Introduction
With >250,000 annual mortalities, pancreatic
carcinoma is one of the most lethal malignancies, ranking 12th
worldwide (1). Mortality resulting
from this disease is high even in developed countries, including
Japan, the United Kingdom, France and the United States (2,3). Overall,
>75% of pancreatic carcinoma cases are histologically
characterized as pancreatic ductal adenocarcinoma (PDAC) (4,5). The
majority of cases of PDAC are incurable due to the necessity of
extensive resection, which is often not feasible, and due to the
fact that the disease is rarely identified at an early stage.
Furthermore, the majority of patients with advanced PDAC either do
not respond, or respond transiently to chemotherapeutic drugs and
radiation (6). Typically, the
majority of patients with PDAC succumb to the disease within one
year of diagnosis, and the overall five-year survival rate is
<5% (7). Even in patients with
resectable carcinoma, the long-term outcome remains unsatisfactory
due to the incidence of early recurrence following surgical
resection.
In order to gain an improved understanding of this
lethal malignant carcinoma, studies that use animal PDAC models
with pancreatic neoplasms that resemble human PDAC are usually
desirable. By focusing on human pancreatic adenocarcinomas that
express a high frequency of KRAS mutation, a transgenic rat
model carrying the human HRASG12V or
KRASG12V oncogene was established (8,9). The
activation of the target transgene is attained by the injection of
a Cre recombinase (Cre)-carrying adenovirus into the pancreatic
ducts of the animal via the common bile duct (8,9). In this
model, the transgenic rats usually develop pre-neoplastic and
neoplastic pancreatic lesions within two weeks of the viral
inoculation (10). These lesions in
the transgenic rats exhibit morphological similarities to those
observed in human pancreatic lesions, including PDAC (11) and intraepithelial neoplasias (PanINs)
(9).
Due to the position of the tumors within the
abdominal cavity, laparotomy is the only technique that is able to
determine the existence and size of pancreatic tumors within the
transgenic rats following virus inoculation, as the tumors cannot
be visually assessed from surface scans of the affected rats. A
previous study determined that in order to serologically detect
early-stage PDAC in the rat models, serum N-ERC levels and the
levels of several serum miRNAs, which are expressed differentially
in PDAC transgenic rats and control rats, could be used (8,12).
However, even in the case of elevated levels of high serum
biomarkers, the exact location and size of pancreatic tumors is
difficult to detect unless exploratory surgery is performed within
the abdominal cavity.
18F-fluorodeoxyglucose-positron emission
tomography ([18F-FDG-PET) is commonly used during the
diagnosis of pancreatic tumors (13,14). Due
to a high sensitivity and penetration depth, PET is considered to
be more accurate for the detection and identification of metastases
in humans and animal models than other imaging systems (15,16).
The objective of the present study was to evaluate
the carcinogenic process in a mammalian model using imaging
modalities, such as PET/computed tomography (CT), which are
applicable for the study of human PDAC.
Materials and methods
Animals
In total, six male KRASG12V
oncogene transgenic rats were used in the present study. Routine
genotyping was performed as previously described (8). The rats were kept in plastic cages in an
air-conditioned room at 24±2°C and 60±5% humidity with a 12-h
light/12-hour dark cycle. A basal diet (Oriental Yeast Co., Ltd.,
Tokyo, Japan) and tap water were available ad libitum
throughout the experiment. All experiments were approved by the
Animal Care and Use Committee of Nagoya City University Graduate
School of Medical Sciences and the National Institute of
Radiological Sciences (Tokyo, Japan).
Procedure of adenovirus
inoculation
The preparation and inoculation of the adenoviruses
was performed as previously described (9). In brief, a Cre-recombinase expressing
adenovirus was amplified in HEK293 cells and then purified using
the Vivapure AdenoPACK (Vivascience, Hannover, Germany) (17). The titer of the adenovirus was then
determined using an Adeno-X rapid titer kit (Clontech, Mountain
View, CA, USA). The virus was prepared to a concentration of
4.0×109 plaque-forming units/ml. The virus (300–400 µl)
was injected using a small syringe into the pancreatic duct of the
rats as previously described (9).
