Introduction
Cervical cancer is the second most prevalent female
cancer worldwide, and 80–90% of cervical cancers are classified as
squamous cell carcinoma (SCC) based on pathological findings
(1,2).
The majority of patients with early-stage cervical cancer can be
treated with radical surgery or radiotherapy (3). Komaki et al (4) reported 5-year overall survival (OS)
rates of 89.4 and 79.3% in patients with International Federation
of Gynecology and Obstetrics (FIGO) stage I and II cervical cancer,
respectively. Pathological factors, including histological type,
tumor diameter, lymph node metastasis, lymph vascular space
invasion, depth of stromal invasion and parametrial extension, are
currently considered independent prognostic factors for survival
(5). However, there is no consensus
regarding the role of these risk factors for the individual patient
(6). The identification of novel
markers to accurately predict the prognosis of patients with
cervical cancer is therefore necessary.
The transcription factor p53 and its two homologs,
p73 and p63, form the p53 gene family. These three genes encode
proteins with high similarity at the structural and functional
level (7,8). The p53 gene is the prototype tumor
suppressor in human cancers due to its proapoptotic and
antiproliferative functions in response to oncogenic stress. The
p53 gene is mutated and its function is lost in ∼50% of human
cancers (9,10). Despite its significant homology to
p53, p73 is not a classic Knudson-type tumor suppressor gene, and
it has several complex isoforms with opposing functions, including
transactivation domain p73 (TAp73) and p73 with inhibitory proteins
lacking TA (ΔTAp73). ΔTAp73 is expressed in four different forms as
follows: ΔNp73, ΔN'p73, ΔEx2p73 and ΔEx2/3p73, of which ΔNp73 is
the predominant form. ΔNp73 is a potent transdominant inhibitor of
TAp73 and wild-type p53. The p73 locus encodes a tumor suppressor
(TAp73) and a putative oncogene (ΔNp73) (11,12).
Recent studies have demonstrated that TAp73 and
ΔNp73 are overexpressed in a number of solid tumors, including
lung, ovarian, hepatocellular, breast and colon cancers, and their
expression levels are associated with prognosis in patients with
these cancers (10–14). However, few studies have investigated
the expression levels of TAp73 and ΔNp73 and their prognostic
significance in cervical cancer, particularly in early-stage
cervical SCC (15). In the current
study, the expression of TAp73 and ΔNp73 was investigated in
cervical squamous cancer cells, and their prognostic significance
was evaluated in patients with FIGO stage I-II cervical SCC.
Patients and methods
Patients and specimen selection
Paraffin-embedded post-operative tissue samples were
obtained from the archives of the Department of Pathology, the
Second Affiliated Hospital of Soochow University (Suzhou, Jiangsu,
China), between January 2009 and December 2010. A total of 59 tumor
samples were retrospectively retrieved from patients with FIGO
stage I-II cervical SCC. Approval for the current project was
obtained from the Ethics Committee of the Second Affiliated
Hospital of Soochow University.
The main characteristics of the 59 patients were
summarized in Table I. The median age
of the patients was 42 years (range, 22–68 years). According to the
FIGO stage, the cohort consisted of 44 patients with stage I and 15
patients with stage II SCC. All patients underwent radical surgery.
A total of 7 patients with a post-operative pathological diagnosis
of pelvic lymph node metastasis received radiotherapy. The target
volume included the whole pelvis, and radiation was delivered in
four or two fields (anterioposterior and posteroanterior beams). A
total dose of 5,000 cGy was delivered in fractions of 200 cGy/day
for 5 days per week. Systemic adjuvant treatment was administered
to 8 patients; these patients received 80 mg/m2
cisplatin and 135 mg/m2 Taxol every 3 weeks for 2–4
cycles.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Patients, n (%) | 59 (100.0) |
| Median age (range),
years | 42 (22–68) |
| Pathological
diagnosis, n (%) |
|
| Cervical
squamous cell carcinoma | 59 (100.0) |
| FIGO stage, n
(%) |
|
| I | 44 (74.6) |
| II | 15 (25.4) |
| Radical surgery, n
(%) | 59 (100.0) |
| Post-operative
radiotherapy, n (%) | 7 (11.9) |
| Post-operative
chemotherapy, n (%) | 8 (13.6) |
Immunohistochemistry
Three serial slides, each 3-µm thick, were cut from
paraffin-embedded tissues. One slide was used for hematoxylin/eosin
staining and the other two were subjected to immunohistochemical
staining using the two-step procedure. Mouse monoclonal anti-human
TAp73 antibody (IMG-246; dilution, 1:100; Imgenex, Novus
Biologicals, Littleton, CO, USA) and mouse monoclonal anti-human
ΔNp73 antibody (ab13649; dilution, 1:100; Abcam, Cambridge, MA,
USA) were used. Following deparaffinization at 65°C and hydration,
the slides were subjected to antigen retrieval by pressure-cooking
for 5 min at 120°C. Endogenous peroxidase activity was neutralized
using peroxide block placement on the slides for 15 min at room
temperature. The slides were then incubated with anti-TAp73 and
anti-ΔNp73 monoclonal antibodies for 30 min at 4°C, followed by
incubation with peroxidase-conjugated polymers (ChemMate
EnVision/HRP; Gene Tech, Shanghai, China) for 30 min at room
temperature. The chromogen reaction was developed in
3,3′-diaminobenzidine tetrahydrochloride (Gene Tech) for 10 min.
