Introduction
Primary gastrointestinal (PGI) lymphoma is an
extremely rare disease that accounts for 1–4% of all
gastrointestinal malignancies (1). In
general, gastrointestinal lymphoma is secondary to widespread nodal
diseases. Lymphomas may originate from any section of the
gastrointestinal tract, however, the most frequently affected
region is the stomach, followed by the small intestine (2). The most commonly diagnosed
histopathological subtype of PGI lymphoma is diffuse large B-cell
lymphoma (DLBCL), which accounts for ∼50% of all cases (3). Due to its rarity, only a limited number
of studies concerning PGI-DLBCL have been published (4–6).
Aberrant DNA methylation, which is characterized by
widespread genome hypomethylation, chromosome instability and
localized DNA hypermethylation, is considered to be an important
event, occurring in early tumor development (7,8).
Methylation patterns are predominantly regulated by independent DNA
methyltransferases (DNMTs), including DNMT1, DNMT3a and DNMT3b
(9,10). DNMT1 mediates maintenance DNA
methylation and DNMT3a and DNMT3b are responsible for de
novo methylation (9). Previous
studies have revealed that DNMT1 and DNMT3b overexpression is
associated with unfavorable prognoses in a number of human cancers,
including breast and hepatocellular carcinomas, lung cancers, acute
myeloid leukemia and epithelial ovarian cancer (11–17).
Increasing evidence suggests that aberrant DNA
methylation is significant in the pathogenesis of lymphomas
(18–22). However, at present, the prognostic
significance of DNMT expression in PGI-DLBCL is yet to be
elucidated. The aims of the current study were to determine the
transcript levels of DNMT1, DNMT3a and DNMT3b in PGI-DLBCL patients
by quantitative polymerase chain reaction (qPCR), and establish
their clinical significance.
Materials and methods
Patients and controls
In total, 62 patients with a histopathological
diagnosis of PGI-DLBCL, and 30 age- and gender-matched healthy
controls were recruited. The study was approved by Tianjin Medical
University Cancer Institute and Hospital Ethics Committee, and all
the patients provided written informed consent prior to study
participation. Fresh samples of cancerous tissues and normal
tissues were collected via surgical resection. The samples were
frozen in liquid nitrogen for 30 min in cryovials, and subsequently
stored at −80°C until further analysis. The diagnosis of PGI-DLBCL
was based upon the World Health Organization classification system
for hematological malignancies (23).
The patients were staged according to the Lugano staging system for
gastrointestinal non-Hodgkin's lymphoma (NHL) (24). The diagnostic work-up consisted of the
patient's history, their performance status according to the
Eastern Cooperative Oncology Group scale, a physical examination, a
baseline endoscopy or barium meal examination, gastric mucosal
biopsies or a gastrectomy, a complete blood cell count, a
biochemical profile, measurement of serum lactate dehydrogenase
(LDH), computed tomography scans of the thorax, abdomen and pelvic
cavity, and a bone marrow aspiration and biopsy. A low hemoglobin
level was defined as <120 g/l, and a high LDH level as >245
U/l. The patients were subsequently grouped according to age,
gender, tumor origin, performance status, Lugano staging system
outcome, clinical stage, B symptoms, LDH level, International
Prognostic Index (IPI) score and chemotherapy response. A
retrospective review of the clinical, pathological and treatment
results of all patients was conducted and the results were entered
into an anonymized database. The clinical characteristics and
histological features of the patients with PGI-DLBCL are shown in
Table I.
 | Table I.Clinical characteristics of primary
gastrointestinal diffuse large B cell lymphoma patients. |
Table I.
Clinical characteristics of primary
gastrointestinal diffuse large B cell lymphoma patients.
