Introduction
Pituitary tumor transforming gene 1 (PTTG1) is the
human homolog of securin and was originally identified in rat
pituitary adenoma cells (1).
PTTG1/securin is highly expressed in a variety of human
malignancies, including those of the breast (2), uterus (3),
lung (4), thyroid (5) and brain (6). A high expression level of securin has
been revealed to be correlated with extremely poor outcomes in
breast cancer, adrenocortical cancer (7,8) and glioma
(6) patients. A previous study
demonstrated that the transgenic overexpression of PTTG1 in the
mouse pituitary caused early multipotential pituitary focal
hyperplasia (9). By contrast, it was
also previously identified that the downregulation of securin
reduced angiogenic activity in pluripotent stem cell-derived
vascular endothelial cells (10). In
addition, a further study established that PTTG1 deficiency
protected against pituitary tumorigenesis induced by retinoblastoma
haploinsufficiency (11). In the
glioma U87 cell line, PTTG-knockdown had an inhibitive effect on
serum-induced proliferation, and PTTG expression was upregulated by
the promalignant ligands, epithelial growth factor and transforming
growth factor-α (12). These results
indicate a promotive role for PTTG1/securin in oncogenic
processes.
Invasion is an important characteristic of malignant
tumors, which involves the proteolytic degradation of extracellular
matrix (ECM) components. Matrix metalloproteinases (MMPs) are
regulators of ECM proteins. MMPs are members of a large family of
proteolytic enzymes that degrade the majority of ECM components. At
present, six groups of MMPs have been identified; these include
collagenases, matrilysins, stromelysins, gelatinases, membrane-type
matrix metalloproteinases, and other MMPs. MMP2 (also known as
gelatinase A) and MMP9 (also known as gelatinase B) cleave
denatured collagens (gelatins), laminins and certain chemokines
(13). In addition, MMP2 activates
MMP1 and MMP9 by cleaving their prodomains (14). Under physiological conditions, MMP
expression is low in the majority of normal cells. By contrast, MMP
expression is markedly increased in a number of different cancers
(15,16) and is directly associated with tumor
invasiveness (17), which contributes
to poor patient outcomes. As two important members of the MMP
family, MMP2 and MMP9 have been identified to be involved in colon
cancer progression, hepatic metastasis (18), the promotion of lung cancer invasion
(19), and hepatocellular carcinoma
cell migration and invasion (20).
MMP2 and 9 have also been revealed to participate in invasion. The
findings of a previous study established that MMP2 exhibited a
positive correlation with β3GnT8 in glioma U251 cells, and that
silencing of the latter decreased cell proliferation, migration and
metastatic ability (21). A further
study identified that the cytotoxic treatment of U251 cells
significantly reduced MMP2 and MMP9 expression (22).
The association between securin and MMPs has
previously been reported. Certain studies identified that PTTG,
MMP2 and MMP9 were simultaneously overexpressed in metastatic
papillary thyroid carcinomas (23,24). In
HEK293 cells, transfection with PTTG cDNA significantly increased
the secretion and expression of MMP2 (25). Furthermore, in a cutaneous squamous
cell carcinoma cell line, the downregulation of PTTG by small
interfering RNA (siRNA) significantly inhibited proliferation and
metastasis, and decreased the expression of MMP2 and MMP9 (26). These findings indicate a positive
correlation between securin and MMPs. In order to further
understand the role of securin in glioma, the present study
established models of securin overexpression and downregulation in
different glioma cells using securin cDNA and siRNA, respectively.
Using these models, the effect of securin on glioma migration and
invasion, and the potential role of MMPs, was investigated.
Materials and methods
Reagents
The chemiluminescence reagent was purchased from
Pierce Biotechnology, Inc. (Rockford, IL, USA) and G418 was
purchased from Sigma-Aldrich (St. Louis, MO, USA). The fetal bovine
serum (FBS), RPMI-1640 medium, Lipofectamine 2000, TRIzol reagent
and SYBR GreenER qPCR SuperMix Universal kit (cat. no. 11762-500)
were purchased from Life Technologies (Grand Island, NY, USA).
M-MLV reverse transcriptase was purchased from Promega Corporation
(Madison, WI, USA). The normal human astrocyte (NHA) cells and
glioma LN-229, U87, U251 and U373 cell lines were purchased from
the American Type Culture Collection (Manassas, VA, USA). The
pGenesil 2 and pcDNA3.1 plasmids were purchased from Jingsai
Shenggong Biological Co., Ltd. (Shanghai, China). Polyclonal
primary rabbit anti-human antibodies including anti-AKT (product
no. 4691), anti-phosphorylated (phospho) AKT (Ser473; product no.
4060), anti-β actin (product no. 4967), anti-MMP2 (product no.
4022), anti-MMP 9 (product no. 3852) or anti-securin (product no.
