduction and clinical applications. Trends Biochem Sci.
26:504–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hendershot LM: The ER function BiP is a
master regulator of ER function. Mt Sinai J Med. 71:289–297.
2004.PubMed/NCBI
|
|
6
|
Lee AS: GRP78 induction in cancer:
therapeutic and prognostic implications. Cancer Res. 67:3496–3499.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pfaffenbach KT and Lee AS: The critical
role of GRP78 inphysiologic and pathologic stress. Curr Opin Cell
Biol. 23:150–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gazit G, Lu J and Lee AS: De-regulation of
GRP stress protein expression in human breast cancer cell lines.
Breast Cancer Res Treat. 54:135–146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernandez PM, Tabbara SO, Jacobs LK,
Manning FC, Tsangaris TN, Schwartz AM, Kennedy KA and Patierno SR:
Overexpression of the glucose regulated stress gene GRP78 in
malignant but not benign human breast lesions. Breast Cancer Res
Treat. 59:15–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsunemi S, Nakanishi T, Fujita Y, Bouras
G, Miyamoto Y, Miyamoto A, Nomura E, Takubo T and Tanigawa N:
Proteomics-based identification of a tumor-associatedantigen and
its corresponding autoantibody in gastric cancer. Oncol Rep.
23:949–956. 2010.PubMed/NCBI
|
|
11
|
Shao Q, Ren P, Li Y, Peng B, Dai L, Lei N,
Yao W, Zhao G, Li L and Zhang J: Autoantibodies against
glucose-regulated protein 78 as serological diagnostic biomarkers
in hepatocellular carcinoma. Int J Oncol. 41:1061–1067.
2012.PubMed/NCBI
|
|
12
|
Zhang LH, Yang XL, Zhang X, Cheng JX and
Zhang W: Association of elevated GRP78 expression with increased
astrocytoma malignancy viaAkt and ERK pathways. Brain Res.
1371:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uramoto H, Sugio K, Oyama T, Nakata S, Ono
K, Yoshimatsu T, Morita M and Yasumoto K: Expression of endoplasmic
reticulum molecular chaperone Grp78 in human lungcancer and its
clinical significance. Lung Cancer. 49:55–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xing X, Li Y, Liu H, Wang L and Sun L:
Glucose regulated protein 78 (GRP78) is overexpressed in
colorectalcarcinoma and regulates colorectal carcinoma cellgrowth
and apoptosis. Acta Histochem. 113:777–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Daneshmand S, Quek ML, Lin E, Lee C, Cote
RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG, et al:
Glucose-regulated protein GRP78 is up-regulated in prostatecancer
and correlates withrecurrence and survival. Hum. Pathol.
38:1547–1552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Jiang Y, Jia Z, Li Q, Gong W,
Wang L, Wei D, Yao J, Fang S and Xie K: Association of elevated
GRP78 expression with increased lymph nodemetastasis and poor
prognosis in patients with gastric cancer. Clin Exp Metastasis.
23:401–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park CH, Choi MS, Ha JY, Kim BH, Park CH
and Kim CI: Effect of overexpression of glucose-regulated protein
78 and bcl-2 onrecurrence and survival in patients with ureter
tumors. Korean J Urol. 54:671–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koomägi R, Mattern J and Volm M:
Glucose-related protein (GRP78) and its relationship to
drug-resistance proteins PI70, GST-pi, LRP56 and angiogenesis in
non-small cell lung carcinomas. Anticancer Res. 19:4333–4336.
1999.PubMed/NCBI
|
|
19
|
Linnik KM and Herscovitz H: Multiple
molecular chaperones interact with apoliprotein B during its
maturation: The network of endoplasmic reticulum-resident
chaperones (ERp72, GRP94, calreticulin, and BiP) interacts with
apoliprotein b regardless of its lipidation state. J Biol Chem.