Finally, hematoxylin was used as a light nuclear counterstain. The
negative control used was an immunoglobulin G2b isotype antibody
(Dako, Shanghai, China), ensuring the same concentration of
immunoglobulins used in the anti-TAp73 and anti-ΔNp73
antibodies.
Assessment of TAp73 and ΔNp73
expression
All slides were evaluated independently by two
experienced pathologists. The intensity of staining was recorded as
negative, weak or strong. In accordance with the description in the
study by Giatromanolaki et al, only strongly-stained cells
were considered positive cells (16).
The extent of staining was expressed as a percentage of positive
cells to total cells and was recorded after examining all optical
fields at ×200 magnification. The mean value was used to score all
samples. Cells were classified into four categories according to
the percentage of positive-staining cells as follows: 1, 0–24%; 2,
25–49%; 3, 50–74%; and 4, 75–100%. The scoring pattern was defined
as follows: 1–2, low expression (<50% positive cells); and 3–4,
high expression (≥50% positive cells).
Statistical analysis
Statistical analysis was performed using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA)The correlation
between the expression of TAp73 and ΔNp73, and the
clincopathological characteristics was examined using the
χ2 test. OS rates were determined using the Kaplan-Meier
method and log-rank test. OS time was defined from the day of
surgery to the day of mortality or last follow-up. For all tests,
two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of TAp73 and ΔNp73 in
cervical SCC cells
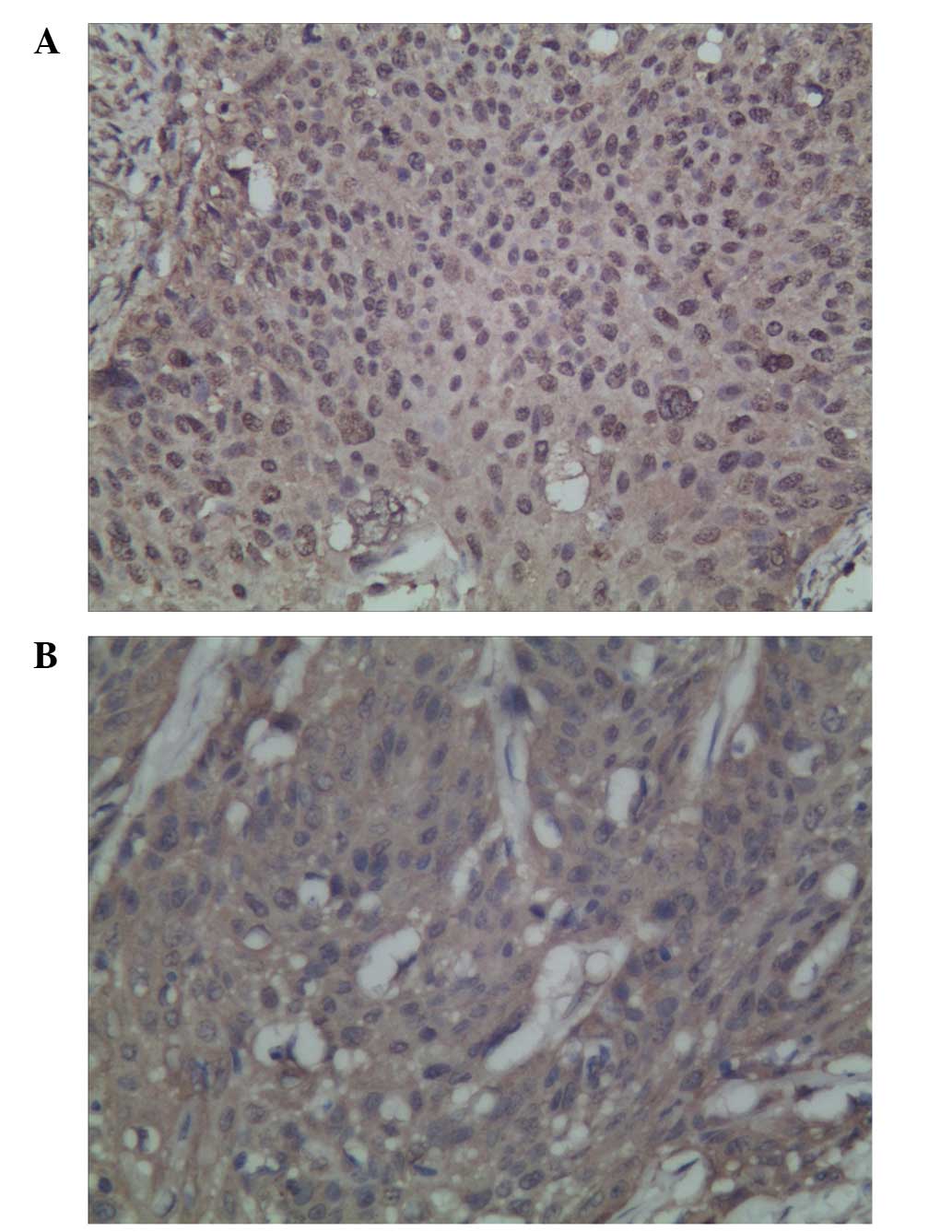
The expression of TAp73 and ΔNp73 was detected in
the nucleus and cytoplasm of the cervical SCC cells. High TAp73 and
ΔNp73 expression was detected in 79.7% (47/59) and 76.3% (45/59) of
patients, respectively (Fig. 1A and
B). No significant correlation between TAp73 and ΔNp73
expression was observed (χ2=0.415; P=0.519).
Association of TAp73 and ΔNp73
expression with clincopathological characteristics
Table II shows the
associations between the expression levels of TAp73 and ΔNp73 in
the 59 patients with FIGO stage I-II cervical SCC and several
clinicopathological characteristics. No significant association was
observed between TAp73 and ΔNp73 expression and age, FIGO stage,
pathological differentiation and lymph node metastasis (Table II).
 | Table II.Association of TAp73 and ΔNp73
expression with clincopathological characteristics. |
Table II.
Association of TAp73 and ΔNp73
expression with clincopathological characteristics.
|
|
| TAp73 expression,
% |
|
| ΔNp73 expression,