| Characteristic | Patients, n (%) |
|---|
| Age, years |
|
| ≤60 | 35 (56.5) |
|
>60 | 27 (43.5) |
| Gender |
|
| Male | 33 (53.2) |
|
Female | 29 (46.8) |
| Origin |
|
|
Stomach | 41 (66.1) |
|
Intestinal | 21 (33.9) |
| Performance
status |
|
| ECOG
0–1 | 45 (72.6) |
| ECOG
2–4 | 17 (27.4) |
| Lugano staging
system |
|
| I-II | 20
(32.3) |
|
IIE-IV | 42 (67.7) |
| Pathological
type |
|
|
Non-GCB | 45 (72.6) |
| GCB | 17 (27.4) |
| LDH |
|
|
Normal | 22 (35.5) |
|
Elevated | 40 (64.5) |
| Hemoglobin |
|
|
Normal | 30 (48.4) |
| Low | 32 (51.6) |
| B symptoms |
|
|
Positive | 13 (21.0) |
|
Negative | 49 (79.0) |
| IPI |
|
| 0–2 | 39 (62.9) |
|
3–5 | 23 (37.1) |
| Therapeutic
evaluation |
|
| CR | 32 (51.6) |
|
PR/SD/PD | 30 (48.4) |
RNA, cDNA preparation and qPCR
For the RNA extraction step, ∼25 mg of tumor tissue
was pulverized under liquid nitrogen using a pestle and mortar. The
RNA was subsequently extracted using RNeasy (Qiagen Inc., Valencia,
CA, USA), according to the manufacturer's instructions. Following
this, reverse transcription reactions were performed using a
PrimeScript 1st Strand cDNA Synthesis Kit (Takara Bio, Inc., Otsu,
Japan), according to the manufacturer's instructions and qPCR was
performed using a CM9600 Sequence Detection System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The amplification step was
performed in a total volume of 20 µl, with 10 µl kit-supplied
QuantiTect™ SYBR® Green RT-PCR Master mix (Applied Biosystems Life
Technologies, Foster City, CA, USA), 0.4 µl of each primer (10 µM),
2 µl of cDNA (50 ng RNA) and 7.2 µl ddH2O. The PCR
cycling parameters were set as follows: 95°C for 30 sec, followed
by 40 cycles of PCR reacting at 95°C for 5 sec, and finally 60°C
for 34 sec. β actin was used as an internal standard. The ΔΔCT
values were calculated using the differences between the target
genes and β actin. The primer sequences are shown in Table II. Each experiment was conducted in
triplicate.
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene | Forward sequence
(5′→3′) | Reverse sequence
(5′→3′) |
|---|
| DNMT1 |
GTGGGGGACTGTGTCTCTGT |
TGAAAGCTGCATGTCCTCAC |
| DNMT3a |
CAGCTTCCACGTTGCCTTCT |
CATCTGCAAGCTGTCTCCCTTT |
| DNMT3b |
CCCATTCGAGTCCTGTCATT |
GGTTCCAACAGCAATGGACT |
| β actin |
CCTGGCACCCAGCACAAT |
GGGCCGGACTCGTCATAC |
Immunohistochemical staining and
scoring
The biopsied tissues were initially fixed in 10%
buffered formalin, embedded in paraffin and cut into 4-µm sections.
Following this, deparaffinization and heat-induced antigen
retrieval steps were performed. The endogenous peroxidase activity
was blocked using 0.5% hydrogen peroxide. Subsequent to washing
with phosphate-buffered saline (PBS), the sections were incubated
with anti-cluster of differentiation (CD)10 (cat. no. ZM0283),
-B-cell lymphoma 6 (BCL-6; cat. no. ZM-0011) and -melanoma
associated antigen 1 (MUM1; cat. no. ZA-0583) antibodies (ZSGB-BIO,
Beijing, China) at 4°C overnight. After washing with PBS again, the
secondary antibodies were added to the sections. The nuclei were
subsequently stained with hematoxylin, and the color was developed
using diaminobenzidine. A positive result was recorded when ≥30% of
the cells were stained. The tissues were also analyzed for CD10,
BCL-6 and MUM1 immunoreactivity according to the immunophenotypic
profile criteria of DLBCL described previously by Hans et al
(25). The intensity of the staining
and the percentage of positive cells were recorded. The staining
intensity was scored between 0 and 3+ as follows: i) 0, Absence of
staining; ii) 1+, >25 % of the tumor cells exhibited weak
staining; iii) 2+, tumor cells exhibited moderate staining; and iv)
3+, tumor cells demonstrated strong staining. Tumors with a score
of 1+, 2+ or 3+ were considered to be positive, while tumors with a
score of 0 were considered to be negative. The immunohistochemical
analysis was performed independently by at least two
histopathologists.
Treatment and response assessment
The patients were treated with four therapeutic
modalities; surgery, chemotherapy, Helicobacter pylori (Hp)
eradication and radiotherapy. The aim of surgery was to remove the
tumor tissue. All patients were treated with 6–8, 21-day cycles of
the CHOP [cyclophosphamide (750 mg/m2, day 1),
doxorubicin (50 mg/m2, day 1), vincristine (1.4
mg/m2, day 1) and prednisone (100 mg, days 1–5)] or
R-CHOP [rituximab (350 mg/m2, day 1), cyclophosphamide
(750 mg/m2, day 1), doxorubicin (50 mg/m2, day 1),
vincristine (1.4 mg/m2, day 1) and prednisone (100 mg,
days 1–5)] regimens. The Hp eradication regimen, which consisted of
proton pump inhibitors [omeprazole (20 mg, twice daily),
lansoprazole (30 mg, twice daily) or rabeprazole (10 mg, twice
daily)] and a combination of antibiotics [amoxicillin (1,000 mg,
twice daily), clarithromycin (500 mg, twice daily) and/or
metronidazole (200 mg, twice daily)], was administered to eight
patients, seven days a month, for three months. In total, six
patients received radiotherapy. The responses were assessed by the
International Workshop for NHL Standardized Criteria (26).