[18F-FDG-PET and CT
procedures, and image analysis
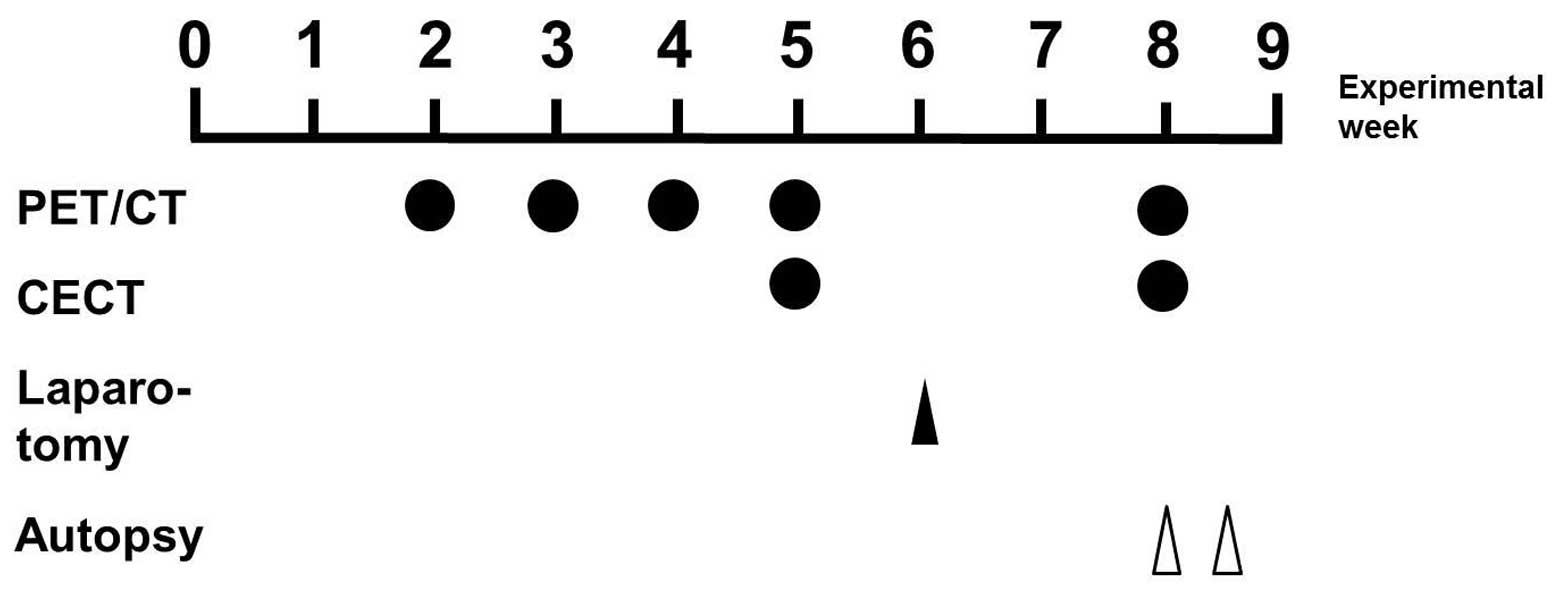
The time course of the experimental protocol is
shown in Fig. 1. For the present
study, 10-week-old male KRASG12V transgenic rats
were used. The rats were divided into two groups, with three rats
per group. The rats in groups 1 and 2 were administered with the
Cre-expressing adenovirus vector or an empty vector (negative
control), respectively. A small-animal multimodality PET system
(Inveon; Siemens Healthcare Inc., Malvern, PA, USA) was used for
PET data acquisition. Following an overnight fast, each rat (body
weight, 403–583 g) was injected with 15 MBq (14.6±1.6 MBq)
18F-FDG (Nihon Medi-Physics Co., Ltd., Tokyo, Japan) via
the tail vein, whilst the rat was under isoflurane anesthesia. The
PET data acquisition was conducted for 10 min, beginning 50 min
after the 18F-FDG injection. Using a lamp, the body
temperature of the rats was maintained at between 36 and 37°C
during the scan. The images were reconstructed using a 3D maximum
a posteriori (18 iterations with 16 subsets; β=0.2), without
attenuation correction. The tracer uptake was expressed as the
standardized uptake value (SUV).
Subsequent to PET scanning, plain or
contrast-enhanced CT (CECT) was conducted with an X-ray source set
at 90 kVp and 200 µA, using a small-animal CT system (R_mCT2;
Rigaku, Tokyo, Japan). For CECT, the rats were intravenously
injected with 10 ml Iopamiron 370 contrast medium (Bayer Yakuhin
Ltd., Osaka, Japan) using an infusion pump (NE-1000; Neuroscience
Inc., Tokyo, Japan) at the rate of 2 ml/min, whilst the rats were
under isoflurane anesthesia. The CECT images were acquired 5 min
subsequent to the injection. In order to reduce the motion
artifacts caused by respiratory and peristaltic movement during the
CT scan, a respiratory gating system was used whilst the rats under
inhalable isoflurane anesthesia. The 18F-FDG-PET
scanning was conducted at two, three, four, five and eight weeks
subsequent to administration of the Cre-expressing adenovirus or
the empty vectors. In order to confirm the results of the PET
analysis, the CECT scan was also conducted at five and eight weeks
subsequent to the virus injection. For the quantitative analysis,
the PET and CT data sets were imported and the fused images were
then obtained using ASIPro VM software (CTI Concorde Microsystems,
Knoxville, TN, USA). Laparotomy was performed six weeks subsequent
to the injection in order to confirm the location and size of the
pancreatic tumors, which were visible to the naked eye. The
experimental rats were sacrificed eight weeks subsequent to the
injection.
Histopathological examination
The rats in groups 1 and 2 survived until the end of
the experimental period. The pancreatic tumors and the normal
pancreatic lobes were removed from the abdomen of the rats, fixed
with 10% buffered formalin and then processed for histopathological
examination using hematoxylin and eosin stain (9). The pancreatic lesions were diagnosed
histopathologically based upon previously described criteria
(8,9).