% |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variables | No. of patients | Low | High | χ2 | P-value | Low | High | χ2 | P-value |
|---|
| Age, years |
|
| ≤42 | 33 | 24.2 | 75.8 | 0.704 | 0.401 | 30.3 | 69.7 | 1.788 | 0.181 |
|
>42 | 26 | 15.4 | 84.6 |
|
| 15.4 | 84.6 |
|
|
| FIGO stage |
|
| I | 44 | 22.7 | 77.3 | 0.609 | 0.435 | 25.0 | 75.0 | 0.155 | 0.694 |
| II | 15 | 13.3 | 86.7 |
|
| 20.0 | 80.0 |
|
|
| Pathological
differentiation |
|
| Low | 12 | 33.3 | 66.7 | 1.600 | 0.449 | 33.3 | 66.7 | 1.215 | 0.545 |
|
Medium | 28 | 17.9 | 82.1 |
|
| 17.9 | 82.1 |
|
|
| High | 19 | 15.8 | 84.2 |
|
| 26.3 | 73.7 |
|
|
| Lymph node
metastasis |
|
| No | 52 | 21.2 | 78.8 | 0.180 | 0.672 | 26.9 | 73.1 | 2.471 | 0.116 |
| Yes | 7 | 14.3 | 85.7 |
|
| 0.0 | 100.0 |
|
|
Correlation between TAp73 and ΔNp73
expression and overall survival
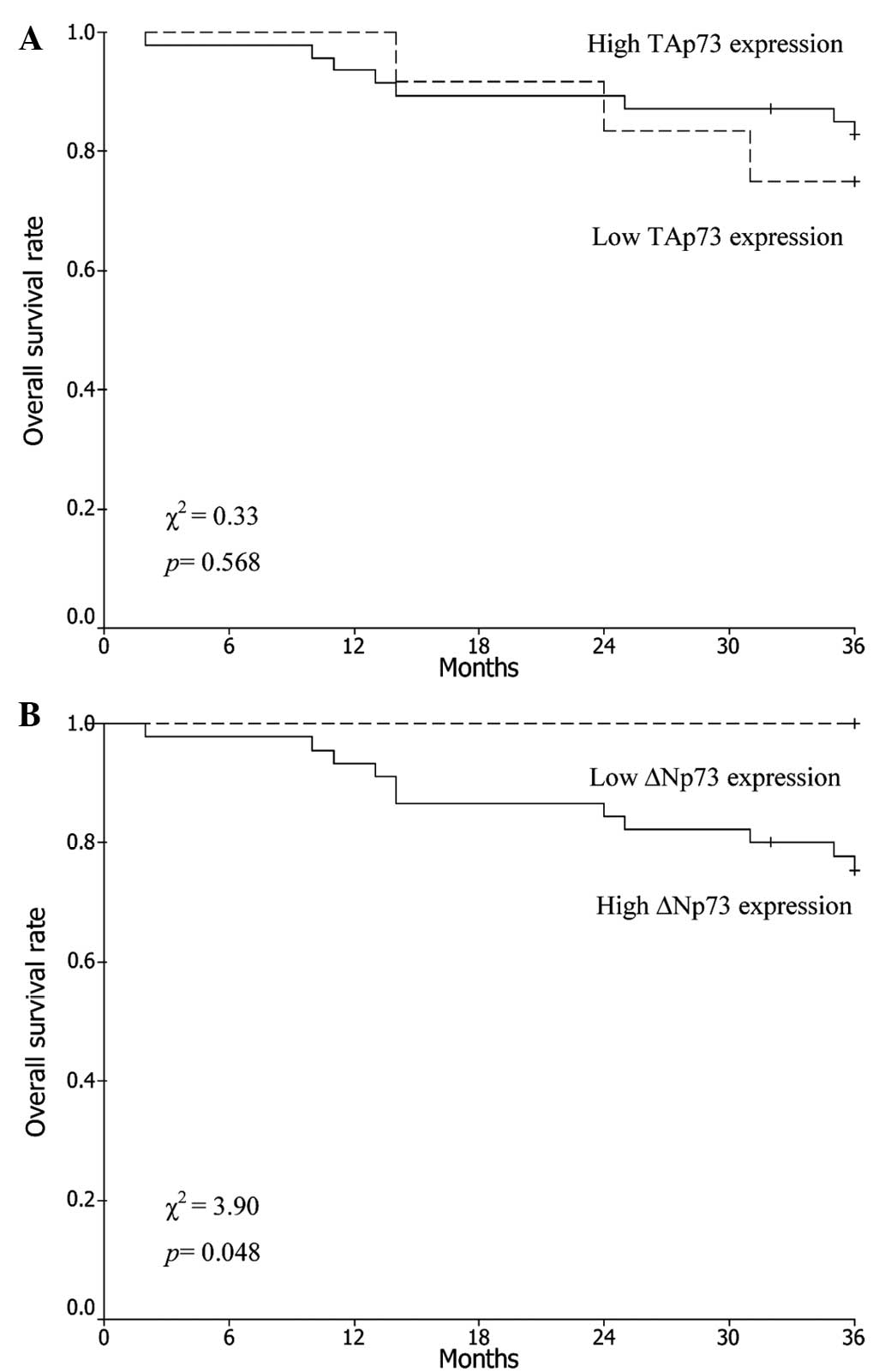
The average duration of follow-up was 41 months
(range, 2–61 months). Analysis of the Kaplan-Meier plots showed
that the 3-year OS rate of all patients was 81.4%. The 3-year OS
rates in patients with low and high expression levels of TAp73 were
75.0 and 83.0%, respectively (χ2=0.33; P=0.568; Fig. 2A), whereas those in patients with low
and high expression levels of ΔNp73 were 100.0 and 75.6%,
respectively (χ2=3.90; P=0.048; Fig. 2B).