13445) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA), while the peroxidase conjugated rabbit IgG was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, Texas,
USA).
Quantitative polymerase chain reaction
(qPCR)
The mRNA levels were investigated using qPCR, as
previously described (13). The total
RNA was extracted using TRIzol reagent according to the
manufacturer's instructions. The RNA was first reverse transcribed
into cDNA using M-MLV reverse transcriptase. Next, qPCR was
performed using the SYBR GreenER qPCR SuperMix Universal kit with
an ABI StepOnePlus real-time PCR system (Applied Biosystems Life
Technologies, Foster City, CA, USA). Subsequent to an initial
denaturation at 95°C for 5 min, the following cycling profile was
used: Denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec
and extension at 72°C for 45 sec. Amplification was performed for
39 cycles. The primers specific for the target genes are shown in
Table I. The results are presented as
the levels of expression relative to those of the controls,
subsequent to normalization to β-actin using the 2−∆∆Ct
method.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
|
| Sense (5′→3′) | Antisense
(5′→3′) | Accession number |
|---|
| β-actin |
GCTCTTTTCCAGCCTTCCTT |
GAGCCAGAGCAGTGATCTCC | HQ154074 |
| MMP2 |
CTCCACTGGATGGAGGAAAA |
ACGGCCACTCAGTAGGTGTC | NM004530 |
| MMP9 |
GAGTTCCCGGAGTGAGTTGA |
ACTCCTCCCTTTCCTCCAGA | NM004994 |
| Securin |
AAAGCTCTGTTCCTGCCTCA |
GAGAGGCACTCCACTCAAGG | NM001282383 |
Western blot analysis
The protein levels of phospho-AKT, MMP2, MMP9 and
securin were analyzed using western blotting, as previously
described (13). Whole tissue or cell
proteins were electrophoretically separated using 4–12% SDS-PAGE
gels and then transferred to nitrocellulose membranes. Subsequent
to 30 min of blocking with 2.5% skimmed milk, the membranes were
incubated with the appropriate primary antibody (dilution, 1:2,000)
overnight at 4°C, followed by a 1-h incubation with a horseradish
peroxidase-conjugated secondary antibody (dilution, 1:2,000).
Subsequent to treatment with each antibody, the membranes were
washed with phosphate-buffered saline (PBS) containing 0.5% Tween
20. Next, the membranes were developed using a chemiluminescence
reagent and then exposed to X-ray film. The protein levels are
expressed as the ratio of the band optical intensity to that of
β-actin or total AKT.
Stable securin transfection in LN-229
cells
Complete securin cDNA was cloned into pcDNA3.1
plasmids and confirmed by DNA sequencing. The stably transfected
LN-229 cells were selected using neomycin. Briefly, the LN-229
cells were transfected with a securin-pcDNA3.1 plasmid or an empty
pcDNA3.1 vector using Lipofectamine 2000 according to the
manufacturer's instructions. Subsequent to 24 h of transfection,
the cells were trypsinized and reseeded into a 6-well plate. These
cells were selected using a selection medium containing 600 µg/ml
G418. The surviving cell clones, which were considered to be the
stably transfected cells, were maintained and passaged in a culture
medium containing 300 µg/ml G418.
Transfection of siRNA in U373
cells
siRNA sequences specific to human securin were
designed according to the following securin target sequences:
Sequence #1, TGGGAGATCTCA AGTTTCA; sequence #2,
GTCTGTAAAGACCAAGGGA; and sequence #3, GCATTCTGTCGACCCTGGA. These
siRNA oligonucleotides were cloned into pGenesil 2 plasmids. In a
6-well plate, 2×105 U373 cells were seeded to 50–60%
confluence in antibiotic-free RPMI-1640 medium and left to grow for
24 h. The cells in each well were then transfected with 200 pmol
securin siRNA or scramble siRNA using Lipofectamine 2000, according
to the manufacturer's instructions. The medium was changed 6 h
after transfection and the cells were incubated for an additional
48 h until further use.
Migration assay
Cell migration was assessed using a wound-healing
scratch assay. The LN-229 cells with stable securin transfection
and the U373 cells with securin siRNA transient transfection were
plated into 6-well plates and allowed to grow until 90–95%
confluence was reached. The cells were then washed in PBS. Next,
the medium was replaced with serum-free medium and the cells were
grown for an additional 24 h. Scratches were then made using a P200
pipette tip (Rainin, Oakland, CA, USA). The images were captured at
the 0-, 18- and 36-h time-points with a microcamera (Olympus,
Waltham, Massachusetts).
Invasion assay
Cell invasion was assessed using the Transwell
invasion assay. The LN-229 and U373 cells were trypsinized and
resuspended in FBS-free RPMI-1640 medium 48 h after transfection.