Results
PET/CT findings and histopathological
examination
The six rats were euthanized eight weeks subsequent
to the injection of the Cre-expressing viral or empty vectors. All
three Cre-expressing transgenic rats in group 1 (100%) developed
orthotopic pancreatic tumors without distant metastasis. By
contrast, no tumors were identified in the negative control rats of
group 2. Upon macroscopic analysis, the tumors appeared nodular and
solid in shape, and were ochre yellow in color. The PET/CT images
obtained eight weeks subsequent to the viral injection revealed the
majority of tumor tissues to be distinguishable from the adjacent
organs, but the tissues were challenging to distinguish from the
normal intestinal tissues, depending on the site of the tumor. The
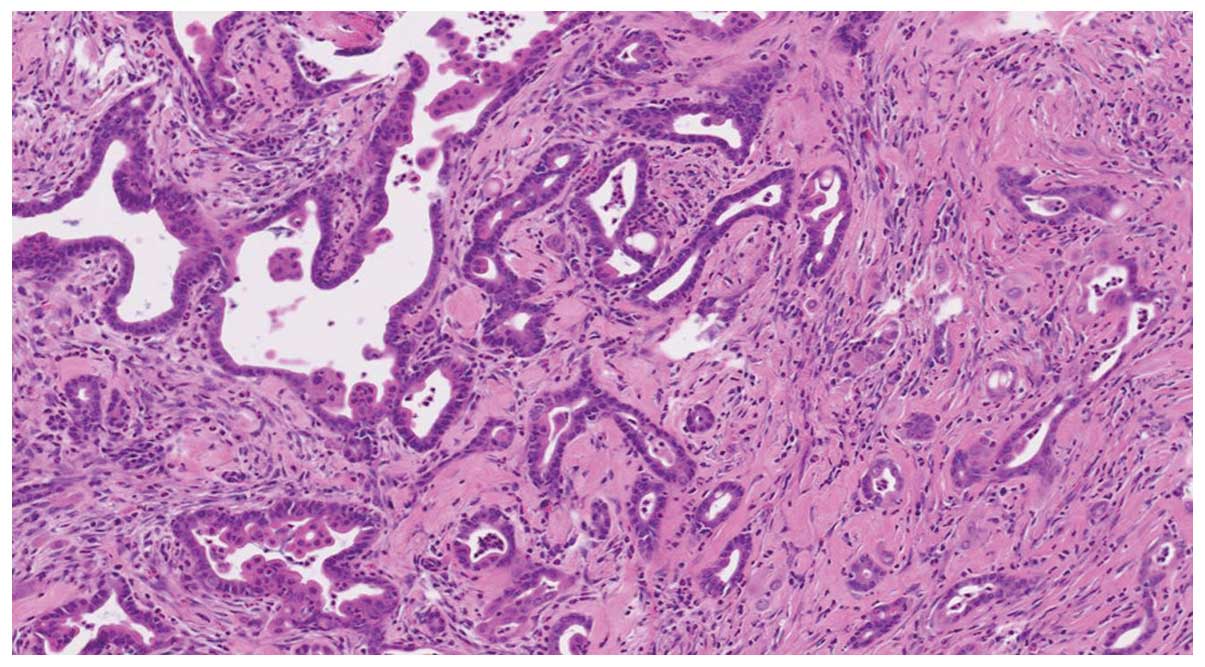
pancreatic tumors were of the ductal adenocarcinoma histological
type. The coexistence of adenocarcinoma and PanIN lesions
surrounded by fibrous tissue with inflammatory cell infiltration
was also identified (Fig. 2).
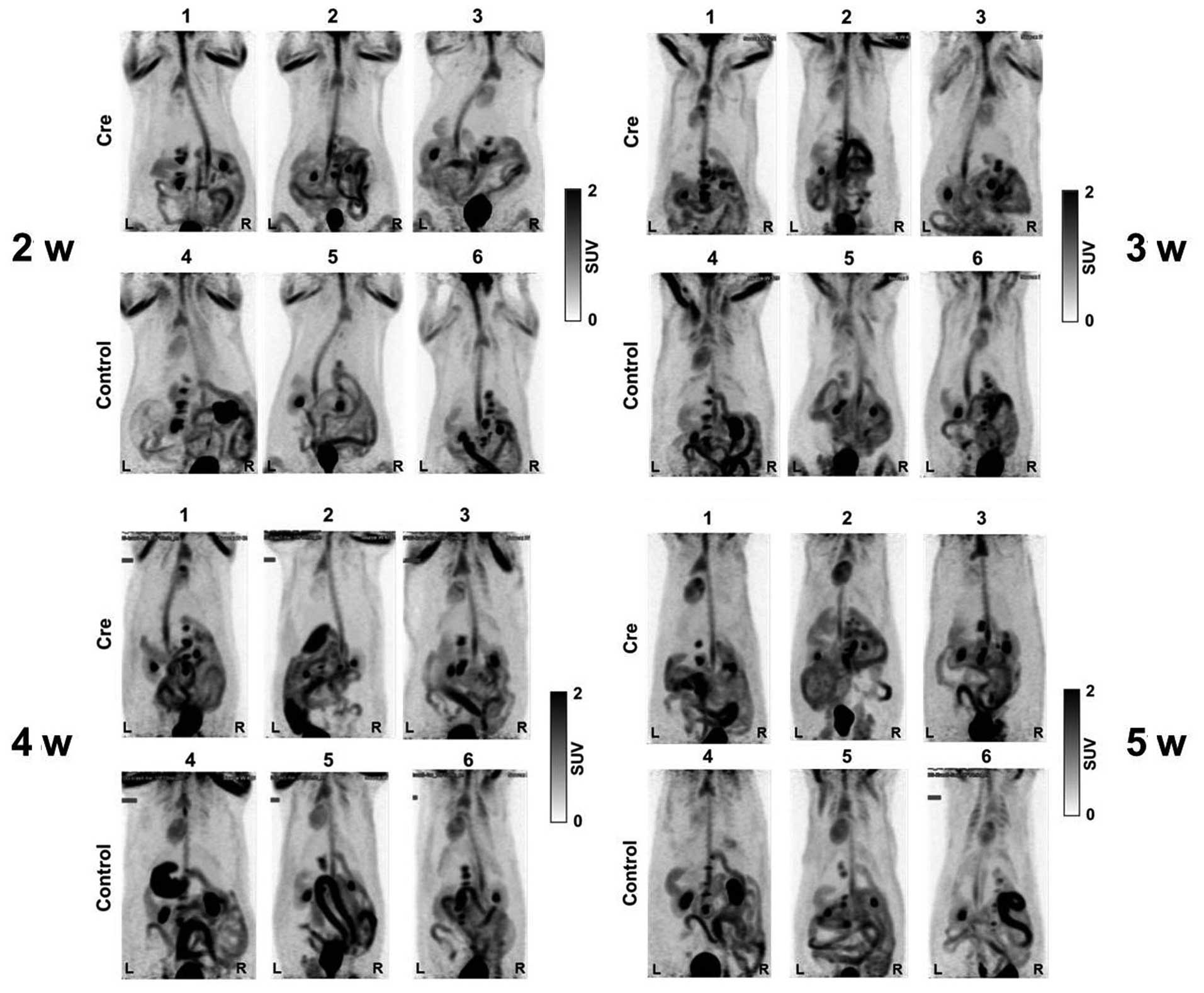
[18F-FDG-PET imaging prior
to experimental week five
