Introduction
It is widely accepted that the histology of ovarian
cancer does not change in a patient. However, cancer stem cells
(CSCs) are the origin of numerous tumors (1). Ovarian CSCs form spheroids and
multicellular colonies that differentiate along epithelial,
granulosa and germ cell lineages in vivo (2). CSCs have been shown to exist in ovarian
carcinomas, and the expression of cluster of differentiation
(CD)44, c-Kit and CD133 have been detected in these tumors
(1). It appears that the histology of
ovarian cancer would be change in the period of therapy with CSCs
existing. To the best of our knowledge, no studies currently exist
regarding the transformation of ovarian cancer from one histology
into another. In the present study, the ovarian cancer transformed
from adenocarcinoma into undifferentiated small cell carcinoma,
supporting the hypothesis that CSCs can differentiate into
different types of mature neoplastic cells.
Case report
A 27-year-old female presented to The Affiliated
Wujing Hospital of Guangzhou Medical College (State Key Laboratory
of Respiratory Disease, Guangzhou, Guangdong, China) with an
abdominal mass in April 2011. Computed tomography (CT) revealed a
large intraperitoneal cystic mass and ascites. The patient
presented with abdominal pain, but no fever. Complete blood count,
and liver and renal function test results were normal. An ascites
sample obtained by abdominocentesis tested positive for
adenocarcinoma. The patient underwent surgery in June 2011, and
multiple pelvic nodules, a large intraperitoneal cystic mass with
ovarian involvement and an abdominal peritoneal implant, measuring
1.0 cm in diameter, were found. The inguinal and retroperitoneal
lymph nodes were negative for cancer cell invasion. The
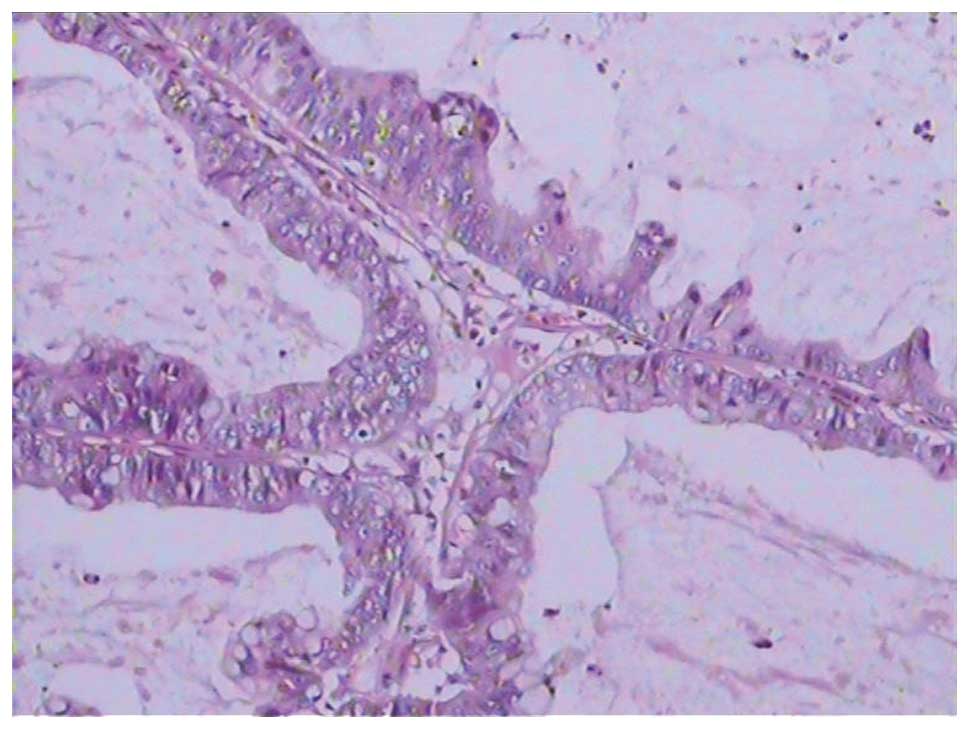
pathological analysis of the mass indicated
moderately-differentiated adenocarcinoma (Fig. 1). The patient was diagnosed with
ovarian cancer, stage IIIb T3bN0M0, according to the International
Federation of Gynecology and Obstetrics staging system (1988)
(3). The patient was treated with
combination chemotherapy consisting of platinum and paclitaxel.