In total, 2×104 cells were plated into the upper chamber
of the Transwell unit, which contained a Matrigel-coated
polycarbonate membrane. Next, fresh medium containing 10% FBS was
added to the lower chamber to act as a chemoattractant. Subsequent
to a 48-h incubation at 37°C with 5% CO2, the cells on
the lower surface of the membrane were fixed with 5% formalin and
stained with 0.2% crystal violet. The non-migrated cells on the
upper surface of the membrane were removed with a cotton swab.
Images of the cells that had migrated to the lower surface were
captured with a microcamera (Olympus). The number of invading cells
was counted from six randomly-selected fields.
Ethics statement
The glioma tissues and normal brain tissues used in
the present study were obtained from glioma and brain trauma
patients, respectively. These patients agreed with the study, and
signed informed consent forms. The study protocol was approved by
the Institutional Review Board of Nanfang Hospital (Guangzhou,
China).
Statistical analysis
Graphpad Prism software version 5 was used for the
data analysis. The data are presented as the mean ± standard error
of the mean. The statistical analysis was performed using a one-way
analysis of variance, followed by Dunnett's test for multiple
comparisons and Student's t-test for comparisons between groups.
P<0.05 was used to indicate a statistically significant
difference.
Results
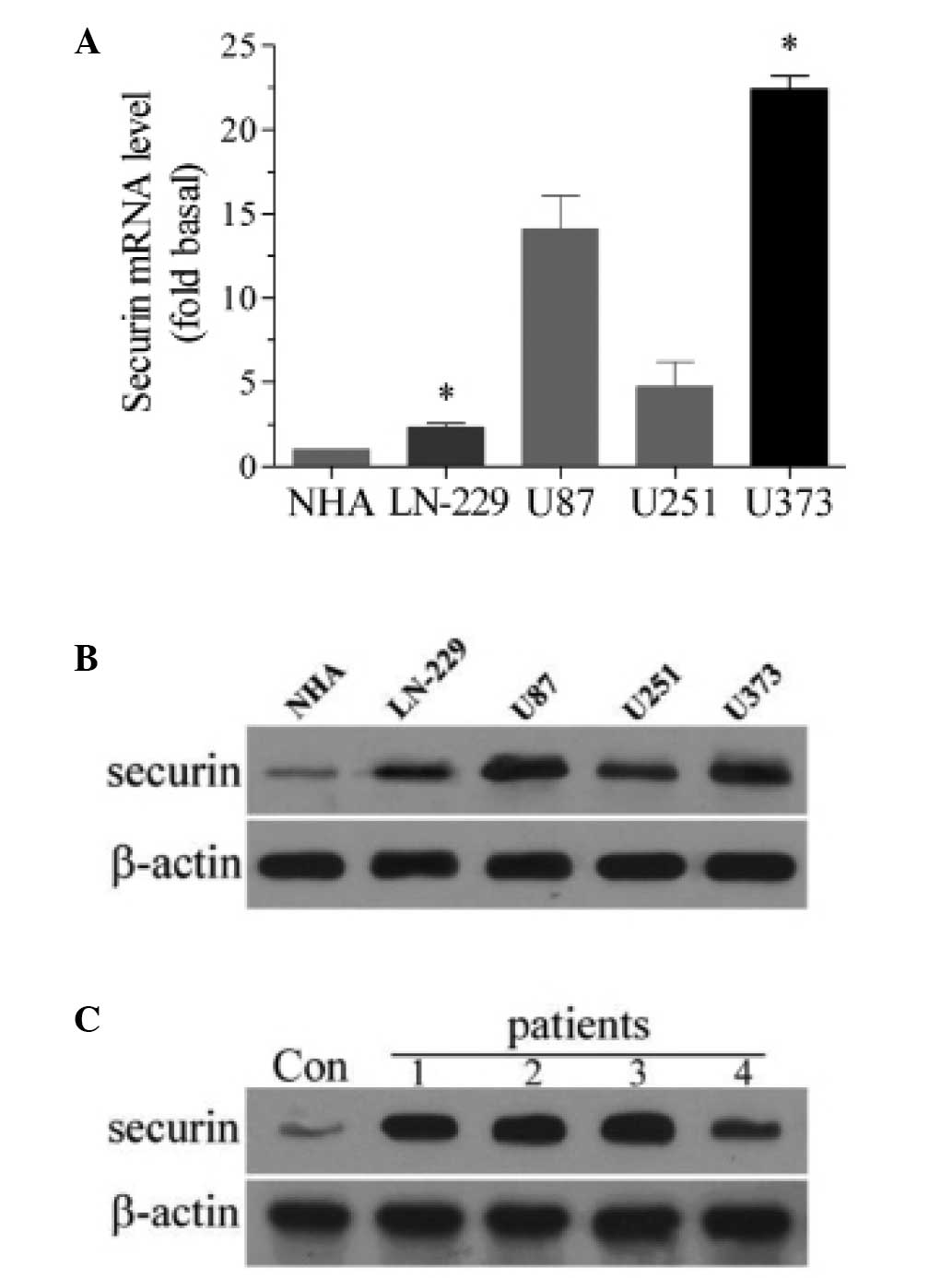
Securin expression in various glioma
cells and human brain glioma tissues
The expression of securin was analyzed in a number
of glioma cells. qPCR revealed that the expression of securin mRNA
was significantly higher in the four types of glioma cells (LN-229,
U87, U251 and U373) compared with the NHA cells (P<0.05;
Fig. 1A). Of the four types of glioma
cells, LN-229 cells exhibited the lowest expression of securin,
whereas U373 cells demonstrated the highest expression of securin.