Discussion
Previous studies reported that the TAp73 gene mimics
p53 suppressor activities, showing proapoptotic effects. However,
the ΔNp73 gene was shown to have an antiapoptotic function, in
which cooperation with oncogenic RAS induces cell transformation,
confers drug resistance and induces the phosphorylation of
retinoblastoma protein (11,12,17). The
different expression levels of the TAp73 and ΔNp73 isoforms may
determine tumorigenesis and resistance to chemo(radio) therapy, as
the predominance of ΔNp73 may confer pro-tumorigenic properties
(18). A previous study showed that
the TAp73 and ΔNp73 isoforms were significantly overexpressed in a
number of solid tumors compared with the corresponding normal
tissues, suggesting that the balance between these two isoforms may
play a role in the regulation of cell proliferation and cell death
(19). Hofstetter et al
(20) showed that TAp73 and ΔNp73
were expressed in 88.0% (73/83) and 57.8% (48/83) of ovarian cancer
samples, respectively. Liu et al (15) reported the positive expression of
TAp73 and ΔNp73 in 41.0 and 30.8% of patients with cervical cancer,
respectively. Moreover, cancers that expressed a higher level of
ΔNp73 tended to express a lower level of TAp73. Müller et al
(11) also reported that TAp73 and
ΔNp73 are inversely regulated and showed that the high expression
of TAp73 is correlated with the low expression of ΔNp73. These
results suggest that the expression of the two isoforms is
upregulated via different mechanisms in different cancers. In the
current study, it was found that 79.7% (47/59) and 76.3% (45/59) of
patients with cervical SCC exhibited high expression levels of
TAp73 and ΔNp73, respectively. However, no significant correlation
was observed between TAp73 and ΔNp73 expression
(χ2=0.415; P=0.519).
With respect to the clinical significance of TAp73
expression in cancers, Castellino et al (21) reported that medulloblastoma patients
with high expression of TAp73 showed favorable disease-free
survival and overall survival times. Similarly, Liu et al
(15) showed that TAp73
overexpression predicted a better survival in patients with
cervical SCC receiving radiotherapy. However, Hofstetter et
al (20) reported that TAp73
expression had no prognostic significance in ovarian cancer. In the
present study, the 3-year OS rates in patients with low and high
expression levels of TAp73 were 75.0 and 83.0%, respectively, in
patients with FIGO stage I-II cervical SCC after radical surgery
(χ2=0.33; P=0.568). Further study is required to
determine the prognostic significance of TAp73 expression.
Emerging evidence suggests that ΔNp73, rather than
TAp73, is the main physiologically relevant component of
tumor-associated p73 overexpression and that it functionally
overrides the frequent concomitant increase in TAp73. Several
studies have shown that a high expression level of ΔNp73 is
correlated with tumor progression and poor survival rates in a
number of cancer types. Uramoto et al (9) showed that ΔNp73 expression in lung
cancer is not correlated with clinicopathological factors,
including histological type, pathological tumor stage and node
stage. However, lung cancer patients with high ΔNp73 expression
exhibited a lower 5-year survival rate than those with low ΔNp73
expression. Similarly, Müller et al (11) reported that a high expression level of
ΔNp73 is correlated with reduced survival in hepatocellular
carcinoma patients. Liu et al (15) found that ΔNp73 overexpression is
significantly associated with resistance to radiotherapy, disease
recurrence and poor survival in patients with cervical SCC. These
findings indicate that ΔNp73 may be a potential marker for
predicting the prognosis and sensitivity to radiotherapy in
patients with cervical SCC. In the current study, it was shown that
the 3-year OS rates of FIGO stage I-II cervical SCC patients with
low and high expression levels of ΔNp73 were 100.0 and 75.6%,
respectively, following radical surgery (χ2=3.90;
P=0.048). Taken together, these findings suggest that ΔNp73 is
associated with an unfavorable prognosis due to its role in
chemo(radio) therapy resistance and tumor aggressiveness.
In conclusion, TAp73 and ΔNp73 were frequently
overexpressed in the cervical SCC cells in the present study. High
expression of ΔNp73 may indicate an unfavorable prognosis in
early-stage cervical SCC. However, this retrospective study was
potentially limited by the relatively small number of patients.