This chemotherapy regimen involved the administration of
placlitaxel (120 mg, i.v.) followed by platinum (120 mg) every
three weeks for six cycles. The treatment outcome was evaluated as
a partial response after 18 weeks. The abdominal mass recurred, and
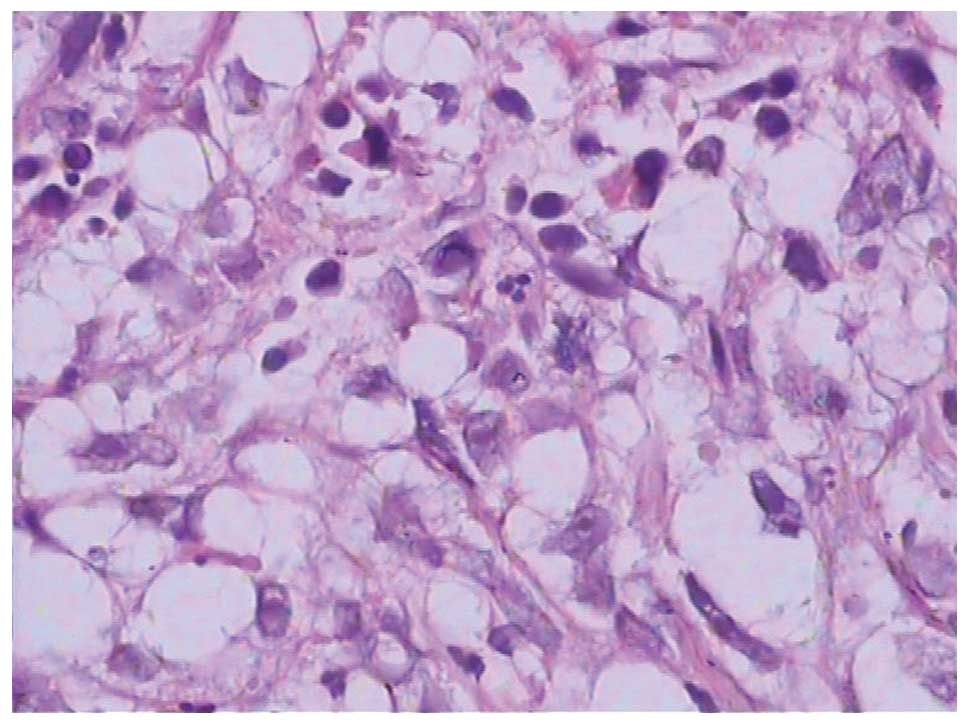
the patient underwent surgery in June 2012. The pathology of the
mass indicated a poorly-differentiated adenocarcinoma in February
2012 (Fig. 2). The ovarian carcinoma
cells were negative for CD133 expression. The patient was treated
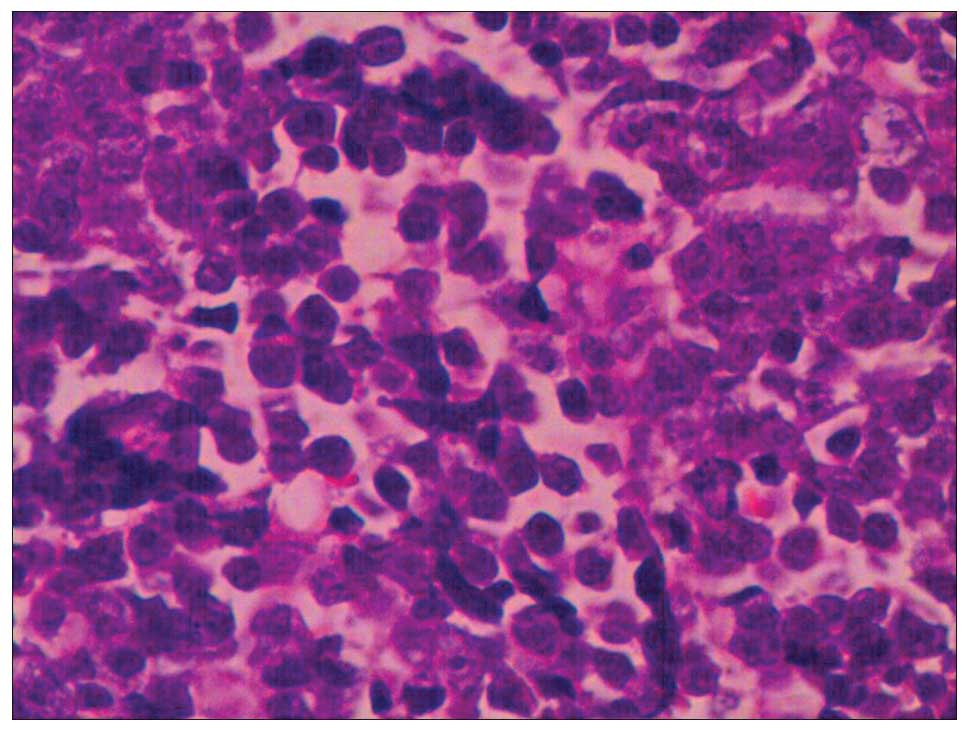
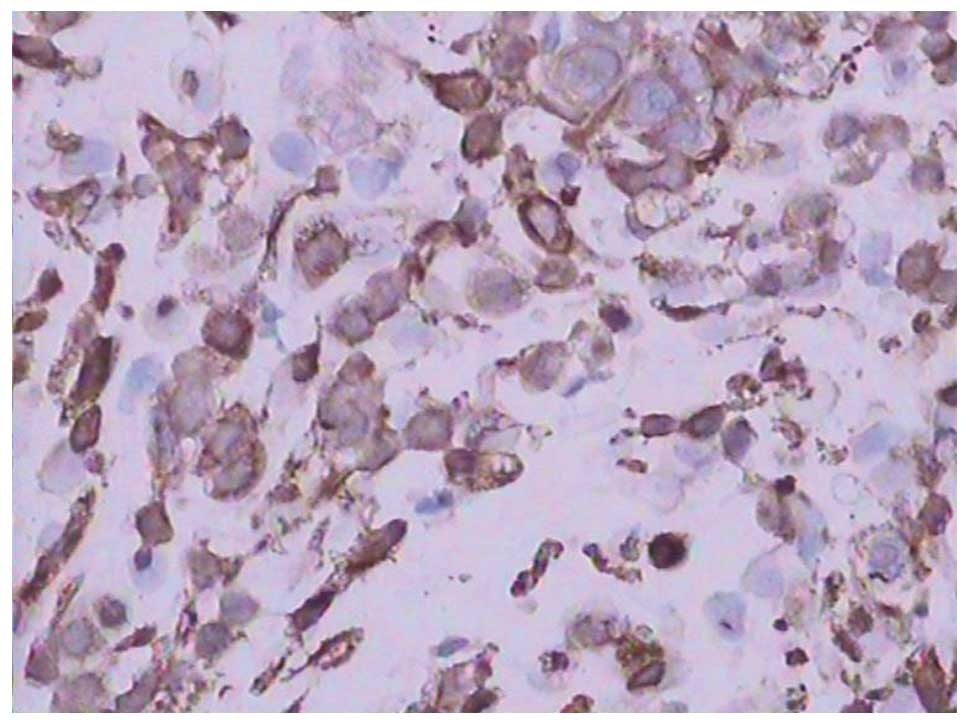
with two cycles of gemcitabine-paclitaxel chemotherapy. Tumor
biopsy results indicated an undifferentiated small cell carcinoma
and positive CD133 expression in the ovarian carcinoma cells
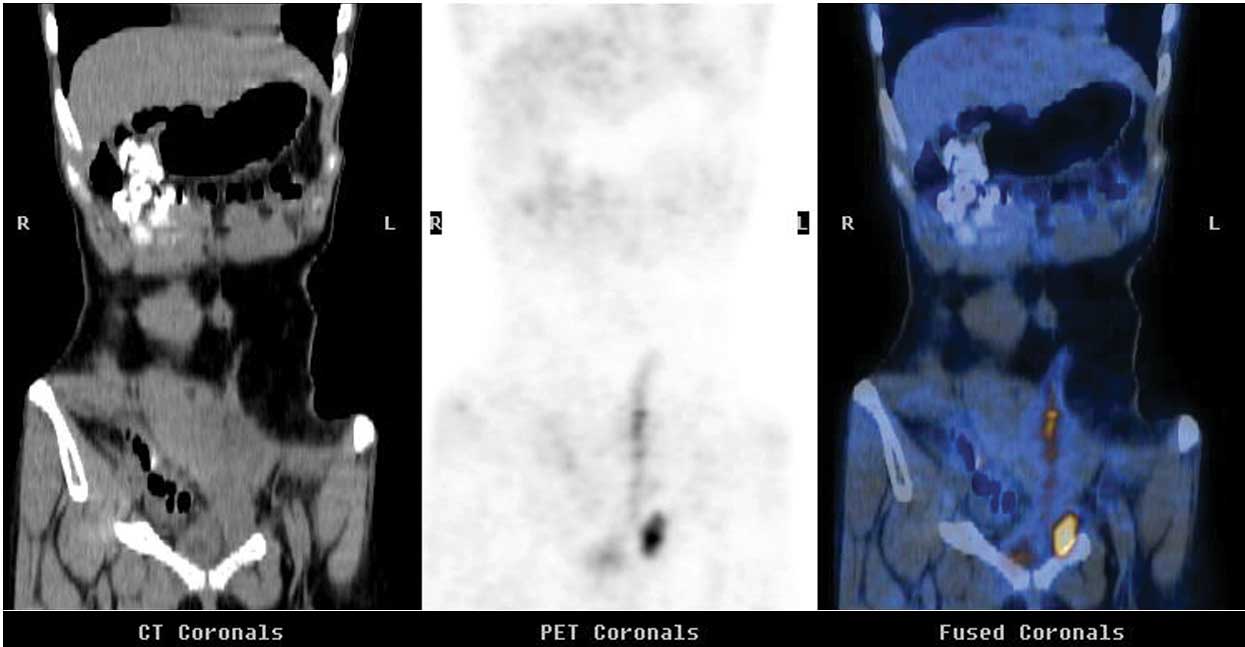
(Figs. 3 and 4). Positron emission tomography-CT revealed
evidence of extensive metastatic disease in the neck, thoracic
lymph nodes and liver, nodules along the diaphragm, and multiple