Therefore, LN-229 and U373 cells were used for the subsequent
analysis of securin overexpression and downregulation. In the
western blot analysis, the protein expression of securin was also
higher in the four types of glioma cells than in the NHA cells
(Fig. 1B). The present study also
used western blot analysis to investigate whether an increased
expression level of securin was evident in human glioma tissues.
Compared with the brain tissues obtained from brain trauma
patients, glioma tissues from glioma patients exhibited a higher
expression level of securin (Fig.
1C). These results indicate that the expression of securin is
increased in glioma.
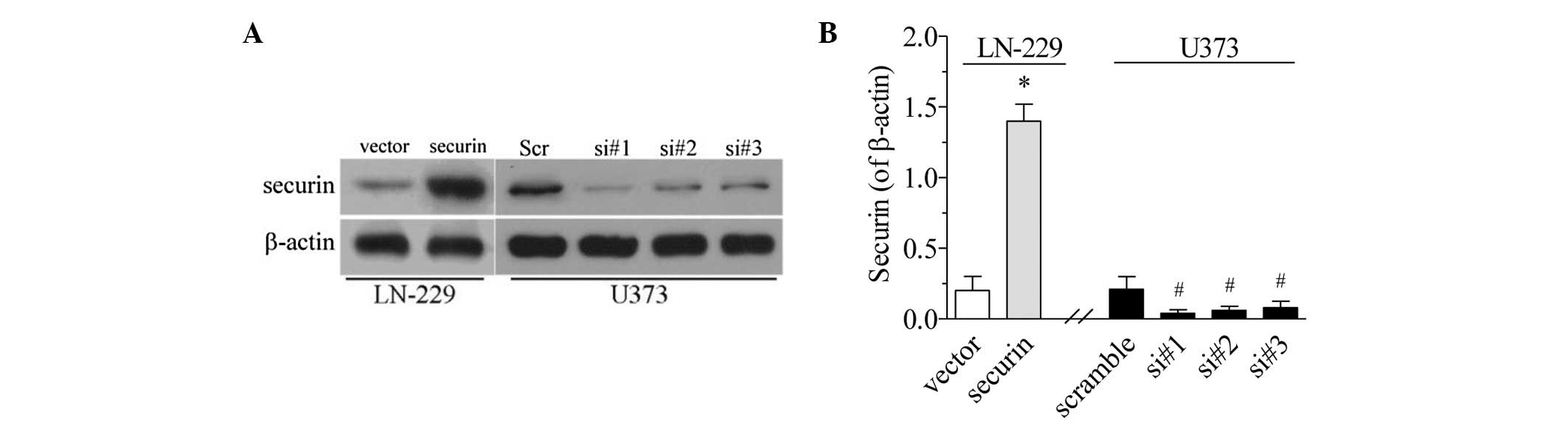
Securin overexpression and
downregulation in glioma cells
In order to examine the role of securin in gliomas,
the present study established securin overexpression and
downregulation models in the LCN-229 and U373 cells, respectively.
For the overexpression model, securin-expressing pcDNA3.1 plasmids
were transfected into the LN-229 cells. Following selection with
G418 antibiotics, surviving cells exhibited increased securin
expression compared with the empty vector controls (P<0.01;
Fig. 2). Therefore, these LN-229
cells were considered to be stable securin mimic cells. For the
securin downregulation model, securin siRNA was transfected into
the U373 cells. It was established that the three securin siRNA
sequences markedly decreased the level of securin expression
(P<0.05; Fig. 2). siRNA sequence
#1 conferred the most marked decrease, and was therefore used in
the subsequent experiments.