The representative maximal-intensity projection
images from 18F-FDG-PET are shown in Fig. 3. PET scanning was performed four times
prior to the five experimental weeks. A marginal or very high
uptake in the gastrointestinal tract and urinary bladder, which was
considered to be physiological FDG uptake, was observed in all
three Cre-expressing transgenic rats and three control rats. At
five weeks post-treatment, the tumors were not clearly visualized
by the indicated imaging system. Following the laparotomy at six
weeks post-treatment, a few small nodules measuring between 1 and 2
mm in diameter, indicative of a carcinoma, were identified in the
pancreas of all Cre-expressing transgenic rats. No metastases were
identified in the rats of the negative control group.
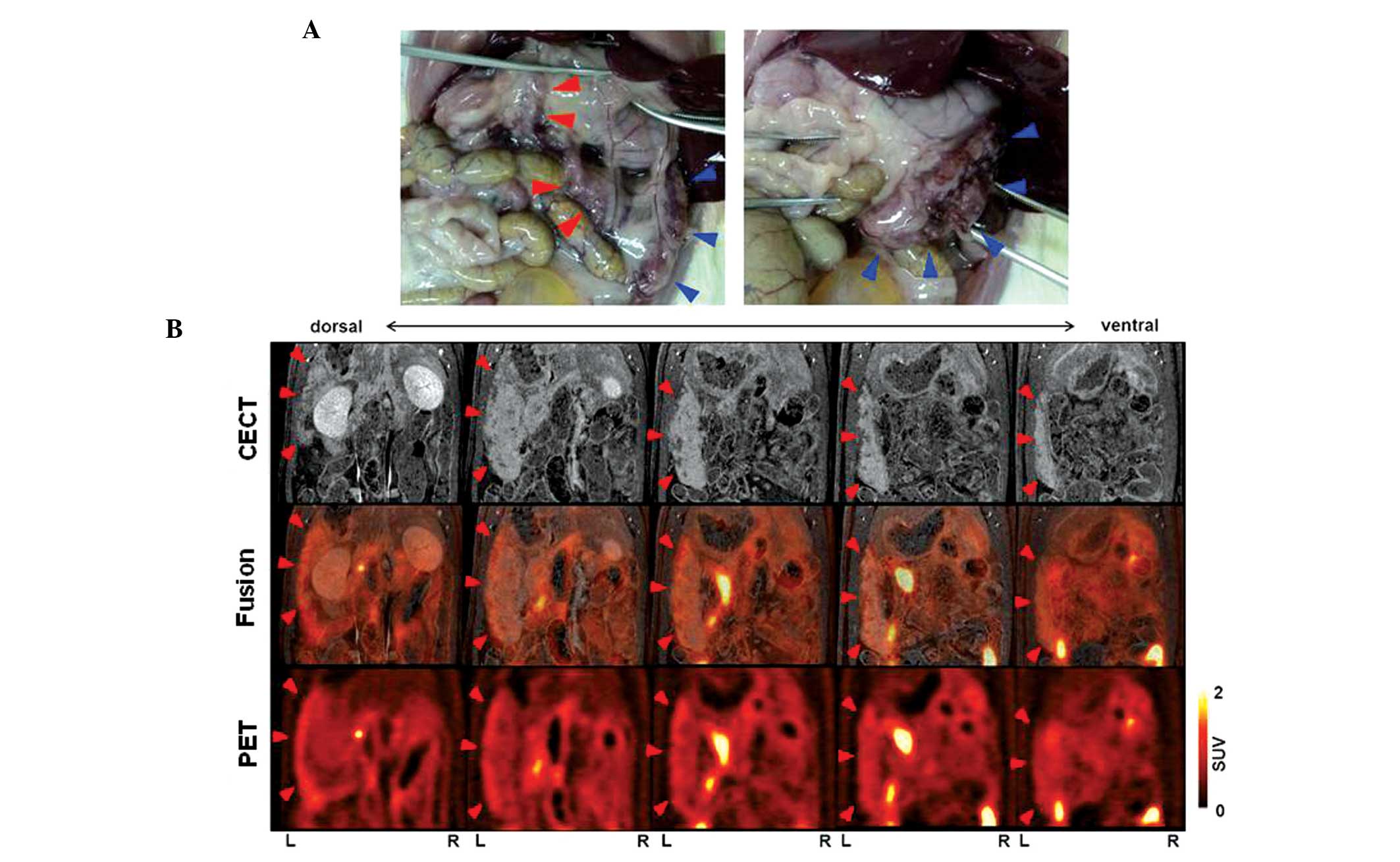
Analysis of CECT, PET and PET/CT
images at eight weeks post-treatment
Representative slices of the
18F-FDG-PET/CT fusion images obtained from the
Cre-expressing transgenic rats are shown in Figs. 4–6. The
SUVmax and SUVmean of the pancreatic tumors
and each organ are shown in Table I.