Statistical analysis
The statistical analysis was performed using SSPS
version 19.0 software (SPSS Inc., Chicago, IL, USA). The results
are presented as the mean ± standard deviation. The data were
analyzed using the non-parametric Mann-Whitney U-test. The
significant differences between the survival curves were calculated
using the log-rank test. A GraphPad Prism software package was used
for the Kaplan-Meier estimate graphs. The Cox proportional hazards
regression model was used for the multivariate analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Clinical characteristics and
histological features
In total, 62 patients with PGI-DLBCL, which included
33 males and 29 females (median age, 54 years; range, 32–76 years),
and 30 age- and gender-matched healthy controls were enrolled in
the present study. Overall, 20 patients (32.3%) were diagnosed with
an advanced Ann Arbor clinical stage (stages IIE-IV), and 45
patients (72.6%) exhibited a good performance status (<2). An
elevated serum LDH level was observed in 40 patients (64.5%); low
or low-intermediate IPI scores were identified in 39 patients
(62.9%). Based upon the results of the immunophenotypical profiles,
17 patients (27.4%) were classified as having a germinal center
B-cell-like (GCB) subtype, and 45 patients (72.6%) with a non-GCB
subtype. In total, 32 patients (51.6%) achieved complete remission
after 6–8 cycles of the CHOP or R-CHOP regimens, 11 (17.8%) were in
partial remission and 19 patients (30.6%) exhibited stable or
progressive disease.
mRNA expression of DNMT1, DNMT3a and
DNMT3b in patients with PGI-DLBCL
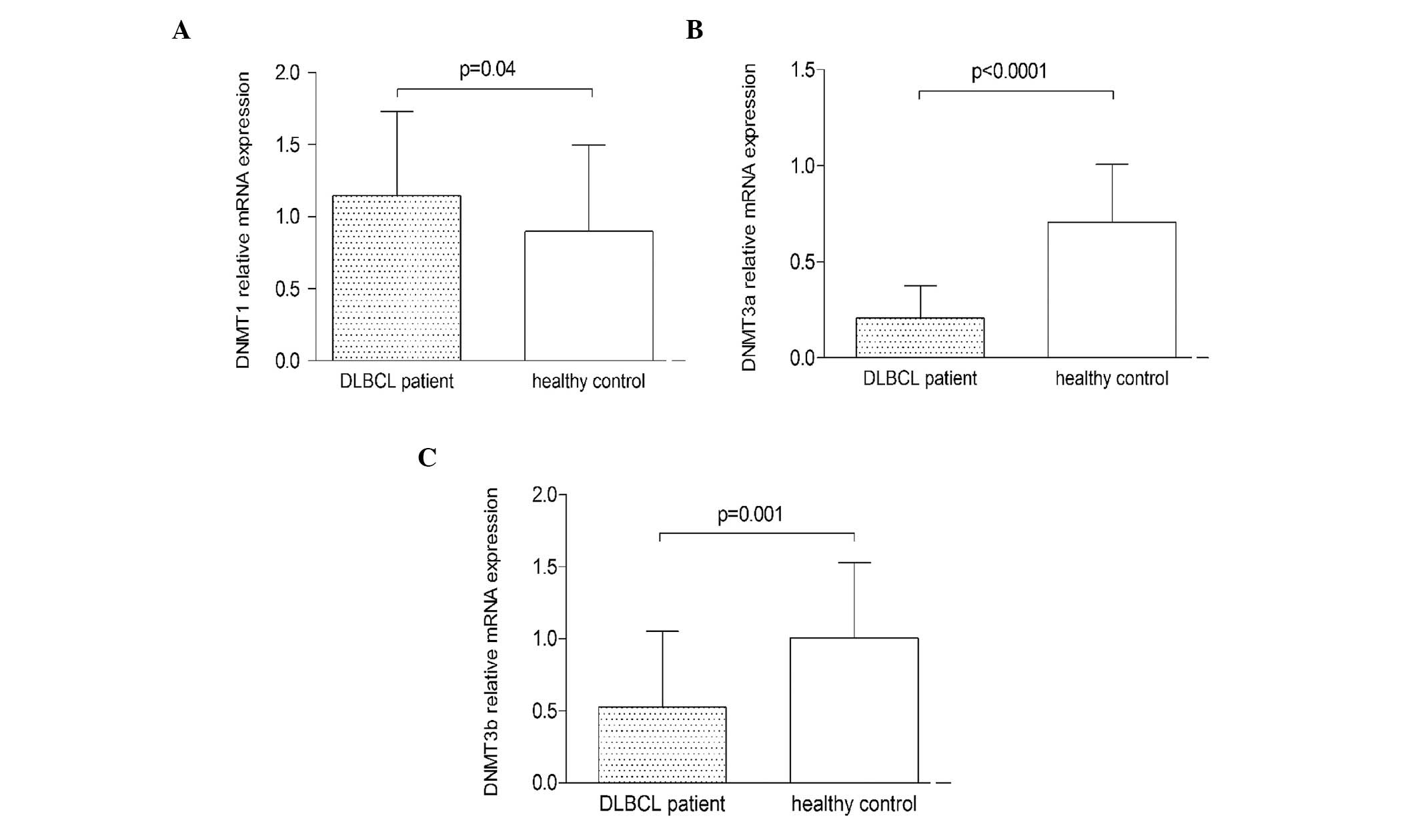
The mRNA expression of DNMT1, DNMT3a and DNMT3b was
determined using qPCR. The expression of DNMT1 mRNA in PGI-DLBCL
patients was significantly higher than that of the healthy controls
(P=0.04), while the expression of DNMT3a and DNMT3b mRNA was
significantly lower (P<0.0001 and P=0.001, respectively)
(Fig. 1).