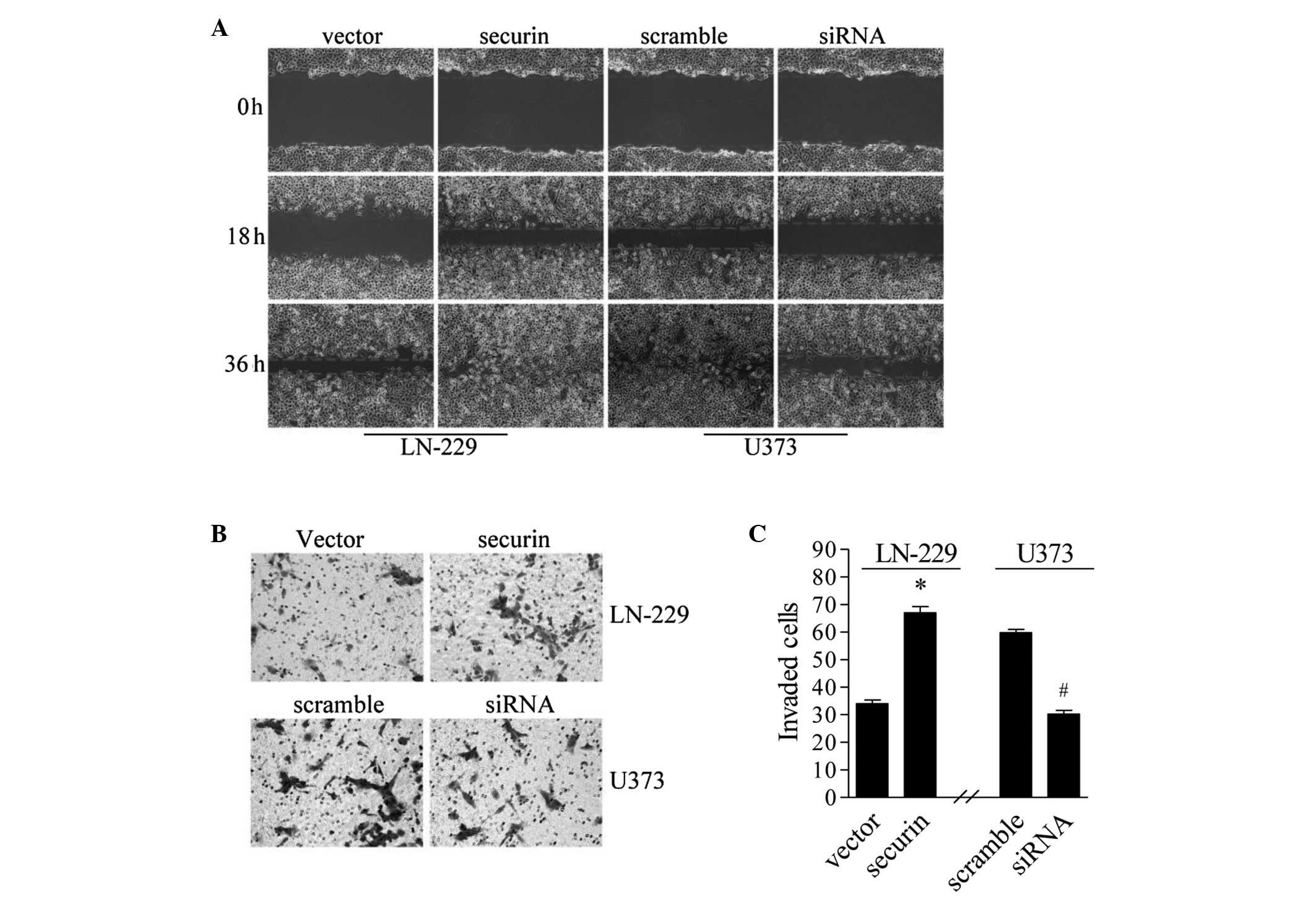
Securin inhibits migration and
invasion in glioma cells
Migration and invasion are important characteristics
of tumor metastasis (27). The
present study investigated whether securin is involved in glioma
metastasis. A classic wound-healing scratch assay was performed in
order to establish the role of securin in regulating the migratory
ability of the glioma cells. The cell monolayers were scratched to
create a wound to monitor the migration ability. The LN-229 cells
that overexpressed securin exhibited an increased rate of healing
at 18 and 36 h compared with the empty vector controls. In U373
cells with siRNA sequence #1-mediated securin-knockdown, healing
was markedly inhibited compared with the scramble control (Fig. 3A). These results demonstrate that
securin promotes the migration of glioma cells.
The effect of securin on cell invasion was
investigated using the Transwell invasion assay. Overall, a higher
number of LN-229 cells with securin overexpression traversed the
Matrigel-coated polycarbonate membrane compared with the empty
vector controls (P<0.05: Fig. 3B and
C). In U3737 cells, securin-knockdown by siRNA significantly
reduced the number of invading cells (P<0.05; Fig. 3B and C). Taken together, these results
indicate that securin not only promotes the migration ability, but
also increases the invasive property of glioma cells.