pelvic nodules (Fig. 5). Whole
abdomen irradiation was administered at a dose of 46 Gy (23
fractions) in May 2012, however, the patient succumbed to the
disease in September 2012.
Written informed consent was obtained from the
patient's parents for publication of this study. The study was
approved by the Ethics Committee of the Affiliated Wujing Hospital
of Guangzhou Medical College.
Discussion
Histologically, ovarian neoplasms are epithelial
tumors, germ cell tumors, sex cord stromal tumors and other tumors
with the same underlying characteristics, and the histology does
not change. Stem cells have the potential for multidifferentiation
and proliferation, and can differentiate into various types of
tissues, such as adipocytes, osteocytes and chondrocytes (4).
It is widely accepted that neoplastic stem cells can
differentiate into different types of mature neoplastic cells.
Multifactorial mechanisms for network formation result in
tumorigenesis in cancers and sarcoma by the activation or
deactivation of signaling pathways (5,6). In the
present study, the pathology indicated a change from
moderately-differentiated adenocarcinoma to poorly-differentiated
adenocarcinoma, and to undifferentiated small cell carcinoma. The
expression of CD133 changed from negative to positive in the
ovarian carcinoma cells. This indicated that the ovarian carcinomas
possessed high proliferative potential and clonogenic efficiency,
with stem-like characteristics.
We hypothesize that neoplastic stem cells can
differentiate into several types of mature neoplastic cells, with
synaptophysin as a marker for adenocarcinoma, small cell cancer and
undifferentiated carcinoma, following chemotherapy. Tumor cells
exhibit potential directional differentiation from one type of cell
to another through cell transformation induced by cell signaling
pathway activation or deactivation (2). This finding is supported by the
previously reported dedifferentiation of mature goblet cells
elicited by Wnt/β-catenin pathway activation (7).