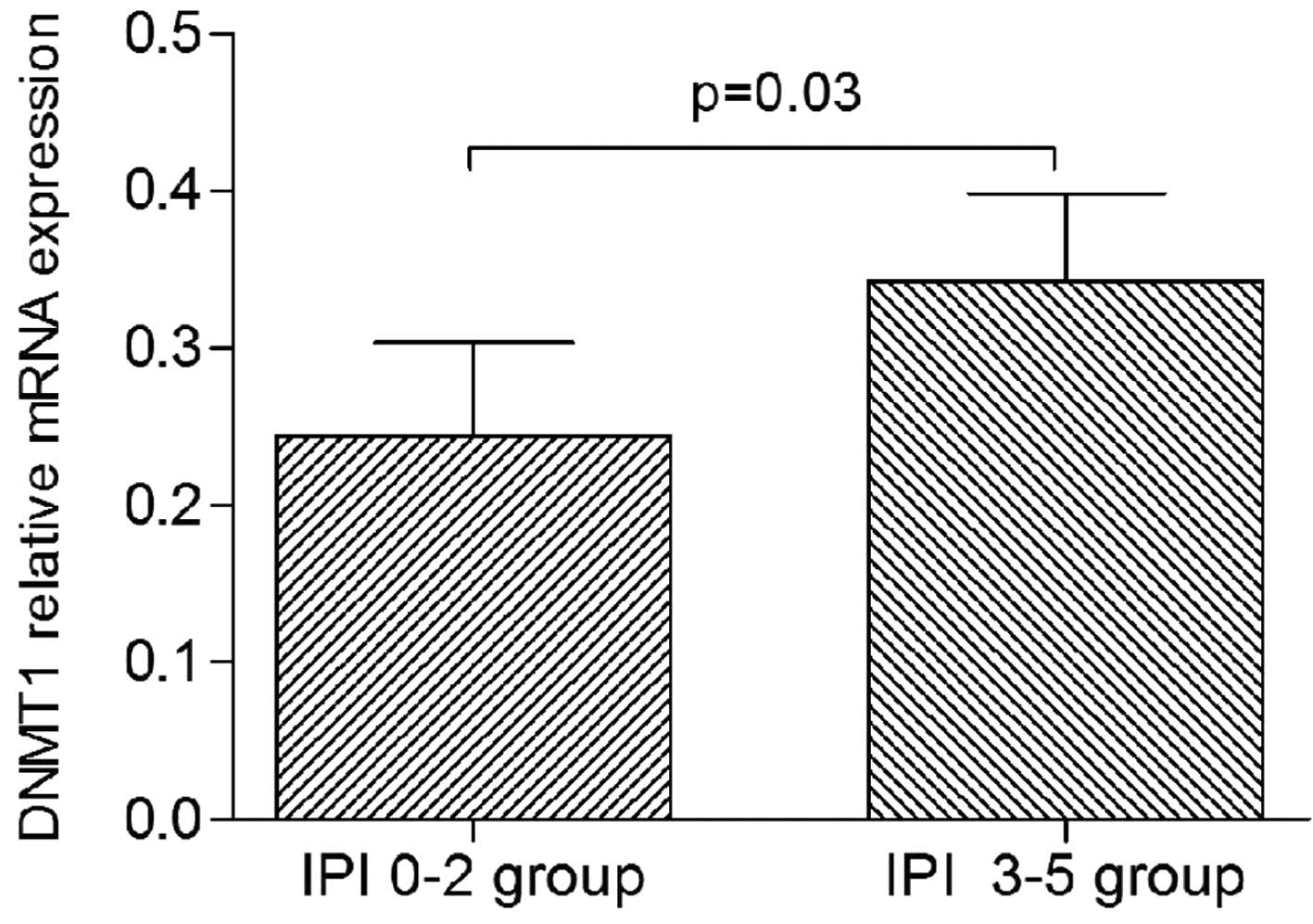
Association of DNMT1 expression with
the clinicopathological characteristics of PGI-DLBCL
The patients with PGI-DLBCL were divided into
subgroups according to specific clinical parameters, such as the
Lugano stage (stages I-II and stages IIE-IV subgroups), the IPI
score (low IPI, 0–2; and high IPI, 3–5 subgroups), and the DLBCL
type (non-GCB and GCB subgroups). It was revealed that DNMT1
expression was significantly higher in the high IPI subgroup than
in the low IPI subgroup (P=0.03; Fig.