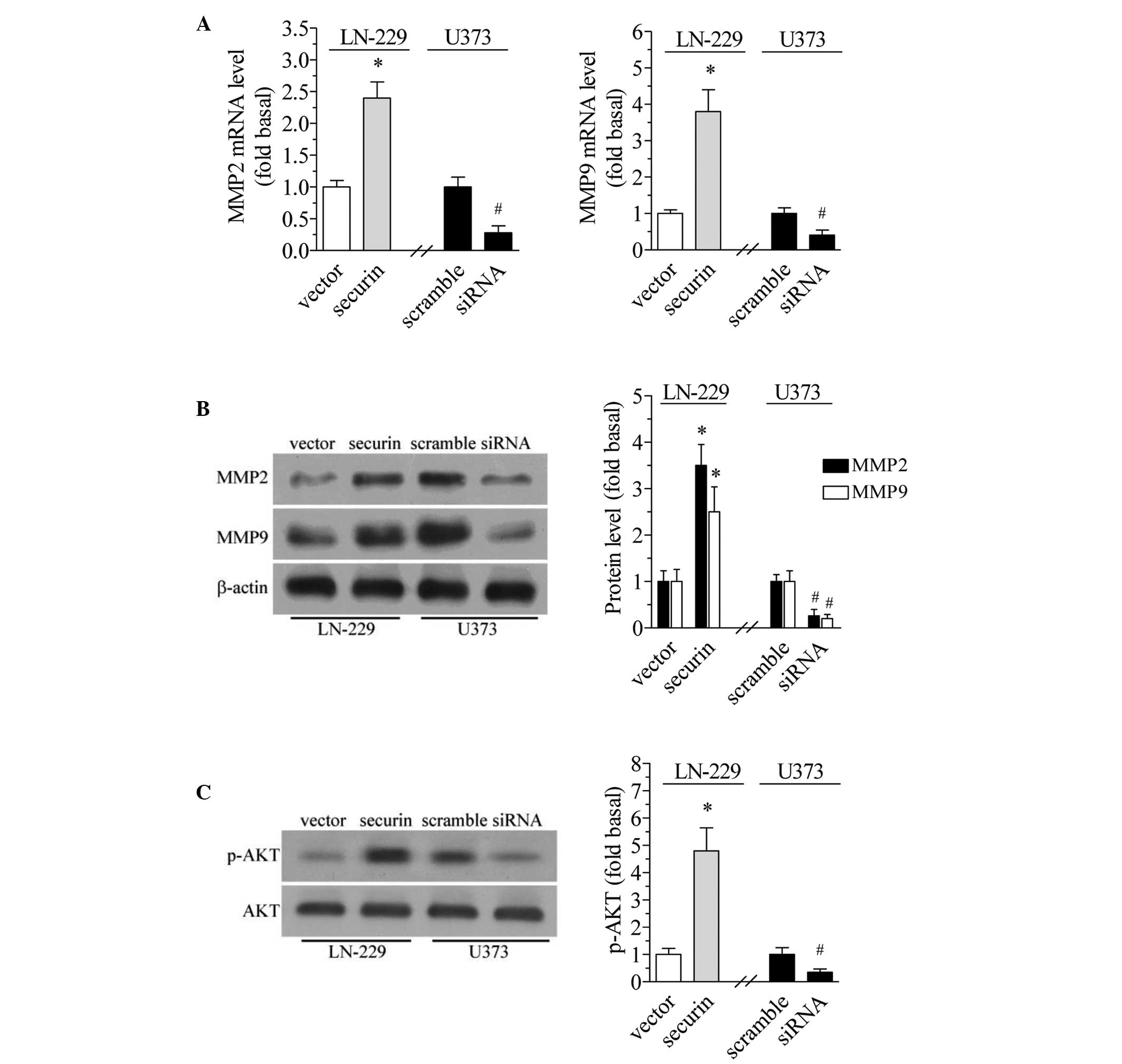
Securin increases MMPs expression
MMPs degrade ECM components and facilitate tumor
invasion and migration (28). The
present study investigated whether the level of MMPs changes in
response to the expression of securin. The results of the qPCR
revealed that securin overexpression in the LCN-229 cells led to a
significant increase in the mRNA expression of MMP2 and MMP9
(P<0.01; Fig. 4A). By contrast,
securin downregulation in U373 cells significantly decreased the
mRNA expression level of MMP2 and MMP9 (Fig. 4A). Similar changes were observed in
the protein expression of MMP2 and MMP9. Securin overexpression and
downregulation increased and decreased the protein expression of
MMP2 and MMP9, respectively (P<0.01 and P<0.05, respectively;
Fig. 4B).
Securin increases phosphorylation of
AKT
High AKT activity is associated with tumor growth
and invasion (29). Therefore, the
present study examined whether securin affected AKT
phosphorylation. The results revealed that securin overexpression
in LCN-229 cells significantly increased the phosphorylation level
of AKT at Ser473 (P<0.01), while securin downregulation in U373
cells significantly decreased the phosphorylation level of AKT
(P<0.05; Fig. 4C). However,
securin overexpression or downregulation did not change the total
AKT level.