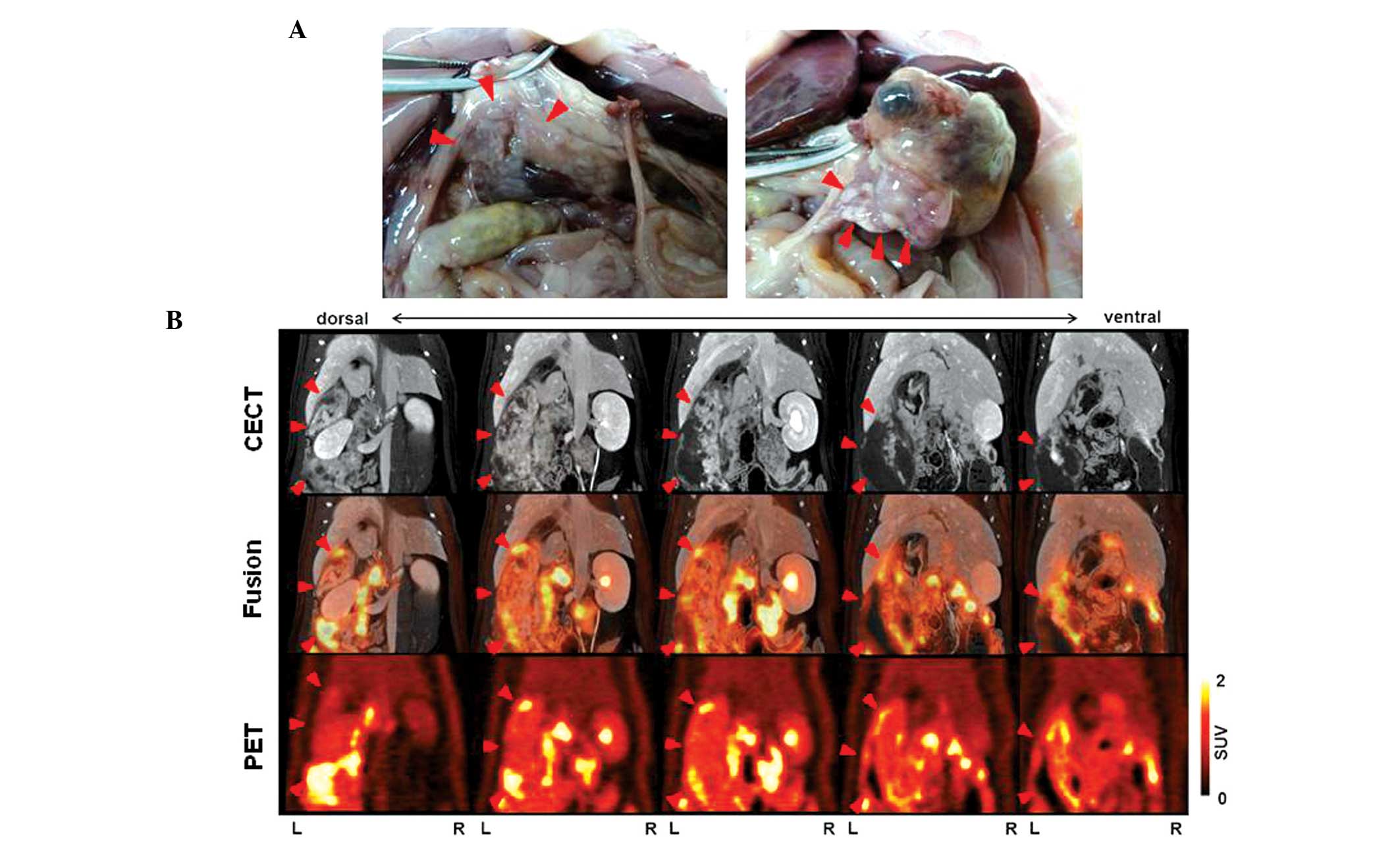
In rat 1 (body weight, 503 g), the CECT images revealed a
heterogeneous lesion measuring 17 mm in the maximum sagittal
diameter in the left side of the abdomen. In addition, the PET and
PET/CT fusion images revealed moderately increased
18F-FDG uptake in the lesion located in the left side of
the abdomen, with a SUVmax of <1.5. Physiological FDG
uptake was observed in the gastrointestinal tract, and the
SUVmax of this organ site was 3.2, as shown in Table I. Autopsy revealed a large tumor in
the splenic lobe, which was detected by CECT and PET/CT. However,
multiple tumors that were present in the duodenal lobe of the
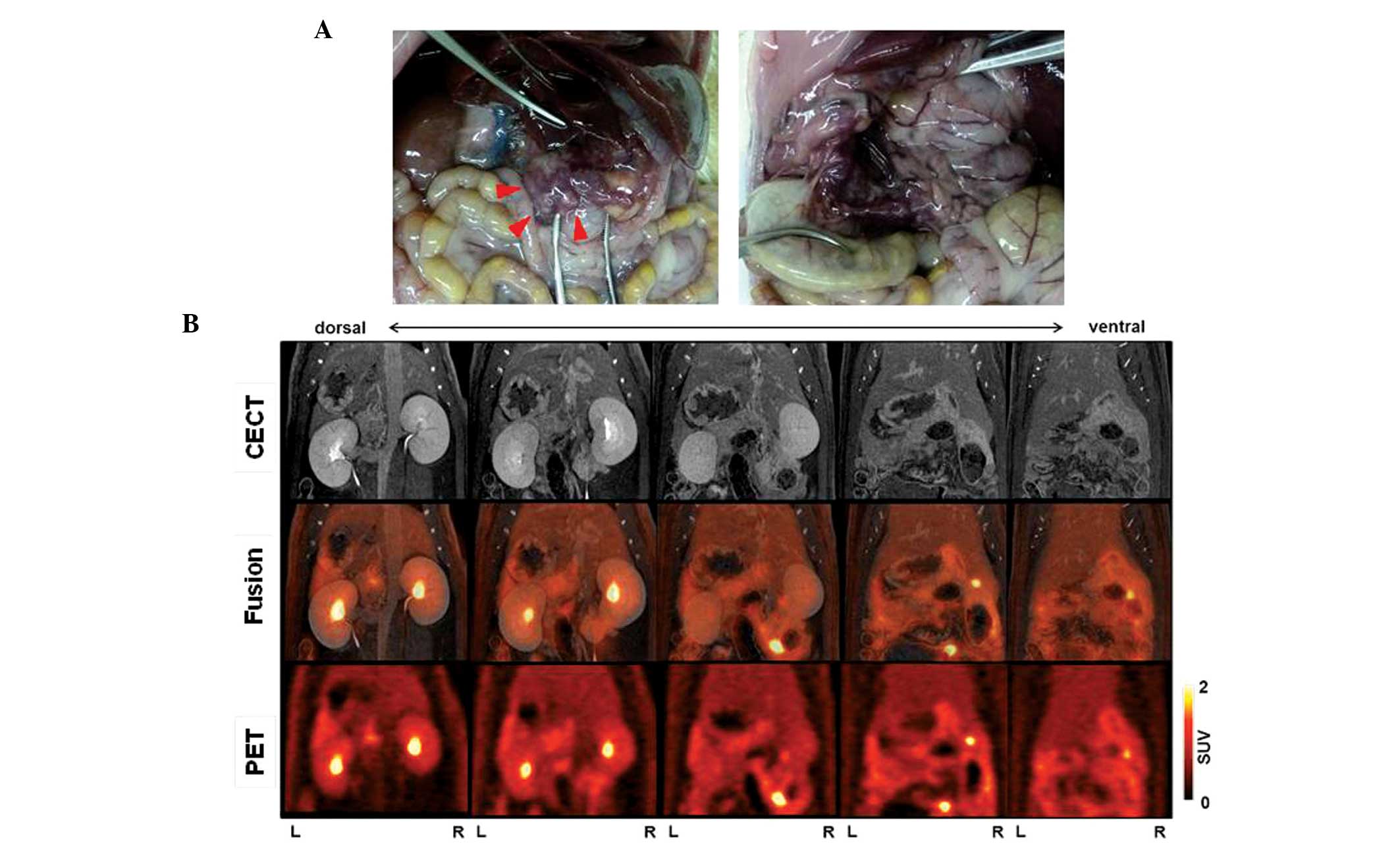
pancreas were not revealed by CECT and PET/CT (Fig. 4). In rat 2 (body weight, 470 g), the
CECT images revealed a heterogeneously-enhanced pancreatic tumor,
measuring 20 mm in the maximum sagittal diameter, in the left side
of the abdomen. PET/CT fusion images revealed moderate uptake of
18F-FDG (SUVmax, 3.0) in the pancreatic
tumor. In addition, physiological FDG uptake in the
gastrointestinal tract (SUVmax, 2.7) was observed.
During autopsy, a large tumor in the splenic lobe was identified by
the imaging analysis. In addition, multiple tumors were identified
in the duodenal lobe of the pancreas, but these tumors were not
visualized on CECT and PET/CT (Fig.