2). By contrast, there were no significant differences in the
expression of DNMT3a and DNMT3b between any of the subgroups (data
not shown).
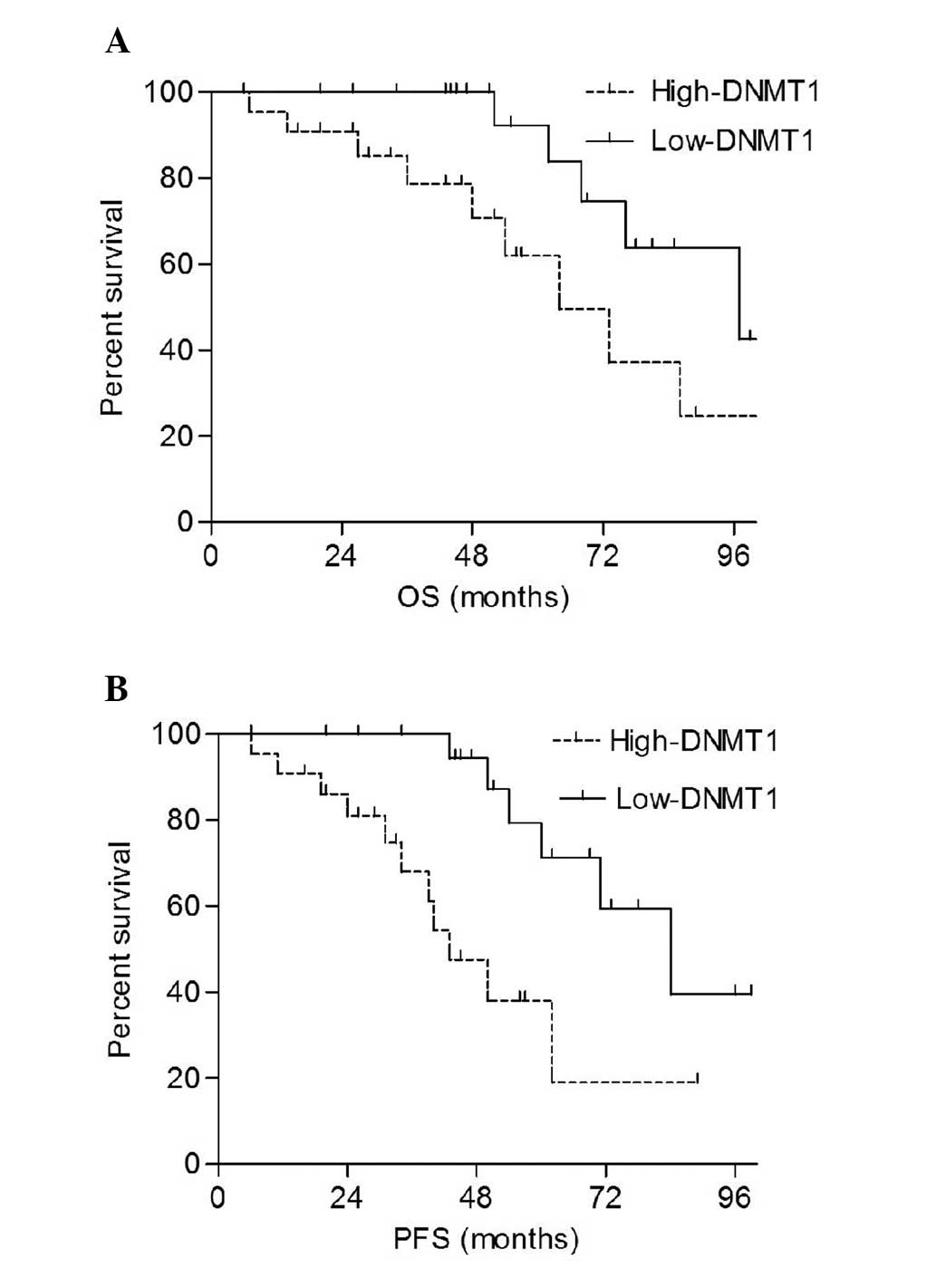
DNMT1 is a negative independent
prognostic factor for patients with PGI-DLBCL
The patients were divided into high-expression
(above the median) and low-expression (below the median) groups
depending on mRNA expression levels. The results demonstrated that
the overall survival (OS) and progression-free survival (PFS) rates
of the high-DNMT1 group were significantly shorter compared with
those of the low-DNMT1 group (P=0.038 and P=0.012, respectively;
Fig. 3). The univariate analysis
revealed that DNMT1 expression, Lugano staging, pathological type
and IPI scores were factors associated with the prognosis of
patients with PGI-DLBCL (Table
III). The multivariate analysis showed that in addition to the
IPI score, DNTM1 expression was also an independent predictive
factor for mortality and disease progression (Table IV).