Discussion
The present study aimed to evaluate the role of
securin in the regulation of migration and invasion in glioma
cells, and to identify the potential underlying mechanisms. It was
revealed that securin expression was significantly increased in a
number of glioma cell lines and tissues. In glioma cells with
securin overexpression, the migration and invasion ability was
significantly increased. By contrast, in glioma cells with securin
downregulation, the migration and invasion ability was
significantly decreased. The overexpression and downregulation of
securin conferred an increase and decrease in the expression level
of MMP2 and MMP9, respectively.
PTTG1/securin is a novel proto-oncogene that serves
as a marker of aggressive phenotypes in several types of cancer. In
a previous study, a high immunoexpression level of PTTG1 was
observed in brain tumor tissues from 88 cases of benign and
malignant brain tumors, and was identified to be correlated with
malignance (30). In the present
study, it was revealed that securin was highly expressed in the
glioma tissues obtained from 40 glioma patients compared with in
normal brain tissues obtained from brain trauma patients. This
finding is consistent with that of a previous study (12) and further increased the sample size in
this field. In the four types of glioma cells (LN-229, U87, U251
and U373), securin expression was significantly higher than that in
the NHA cells. Therefore, the results demonstrate that securin is
highly expressed in glioma.
Migration and invasion are two important steps
involved in cancer cell metastasis. The latter ultimately leads to
a poor prognosis. Previous studies have reported that an increased
expression level of PTTG-1 was correlated with a poor prognosis in
patients with glioma (6), esophageal
squamous cell cancer (31), medullary
thyroid carcinoma (32) and
endometrial cancer (33). Therefore,
the present study aimed to determine whether securin affects
migration and invasion. The results of the Matrigel Transwell
invasion assay and wound-healing scratch assay revealed that the
overexpression and downregulation of securin increased and
decreased glioma cell invasion and migration, respectively. These
results indicated a promotive role for securin in the migration and
invasion of glioma cells. The promotive effect of securin on
migration and invasion has also been observed in other types of
cancers. In pituitary macroadenomas, the expression levels of PTTG
were significantly higher in invasive pituitary adenomas than in
non-invasive pituitary adenomas (34). In a different study, the
overexpression of PTTG induced lymph node metastasis in human
esophageal squamous cell carcinoma (35). Furthermore, conditioned medium
collected from HEK293 cells overexpressing PTTG significantly
increased the migration, invasion and tubule formation of human
umbilical vein endothelial cells (HUVECs) (25). These previous findings and the data
obtained from the present study indicate an important role for
securin in glioma migration and invasion.
The invasion of malignant tumors requires the
proteolytic degradation of ECM components. Previous data has
established that MMPs are major regulators of ECM proteins. In a
study by Xia et al (26), the
downregulation of PTTG by siRNA markedly decreased the expression
of MMP2 and MMP9 in the cutaneous squamous cell carcinoma SCL-1
cell line, and also inhibited cell proliferation. A different study
demonstrated that the overexpression of PTTG in metastatic
papillary thyroid carcinomas was accompanied by an increased
expression level of MMP2 and MMP9 (24). Furthermore, the transient or stable
transfection of HEK293 cells with PTTG cDNA has been demonstrated
to confer a significant increase in the secretion and expression of
MMP2. This secretion in the medium may directly promote the
migration and invasion of HUVECs (25). In the present study, the
overexpression and downregulation of securin in glioma cells
increased and decreased the expression of MMP2 and MMP9,
respectively. These results suggest that the increased expression
of MMP2 and MMP9 by securin may mediate the migration and invasion
of glioma.
The present study also identified that the
overexpression and downregulation of securin in glioma cells
increased and decreased the phosphorylation level of AKT,
respectively. AKT activation has been revealed to be correlated
with cell migration and invasion in a number of different tumors.
In a previous study concerning glioma, microRNA-149 inhibited cell
proliferation and invasion via the blockade of AKT1 signaling
(36). In addition, the reduction of
AKT phosphorylation by parthenolide has been identified to be
accompanied by decreased proliferation, invasion and tumor-induced
angiogenesis in glioblastoma cells (37). The results of a further study
established that AKT activation also mediated invasion in brain
glioblastoma cancer (38). However,
data regarding the association between AKT activity and securin is
limited. In the human breast cancer MCF-7 cell line, the activation
of the PI3K/AKT cascade was reported to mediate the overexpression
of PTTG by insulin and insulin growth factor-1 (39). In a study by Pan et al
(40), the overexpression of
γ-catenin increased cell mobility and migration, enhanced AKT
phosphorylation and led to an increase in the protein level of
PTTG. These results indicate a possible interaction between AKT and
securin. In the present study, securin exhibited a promotive effect
on the phosphorylation of AKT in securin overexpression and
downregulation models. These results suggest the existence of a
further mechanism in the progression of glioma, which may involve
the activation of AKT by securin.