If a signal pathway is terminated or weakened by
medicine or other interventions, such as pre-operative
chemotherapy, radiation, its impaired function would be replaced by
other signaling pathways (8). This is
a likely cause of the change in malignant pathological type
following treatment. In the present study, the mass was complete
resected by surgery and no residual tumor tissue was left. The
biopsy results showed a different pathology at all incidences of
tumor progression following surgery or the different chemotherapy
regimens. Considerable evidence supports the ability of neoplastic
stem cells to differentiate into various types of mature tumor
cells (1).
It is tempting to speculate that chemotherapy may
result in differentiation into one particular type of mature tumor
cell, which could lead to a shift in the phenotypical appearance.
The neoplastic stem cell genotype and phenotype may evolve
dynamically under the selective pressure of pre-operative
chemotherapy, radiation, and targeted therapy (2).
In the present study, the malignancies exhibited
pathological changes following chemotherapy. This finding is
supported by previous observations of chemotherapy-resistant tumors
that transformed from non-small cell lung cancer (NSCLC) into SCLC
(9), which was attributable to a
genetic or epigenetic event that concurrently led to a shift in
phenotypic appearance. To the best of our knowledge, the genetic
resistance to epidermal growth factor receptor (EGFR) tyrosine
kinase inhibitors has persisted, as EGFR genetic mutations resulted
in the transformation of NSCLC to SCLC (10,11).
The present study indicated that epithelial tumors,
germ cell tumors, sex cord stromal tumors and other tumors may
histologically originate from neoplastic stem cells in ovarian
neoplasms. This study also demonstrates the importance of
repeatedly checking the appropriate therapy by tumor biopsy
throughout the course of ovarian cancer progression.
References
|
1
|
López J, Valdez-Morales FJ,
Benítez-Bribiesca L, Cerbón M and Carrancá AG: Normal and cancer
stem cells of the human female reproductive system. Reprod Biol
Endocrinol. 11:532013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bapat SA, Mali AM, Koppikar CB and Kurrey
NK: Stem and progenitor-like cells contribute to the aggressive
behavior of human epithelial ovarian cancer. Cancer Res.
65:3025–3029. 2005.PubMed/NCBI
|
|
3
|
Shepherd JH: Revised FIGO staging for
gynaecological cancer. Br J Obstet Gynaecol. 96:889–892. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H, Zhang B, Tao Y, Cheng M, Hu J, Xu
M and Chen H: Isolation and characterization of mesenchymal stem
cells from whole human umbilical cord applying a single enzyme
approach. Cell Biochem Funct. 30:643–649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Takano Y and Zheng HC: The
pathobiological features of gastrointestinal cancers (Review).
Oncol Lett. 3:961–969. 2012.PubMed/NCBI
|
|
6
|
Bosland MC: Sexsteroids and
prostatecarcinogenesis: integrated, multifactorial working
hypothesis. Ann NY Acad Sci. 1089:168–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Finch AJ, Soucek L, Junttila MR, Swigart
LB and Evan GI: Acute overexpression of Myc in intestinal
epithelium recapitulates some but not all the changes elicited by
Wnt/beta-catenin pathway activation. Mol Cell Biol. 29:5306–5315.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu W, Madan E, Yee M and Zhang H: Progress
of molecular targeted therapies for prostate cancers. Biochim
Biophys Acta. 1825:140–152. 2012.PubMed/NCBI
|
|
9
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG,
Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ,
Mino-Kenudson M and Engelman JA: Genotypic and histological
evolution of lung cancers acquiring resistance to EGFR inhibitors.
Sci Transl Med. 3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zakowski MF, Ladanyi M and Kris MG:
Memorial Sloan-Kettering Cancer Center Lung Cancer OncoGenome
Group: EGFR mutations in small-cell lung cancers in patients who
have never smoked. N Engl J Med. 355:213–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tatematsu A, Shimizu J, Murakami Y, Horio
Y, Nakamura S, Hida T, Mitsudomi T and Yatabe Y: Epidermal growth
factor receptor mutations in small cell lung cancer. Clin Cancer
Res. 14:6092–6096. 2008. View Article : Google Scholar : PubMed/NCBI
|