Additional larger studies are required to reach a definitive
conclusion.
Acknowledgements
This study was supported by grants from the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (grant no. PAPD201105) and Jiangsu Province's Key
Medical Department in 2011 (grant no. RC2011144).
References
|
1
|
Lee MY and Shen MR: Epithelial-mesenchymal
transition in cervical carcinoma. Am J Transl Res. 4:1–13.
2012.PubMed/NCBI
|
|
2
|
Sun Y, Liu JH, Jin L, et al:
Over-expression of the Beclin1 gene upregulates chemosensitivity to
anti-cancer drugs by enhancing therapy-induced apoptosis in cervix
squamous carcinoma CaSki cells. Cancer Lett. 294:204–210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Landoni F, Maneo A, Colombo A, et al:
Randomised study of radical surgery versus radiotherapy for stage
Ib-IIa cervical cancer. Lancet. 350:535–540. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Komaki R, Brickner TJ, Hanlon AL, Owen JB
and Hanks GE: Long-term results of treatment of cervical carcinoma
in the United States in 1973, 1978 and 1983: Patterns of Care Study
(PCS). Int J Radiat Oncol Biol Phys. 31:973–982. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Biewenga P, van der Velden J, Mol BW, et
al: Prognostic model for survival in patients with early stage
cervical cancer. Cancer. 117:768–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biewenga P, van der Velden J, Mol BW, et
al: Validation of existing prognostic models in patients with
early-stage cervical cancer. Gynecol Oncol. 115:277–284. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bourdon JC: p53 family isoforms. Curr
Pharm Biotechnol. 8:332–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salomoni P and Dyer MJS: Delta N-p73: the
enemy within. Cell Death Differ. 12:1553–1554. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uramoto H, Sugio K, Oyama T, et al:
Expression of the p53 family in lung cancer. Anticancer Res.
26:1785–1790. 2006.PubMed/NCBI
|
|
10
|
Khoury MP and Bourdon JC: p53 isoforms: an
intracellular microprocessor? Genes Cancer. 2:453–465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Müller M, Schilling T, Sayan AE, et al:
TAp73/Delta Np73 influences apoptotic response, chemosensitivity
and prognosis in hepatocellular carcinoma. Cell Death Differ.
12:1564–1577. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Domínguez G, García JM, Peña C, et al:
Delta TAp73 upregulation correlates with poor prognosis in human
tumors: putative in vivo network involving p73 isoforms, p53, and
E2F-1. J Clin Oncol. 24:805–815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moll UM: The role of p63 and p73 in tumor
formation and progression: coming of age toward clinical
usefulness. Clin Cancer Res. 9:5437–5441. 2003.PubMed/NCBI
|
|
14
|
Becker K, Pancoska P, Concin N, et al:
Patterns of p73 N-terminal isoform expression and p53 status have
prognostic value in gynecological cancers. Int J Oncol. 29:889–902.
2006.PubMed/NCBI
|
|
15
|
Liu SS, Chan KY, Cheung AN, Liao XY, Leung
TW and Ngan HY: Expression of deltaNp73 and TAp73alpha
independently associated with radiosensitivities and prognoses in
cervical squamous cell carcinoma. Clin Cancer Res. 12:3922–3927.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giatromanolaki A, Koukourakis MI,
Koutsopoulos A, Chloropoulou P, Liberis V and Sivridis E: High
Beclin 1 expression defines a poor prognosis in endometrial
adenocarcinomas. Gynecol Oncol. 123:147–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grob TJ, Fey MF and Tobler A: The two
faces of p73. Cell Death Differ. 9:229–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soldevilla B, Millàn CS, Bonilla F and
Domínguez G: The TP73 complex network: ready for clinical
translation in cancer? Genes Chromosomes Cancer. 52:989–1006. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melino G, Laurenzi V and Vousden KH: p73:
Friend or foe in tumorigenesis. Nat Rev Cancer. 2:605–615. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hofstetter G, Berger A, Chamson M, et al:
Clinical relevance of TAp73 and ΔNp73 protein expression in ovarian
cancer: a series of 83 cases and review of the literature. Int J
Gynecol Pathol. 30:527–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castellino RC, De Bortoli M, Lin LL, et
al: Overexpressed TP73 induces apoptosis in medulloblastoma. BMC
Cancer. 7:1272007. View Article : Google Scholar : PubMed/NCBI
|