 | Table III.Univariate analysis revealing the
significance of different prognostic variables for primary
gastrointestinal diffuse large B cell lymphoma. |
Table III.
Univariate analysis revealing the
significance of different prognostic variables for primary
gastrointestinal diffuse large B cell lymphoma.
|
| Overall
survival | Progression-free
survival |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| DNMT1 expression
(low vs. high) | 5.87
(2.31–12.68) | 0.015 | 3.78
(1.24–8.62) | 0.012 |
| ECOG (0–1 vs.
−4) | 3.24
(1.67–8.90) | 0.072 | 2.56
(1.24–9.78) | 0.145 |
| Lugano stage (I-II
vs. IIE-IV) | 2.13
(0.45–7.89) | 0.032 | 3.02
(0.24–8.34) | 0.024 |
| Pathological type
(non-GCB vs. GCB) | 3.56
(0.83–10.76) | 0.027 | 1.56
(0.45–6.76) | 0.032 |
| LDH (normal vs.
elevated) | 0.67
(0.06–1.18) | 0.124 | 0.92
(0.06–3.54) | 0.294 |
| IPI (0–2 vs.
3–5) | 3.45
(0.23–11.92) | 0.016 | 2.30
(0.23–10.34) | 0.031 |
| Treatment
(surgery+R-CHOP vs. surgery+CHOP) | 2.01
(0.54–5.72) | 0.569 | 1.03
(0.78–8.45) | 0.756 |
| Therapeutic
evaluation (SD vs. PR/SD/PD) | 2.69
(0.35–6.97) | 0.187 | 1.45
(0.56–12.46) | 0.357 |
 | Table IV.Multivariate analysis revealing the
significance of independent prognostic variables for primary
gastrointestinal diffuse large B cell lymphoma. |
Table IV.
Multivariate analysis revealing the
significance of independent prognostic variables for primary
gastrointestinal diffuse large B cell lymphoma.
|
| Overall
survival | Progression-free
survival |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| DNMT1 expression
(low vs. high) | 4.32
(1.25–8.23) | 0.018 | 4.02
(1.42–12.63) | 0.008 |
| Lugano stage (I-II
vs. IIE-IV) | 1.34
(0.69–6.90) | 0.082 | 1.56
(0.54–7.78) | 0.160 |
| Pathological type
(non-GCB vs. GCB) | 1.13
(0.45–4.56) | 0.143 | 1.02
(0.24–3.34) | 0.237 |
| IPI (0–2 vs.
3–5) | 2.96
(0.58–5.76) | 0.017 | 2.56
(0.83–5.76) | 0.020 |
Discussion
The processes that underlie the pathogenesis of
PGI-DLBCL are yet to be elucidated. Previous studies have
hypothesized that abnormal DNA methylation is an important event
that occurs early in tumor development (27,28). DNA
methylation is regulated by DNMTs, predominantly DNMT1, DNMT3a and
DNMT3b (9,10). Therefore, we hypothesize that the
dysregulated expression and function of DNMT1, DNMT3a and DNMT3b is
significant in the pathogenesis of PGI-DLBCL.
Increasing evidence indicates that high DNMT1 and/or
DNMT3b expression has a modulating effect upon the clinical outcome
of cancer patients. A previous study revealed that high DNMT1
expression was associated with a subgroup of gastric cancer
patients with poorer outcomes following platinum/fluorouracil-based
neoadjuvant chemotherapy (29).
Another study identified that the level of DNMT1 mRNA was
significantly higher in gastric cancer patients with methylated
Runx3, compared with those with unmethylated Runx3 (30). Furthermore, increased expression of
DNMT1 and DNMT3b have been detected in the tumor tissues of
patients with lung cancer, and were also associated with poorer
prognoses (31–34). In a study concerning pancreatic cancer
patients, those with a higher DNMT1 expression exhibited a lower
overall survival rate (35). In
addition, DNMT1 and DNMT3b were markedly upregulated in malignant
glioma tumor tissues (36), and
DNMT3b overexpression was identified to be an independent
prognostic factor for predicting the reduced survival of DLBCL
patients (37).