5). In rat 3 (body weight, 564 g), the presence of a tumor was
not observed on either CECT or PET scans (Fig. 6). Next, an incision was made in the
abdomen of the rat, and a number of nodules, which were smaller in
size than those observed in rats no. 1 and 2, were identified in
the duodenal lobe of the pancreas. No tumor was identified in the
splenic lobe of the pancreas. No macroscopic metastasis was
identified in rats 1–3.
 | Table I.Mean SUV of the tumor and each organ
in the experimental rats. |
Table I.
Mean SUV of the tumor and each organ
in the experimental rats.
| Rat | Tumor
(SUVmax) | GI tract
(SUVmax) | Liver | Kidney (cortex and
medulla) |
|---|
| 1 | 0.7–1.2 (1.4) | 0.5–1.0 (3.2) | 0.4–0.5 | 0.8–0.9 |
| 2 | 0.9–2.0 (3.0) | 0.5–1.8 (2.7) | 0.5–0.6 | 0.9–1.1 |
| 3 | ND | 0.6–1.4 (4.2) | 0.6–0.7 | 0.9–1.1 |
Discussion
A limited number of the documented studies that
involve imaging analysis of pancreatic tumors in animal models used
the FDG-PET/CT system (18,19). In the present study, a
KRAS-mediated transgenic rat model was used to develop
multiple pancreatic tumors that resembled the developmental and
histological features of human PDAC within two weeks (8). In living rats at eight weeks
post-treatment, the pancreatic tumors were clearly enhanced in the
CECT images following the administration of a contrast media, and
were distinguishable from the gastrointestinal tract. In the
absence of imaging analysis, calipers are used to determine the
location and size of a pancreatic tumor following a laparotomy or
autopsy of an animal. Imaging analysis therefore allows each animal
to be scanned sequentially in a sectional plane of interest, such
as transverse, coronal or sagittal, and be monitored over time
without the need to be sacrificed. In addition, PET/CT enables the
accurate measurement of irregularly-shaped tumors in a pancreatic
tumor model. According to the Three Rs principle (20), which aims to replace existing
experimental methods with those that do not use animals, reduce the
number of test animals used and refine methods in order to minimize
the suffering of test animals, the PET/CT system reduces the number
of animals required for experimental treatment and control groups.
This indicates that imaging systems should be recommended for use
in animal experiments. Recently, inoculation efficacy has been
improved by clamping the common bile duct and increasing the amount
of virus that is administered. Using this technique, studies may be
able to control the size of the pancreatic tumor quantitatively
within an appropriate time period, a factor that demonstrates the
usefulness of this model.
With regard to studies that have used small animal
models, Kitahashi et al (21)
used micro-CT to detect chemically-induced pancreatic tumors of
>4 mm in diameter in Syrian hamsters. Another study by Fendrich
et al (18) detected precursor
pancreatic adenocarcinoma lesions with an activity of 9.6±0.5 MBq
in a five-month-old transgenic mouse model by FDG-PET/CT. Kayed
et al (22) measured the
anticancer effects of cyclopamine in a pancreatic carcinoma
xenograft with an activity of 7.4 MBq model using
18F-PET/CT. The study examined the size and SUV of each
tumor that was grown in the hip region of nude mice during a
seven-day treatment period. A technical limitation in baseline
calibration occurred, but the system was believed to be suitable
for practical use. The SUV and SUVmax parameters have
been demonstrated in a previous study to be potential predictors of
early recurrence following the curative resection of lung
carcinomas (23).
It was hypothesized that the amount of FDG uptake
into the pancreatic tumors would be higher compared with other
abdominal organs. However, the results shown in Table I indicate that each value was not
necessarily specific to the region of interest. This indicates a
limitation in the use of this parameter for differential diagnoses
that are based upon imaging techniques. To avoid bias during the
measurement of the SUV, representative areas of tumor tissues that
demonstrated moderate FDG uptake were selected. Therefore, the
potential limitations of this methodology should be considered when
calculating the SUV or SUVmax. This aspect warrants
further investigation.
PET images did not identify tumor masses in any
organ of the Cre-expressing rats until five weeks post-treatment
(Fig. 3). When the laparotomy was
performed six weeks subsequent to the viral inoculation, multiple
tumors measuring <2 mm in diameter were identified in the
pancreas of all three Cre-expressing transgenic rats. This
indicated that the transgenic rats developed macroscopically
visible tumors by six weeks post-treatment. At eight weeks
post-treatment, the PET/CT images revealed pancreatic tumor masses
in two of the three rats, which indicated a potential limitation in
the detection of tumors prior to the eighth week by current imaging
techniques. Pancreatic tumors were primarily identified in the
splenic lobe of the pancreas by PET/CT. Tumors in the duodenal
lobe, however, could not be detected by such imaging analyses, even
if these tumors were confirmed by laparotomy. In addition, the
presence of smaller tumors, and a specific anatomical location
within the gastrointestinal tract, may affect the visibility of the
tumor. In the PET and PET/CT fusion images, the pancreatic tumors
were visible, but the physiological 18F-FDG uptake in
the intestine reduced the appearance of the lesions.