273:21368–21373. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Song H, Luo J, Liang J, Zhao S and
Su R: Knockdown of glucose-regulated protein 78 decreases
theinvasion, metalloproteinaseexpression and ECM degradation in
hepatocellular carcinoma cells. J Exp Clin Cancer Res. 31:392012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ermakova SP, Kang BS, Choi BY, Choi HS,
Schuster TF, Ma WY, Bode AM and Dong Z: (-)-Epigallocatechin
gallate overcomes resistance to etoposide-induced cell death by
targeting the molecular chaperone glucose-regulated protein 78.
Cancer Res. 66:9260–9269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng WG, Ruan KH, Du M, Saunders MA and Wu
KK: Aspirin and salicylate bind to immunoglobulin heavy chain
binding protein (BiP) and inhibit its ATPase activity in human
fibroblasts. FASEB J. 15:2463–2470. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin S, Lamb HK, Brady C, Lefkove B,
Bonner MY, Thompson P, Lovat PE, Arbiser JL, Hawkins AR and Redfern
CP: Inducing apoptosis of cancer cells using small-molecule plant
compounds that bind to GRP78. Br J Cancer. 109:433–443. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hao YK, Zhang YK, Yang ZP, Li X, Yang Q
and Li JM: The accuracy of magnetic resonance imaging in
determining osteotomy plane in osteosarcoma. Orthopedics.
31:5442008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong D, Dubeau L, Bading J, Nguyen K, Luna
M, Yu H, Gazit-Bornstein G, Gordon EM, Gomer C, Hall FL, Gambhir SS
and Lee AS: Spontaneous and controllable activation of suicide gene
expression driven by the stress-inducible grp78 promoter resulting
in eradication of sizable human tumors. Hum Gene Ther. 15:553–561.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsuo K, Gray MJ, Yang DY, Srivastava SA,
Tripathi PB, Sonoda LA, Yoo EJ, Dubeau L, Lee AS and Lin YG: The
endoplasmic reticulum stressmarker, glucose-regulated protein-78
(GRP78) in visceral adipocytes predicts endometrial
cancerprogression and patient survival. Gynecol Oncol. 128:552–559.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuroda K, Horiguchi A and Asano T, Ito K,
Asakuma J, Sato A, Yoshii H, Hayakawa M, Sumitomo M and Asano T:
Glucose-regulated protein 78 positivity as a predictor of poor
survival in patients with renal cell carcinoma. Urol Int.
87:450–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hardy B, Raiter A, Yakimov M, Vilkin A and
Niv Y: Colon cancer cells expressing cell surface GRP78 as a marker
for reduced tumorigenicity. Cell Oncol (Dordr). 35:345–354. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park HR, Tomida A, Sato S, Tsukumo Y, Yun
J, Yamori T, Hayakawa Y, Tsuruo T and Shin-ya K: Effect on tumor
cells of blocking survival response to glucose deprivation. J Natl
Cancer Inst. 96:1300–1310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gupta P, Walter MR, Su ZZ, Lebedeva IV,
Emdad L, Randolph A, Valerie K, Sarkar D and Fisher PB: BiP/GRP78
is an intracellular target for MDA-7/IL-24 induction of
cancer-specific apoptosis. Cancer Res. 66:8182–8191. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gonzalez-Gronow M, Cuchacovich M, Llanos
C, Urzua C, Gawdi G and Pizzo SV: Prostate cancer cell
proliferation in vitro is modulated by antibodies against
glucose-regulated protein 78 isolated from patient serum. Cancer
Res. 66:11424–11431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuo LJ, Hung CS, Chen WY, Chang YJ and Wei
PL: Glucose-regulated protein 78 silencing down-regulates vascular
endothelial growth factor/vascular endothelial growth factor
receptor 2 pathway to suppress human colon cancer tumor growth. J
Surg Res. 185:264–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng CC, Lu N, Peng CL, Chang CC, Mai FD,
Chen LY, Liao MH, Wang WM and Chang J: Targeting to overexpressed
glucose-regulated protein 78 in gastric cancer discovered by 2D
DIGE improves thediagnostic and therapeutic efficacy of
micelles-mediated system. Proteomics. 12:2584–2597. 2012.
View Article : Google Scholar : PubMed/NCBI
|