The present study revealed that DNMT1 mRNA
expression in PGI-DLBCL patients was higher than that of the
healthy controls, and that it was increased in the high IPI
subgroup compared with the low IPI subgroup. The Kaplan-Meier
analysis showed that the high-DNMT1 subgroup exhibited shorter OS
and PFS rates compared with the low-DNMT1 subgroup. This suggests
that DNMT1 is a negative predictive factor for PGI-DLBCL. The Cox
regression analysis revealed that DNMT1 was an independent
prognostic factor, which suggests that DNMT1 expression may be a
suitable prognostic marker for patients with PGI-DLBCL. These
findings are consistent with those of other previous studies
(28–36). Although previous studies have reported
that the levels of DNMT3a and DNMT3b were significantly higher in
cancer patients (31,33,35,36), the
present study indicated that the expression of DNMT3a and DNMT3b in
PGI-DLBCL patients was markedly lower than that of the healthy
controls. This may be due to the small number of samples used in
the present study. Future studies with larger patient cohorts are
therefore required in order to address this inconsistency.
In conclusion, the results of the present study
suggest that DNMT1, DNMT3a and DNMT3b may be involved in the
etiology of PGI-DLBCL. In addition, the results indicate that DNMT1
is a negative and independent prognostic parameter for patients
with PGI-DLBCL, and, therefore, may be used as a biomarker in order
to guide prognosis.
References
|
1
|
Wang T, Gui W and Shen Q: Primary
gastrointestinal non-Hodgkin's lymphoma: clinicopathological and
prognostic analysis. Med Oncol. 27:661–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herrmann R, Panahon AM, Barcos MP, Walsh D
and Stutzman L: Gastrointestinal involvement in non-Hodgkin's
lymphoma. Cancer. 46:215–222. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Krieken JH, Otter R, Hermans J, et al:
Malignant lymphoma of the gastrointestinal tract and mesentery. A
clinico-pathologic study of the significance of histologic
classification. NHL Study Group of the Comprehensive Cancer Center
West. Am J Pathol. 135:281–289. 1989.PubMed/NCBI
|
|
4
|
Chen F, Yang G and Xia B: Increased
expression of the spindle checkpoint protein BubR1 is associated
with high cell proliferation in primary gastrointestinal diffuse
large B cell lymphoma. Cell Biochem Biophys. 66:747–752. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding D, Pei W, Chen W, Zuo Y and Ren S:
Analysis of clinical characteristics, diagnosis, treatment and
prognosis of 46 patients with primary gastrointestinal non-Hodgkin
lymphoma. Mol Clin Oncol. 2:259–264. 2014.PubMed/NCBI
|
|
6
|
Li X, Shen W, Cao J, et al: Treatment of
gastrointestinal diffuse large B cell lymphoma in China: a 10-year
retrospective study of 114 cases. Ann Hematol. 91:1721–1729. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Robertson KD: DNA methylation,
methyltransferases, and cancer. Oncogene. 20:3139–3155. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI
|
|
9
|
Fitzpatrick DR and Wilson CB: Methylation
and demethylation in the regulation of genes, cells, and responses
in the immune system. Clin Immunol. 109:37–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fatemi M, Hermann A, Gowher H and Jeltsch
A: Dnmt3a and Dnmt1 functionally cooperate during de novo
methylation of DNA. Eur J Biochem. 269:4981–4984. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin RK, Hsu HS, Chang JW, Chen CY, Chen JT
and Wang YC: Alteration of DNA methyltransferases contributes to
5′CpG methylation and poor prognosis in lung cancer. Lung Cancer.
55:205–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito Y, Kanai Y, Nakagawa T, et al:
Increased protein expression of DNA methyltransferase (DNMT) 1 is
significantly correlated with the malignant potential and poor
prognosis of human hepatocellular carcinomas. Int J Cancer.
105:527–532. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oue N, Kuraoka K, Kuniyasu H, et al: DNA
methylation status of hMLH1, p16(INK4a), and CDH1 is not associated
with mRNA expression levels of DNA methyltransferase and DNA
demethylase in gastric carcinomas. Oncol Rep. 8:1085–1089.
2001.PubMed/NCBI
|
|
14
|
Girault I, Tozlu S, Lidereau R and Bieche
I: Expression analysis of DNA methyltransferases 1, 3A, and 3B in
sporadic breast carcinomas. Clin Cancer Res. 9:4415–4122.
2003.PubMed/NCBI
|
|
15
|
Xing J, Stewart DJ, Gu J, Lu C, Spitz MR
and Wu X: Expression of methylation-related genes is associated
with overall survival in patients with non-small cell lung cancer.