In conclusion, the present study demonstrated that
pancreatic tumors can be detected in rats using imaging modalities
eight weeks after viral inoculation. The FDG-PET/CT imaging system
is a valuable approach for the evaluation of the carcinogenic
process and potential treatment or prevention methods for
pancreatic tumors in mammalian models. Therefore, it is proposed
that this experimental system can also be applied to studies that
examine cases of human PDAC.
Acknowledgements
The authors would like to thank Maki Okada for
providing technical assistance with the CECT scanning, Hidekatsu
Wakizaka for providing operational and quality control support for
the PET system and Nobuyuki Miyahara for providing quality control
support for the CT system.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer
in: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsuda A, Matsuda T, Shibata A, Katanoda
K, Sobue T and Nishimoto H: Japan Cancer Surveillance Research
Group: Cancer incidence and incidence rates in Japan in 2007: a
study of 21 population-based cancer registries for the Monitoring
of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol.
43:328–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosetti C, Bertuccio P, Negri E, La
Vecchia C, Zeegers MP and Boffetta P: Pancreatic cancer: overview
of descriptive epidemiology. Mol Carcinog. 51:3–13. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cubilla AL and Fitzgerald PJ: Cancer of
the exocrine pancreas: the pathologic aspects. CA Cancer J Clin.
35:2–18. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morohoshi T, Held G and Klöppel G:
Exocrine pancreatic tumours and their histological classification.
A study based on 167 autopsy and 97 surgical cases. Histopathology.
7:645–661. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolfgang CL, Herman JM, Laheru DA, et al:
Recent progress in pancreatic cancer. CA Cancer J Clin. 63:318–348.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukamachi K, Tanaka H, Hagiwara Y, et al:
An animal model of preclinical diagnosis of pancreatic ductal
adenocarcinomas. Biochem Biophys Res Commun. 390:636–641. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ueda S, Fukamachi K, Matsuoka Y, et al:
Ductal origin of pancreatic adenocarcinomas induced by conditional
activation of a human Ha-ras oncogene in rat pancreas.
Carcinogenesis. 27:2497–2510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yabushita S, Fukamachi K, Tanaka H, et al:
Metabolomic and transcriptomic profiling of human K-ras oncogene
transgenic rats with pancreatic ductal adenocarcinomas.
Carcinogenesis. 34:1251–1259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yabushita S, Fukamachi K, Kikuchi F, et
al: Twenty-one proteins up-regulated in human H-ras oncogene
transgenic rat pancreas cancers are up-regulated in human pancreas
cancer. Pancreas. 42:1034–1039. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yabushita S, Fukamachi K, Tanaka H, et al:
Circulating microRNAs in serum of human K-ras oncogene transgenic
rats with pancreatic ductal adenocarcinomas. Pancreas.
41:1013–1018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asagi A, Ohta K, Nasu J, et al: Utility of
contrast-enhanced FDG-PET/CT in the clinical management of
pancreatic cancer: impact on diagnosis, staging, evaluation of
treatment response, and detection of recurrence. Pancreas.
42:11–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomimaru Y, Takeda Y, Tatsumi M, et al:
Utility of 2-[18F] fluoro-2-deoxy-D-glucose positron emission
tomography in differential diagnosis of benign and malignant
intraductal papillary-mucinous neoplasm of the pancreas. Oncol Rep.
24:613–620. 2010.PubMed/NCBI
|
|
15
|
Lan BY, Kwee SA and Wong LL: Positron
emission tomography in hepatobiliary and pancreatic malignancies: a
review. Am J Surg. 204:232–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Studwell AJ and Kotton DN: A shift from
cell cultures to creatures: in vivo imaging of small animals in
experimental regenerative medicine. Mol Ther. 19:1933–1941. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanegae Y, Lee G, Sato Y, et al: Efficient
gene activation in mammalian cells by using recombinant adenovirus
expressing site-specific Cre recombinase. Nucleic Acids Res.
23:3816–3821. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fendrich V, Schneider R, Maitra A,
Jacobsen ID, Opfermann T and Bartsch DK: Detection of precursor
lesions of pancreatic adenocarcinoma in PET-CT in a genetically
engineered mouse model of pancreatic cancer. Neoplasia. 13:180–186.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Kouwen MC, Laverman P, van Krieken JH,
Oyen WJ, Jansen JB and Drenth JP: FDG-PET in the detection of early
pancreatic cancer in a BOP hamster model. Nucl Med Biol.
32:445–450. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Russell WMS and Burch RL: The Principles
of Humane Experimental Technique2nd. Methuen & Co; London:
1992
|
|
21
|
Kitahashi T, Mutoh M, Tsurusaki M, et al:
Imaging study of pancreatic ductal adenocarcinomas in Syrian
hamsters using X-ray micro-computed tomography (CT). Cancer Sci.
101:1761–1766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kayed H, Meyer P, He Y, et al: Evaluation
of the metabolic response to cyclopamine therapy in pancreatic
cancer xenografts using a clinical PET-CT system. Transl Oncol.
5:335–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakai T, Tsushima T, Kimura D, Hatanaka R,
Yamada Y and Fukuda I: A clinical study of the prognostic factors
for postoperative early recurrence in patients who underwent
complete resection for pulmonary adenocarcinoma. Ann Thorac
Cardiovasc Surg. 17:539–543. 2011. View Article : Google Scholar : PubMed/NCBI
|