Br J Cancer. 98:1716–1722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayette S, Thomas X, Jallades L, et al:
High DNA methyltransferase DNMT3B levels: poor prognostic marker in
acute myeloid leukemia. PLoS One. 7:e515272012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai X, Song Z, Fu Y, et al:
Clinicopathological significance and prognostic value of DNA
methyltransferase 1, 3a, and 3b expressions in sporadic epithelial
ovarian cancer. PLoS One. 7:e400242012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shaknovich R and Melnick A: Epigenetics
and B-cell lymphoma. Curr Opin Hematol. 18:293–299. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taylor KH, Briley A, Wang Z, Cheng J, Shi
H and Caldwell CW: Aberrant epigenetic gene regulation in lymphoid
malignancies. Semin Hematol. 50:38–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De S, Shaknovich R, Riester M, et al:
Aberration in DNA methylation in B-cell lymphomas has a complex
origin and increases with disease severity. PLoS Genet.
9:e10031372013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pike BL, Greiner TC, Wang X, et al: DNA
methylation profiles in diffuse large B-cell lymphoma and their
relationship to gene expression status. Leukemia. 22:1035–1043.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Asmar F, Punj V, Christensen J, et al:
Genome-wide profiling identifies a DNA methylation signature that
associates with TET2 mutations in diffuse large B-cell lymphoma.
Haematologica. 98:1912–1920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tomonaga M.: Outline and direction of
revised WHO classification of tumors of haematopoietic and lymphoid
tissues. Rinsho Ketsueki. 50:1401–1406. 2009.(In Japanese).
PubMed/NCBI
|
|
24
|
No authors listed: 5th International
Conference on Malignant Lymphoma, Part 2. Proceedings. Lugano,
Switzerland, June 9–12, 1993. Ann Oncol. 5 (Suppl 2):S1–S163.
1994.PubMed/NCBI
|
|
25
|
Hans CP, Weisenburger DD, Greiner TC, et
al: Confirmation of the molecular classification of diffuse large
B-cell lymphoma by immunohistochemistry using a tissue microarray.
Blood. 103:275–282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheson BD, Horning SJ, Coiffier B, et al:
Report of an international workshop to standardize response
criteria for non-Hodgkin's lymphomas. NCI Sponsored International
Working Group. J Clin Oncol. 17:12441999.PubMed/NCBI
|
|
27
|
Fukushige S and Horii A: DNA methylation
in cancer: a gene silencing mechanism and the clinical potential of
its biomarkers. Tohoku J Exp Med. 229:173–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sincic N and Herceg Z: DNA methylation and
cancer: ghosts and angels above the genes. Curr Opin Oncol.
23:69–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mutze K, Langer R, Schumacher F, et al:
DNA methyltransferase 1 as a predictive biomarker and potential
therapeutic target for chemotherapy in gastric cancer. Eur J
Cancer. 47:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen W, Gao N, Shen Y and Cen JN:
Hypermethylation downregulates Runx3 gene expression and its
restoration suppresses gastric epithelial cell growth by inducing
p27 and caspase3 in human gastric cancer. J Gastroenterol Hepatol.
25:823–831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao P, Yang X, Xue YW, Zhang XF, Wang Y,
Liu WJ and Wu XJ: Promoter methylation of glutathione S-transferase
pi1 and multidrug resistance gene 1 in bronchioloalveolar carcinoma
and its correlation with DNA methyltransferase 1 expression.
Cancer. 115:3222–3232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xing J, Stewart DJ, Gu J, Lu C, Spitz MR
and Wu X: Expression of methylation-related genes is associated
with overall survival in patients with non-small cell lung cancer.
Br J Cancer. 98:1716–1722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim H, Kwon YM, Kim JS, Han J, Shim YM,
Park J and Kim DH: Elevated mRNA levels of DNA methyltransferase-1
as an independent prognostic factor in primary non-small cell lung
cancer. Cancer. 107:1042–1049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vallbohmer D, Brabender J, Yang D, et al:
DNA methyltransferases messenger RNA expression and aberrant
methylation of CpG islands in non-small-cell lung cancer:
association and prognostic value. Clin Lung Cancer. 8:39–44. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang W, Gao J, Man XH, Li ZS and Gong YF:
Significance of DNA methyltransferase-1 and histone deacetylase-1
in pancreatic cancer. Oncol Rep. 21:1439–1447. 2009.PubMed/NCBI
|
|
36
|
Kreth S, Thon N, Eigenbrod S, et al:
O-methylguanine-DNA methyltransferase (MGMT) mRNA expression
predicts outcome in malignant glioma independent of MGMT promoter
methylation. PLoS One. 6:e171562011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amara K, Ziadi S, Hachana M, Soltani N,
Korbi S and Trimeche M: DNA methyltransferase DNMT3b protein
overexpression as a prognostic factor in patients with diffuse
large B-cell lymphomas. Cancer Sci. 101:1722–1730. 2010. View Article : Google Scholar : PubMed/NCBI
|