In conclusion, the present study established cell
models with securin overexpression or downregulation in order to
examine the effect of securin on glioma cell invasion and
migration. The results demonstrated an important promotive role for
securin in the migration and invasion of glioma cells, which may
involve MMPs and AKT phosphorylation.
References
|
1
|
Pei L and Melmed S: Isolation and
characterization of a pituitary tumor-transforming gene (PTTG). Mol
Endocrinol. 11:433–441. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Puri R, Tousson A, Chen L and Kakar SS:
Molecular cloning of pituitary tumor transforming gene 1 from
ovarian tumors and its expression in tumors. Cancer Lett.
163:131–139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kakar SS: Molecular cloning, genomic
organization and identification of the promoter for the human
pituitary tumor transforming gene (PTTG). Gene. 240:317–324. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Horwitz GA, Prezant TR, Valentini
A, Nakashima M, Bronstein MD and Melmed S: Structure, expression
and function of human pituitary tumor-transforming gene (PTTG). Mol
Endocrinol. 13:156–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boelaert K, McCabe CJ, Tannahill LA,
Gittoes NJ, Holder RL, Watkinson JC, Bradwell AR, Sheppard MC and
Franklyn JA: Pituitary tumor transforming gene and fibroblast
growth factor-2 expression: potential prognostic indicators in
differentiated thyroid cancer. J Clin Endocrinol Metab.
88:2341–2347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Genkai N, Homma J, Sano M, Tanaka R and
Yamanaka R: Increased expression of pituitary tumor-transforming
gene (PTTG)-1 is correlated with poor prognosis in glioma patients.
Oncol Rep. 15:1569–1574. 2006.PubMed/NCBI
|
|
7
|
Karra H, Repo H, Ahonen I, Löyttyniemi E,
Pitkӓnen R, Lintunen M, Kuopio T, Söderström M and Kronqvist P:
Cdc20 and securin overexpression predict short-term breast cancer
survival. Br J Cancer. 110:2905–2913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demeure MJ, Coan KE, Grant CS, Komorowski
RA, Stephan E, Sinari S, Mount D and Bussey KJ: PTTG1
overexpression in adrenocortical cancer is associated with poor
survival and represents a potential therapeutic target. Surgery.
154:1405–1416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abbud RA, Takumi I, Barker EM, Ren SG,
Chen DY, Wawrowsky K and Melmed S: Early multipotential pituitary
focal hyperplasia in the alpha-subunit of glycoprotein
hormone-driven pituitary tumor-transforming gene transgenic mice.
Mol Endocrinol. 19:1383–1391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hitomi T, Habu T, Kobayashi H, et al:
Downregulation of Securin by the variant RNF213 R4810K
(rs112735431, G>A) reduces angiogenic activity of induced
pluripotent stem cell-derived vascular endothelial cells from
moyamoya patients. Biochem Biophys Res Commun. 438:13–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chesnokova V, Kovacs K, Castro AV, Zonis S
and Melmed S: Pituitary hypoplasia in Pttg-/- mice is protective
for Rb+/- pituitary tumorigenesis. Mol Endocrinol. 19:2371–2379.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tfelt-Hansen J, Yano S, Bandyopadhyay S,
Carroll R, Brown EM and Chattopadhyay N: Expression of pituitary
tumor transforming gene (PTTG) and its binding protein in human
astrocytes and astrocytoma cells: function and regulation of PTTG
in U87 astrocytoma cells. Endocrinology. 145:4222–4231. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Wang J, Wang Y, Fu Q, Lei YH, Nie
ZY, Qiu J and Bao TY: Protective effect of exogenous matrix
metalloproteinase-9 on chronic renal failure. Exp Ther Med.
7:329–334. 2014.PubMed/NCBI
|
|
14
|
Toth M, Chvyrkova I, Bernardo MM,
Hernandez-Barrantes S and Fridman R: Pro-MMP-9 activation by the
MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes.
Biochem Biophys Res Commun. 308:386–395. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gouda HM, Khorshied MM, El Sissy MH,
Shaheen IA and Mohsen MM: Association between matrix
metalloproteinase 2 (MMP2) promoter polymorphisms and the
susceptibility to non-Hodgkin's lymphoma in Egyptians. Ann Hematol.
93:1313–1318. 2014.PubMed/NCBI
|
|
16
|
Iizuka S, Ishimaru N and Kudo Y: Matrix
metalloproteinases: the gene expression signatures of head and neck
cancer progression. Cancers (Basel). 6:396–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai X, Liao J, Lin W, et al: Inhibitor of
DNA-binding protein 1 knockdown arrests the growth of colorectal
cancer cells and suppresses hepatic metastasis in vivo. Oncol Rep.
32:79–88. 2014.PubMed/NCBI
|
|
19
|
Xia H, Ma YF, Yu CH, Li YJ, Tang J, Li JB,
Zhao YN and Liu Y: Aquaporin 3 knockdown suppresses tumor growth
and angiogenesis in experimental non-small cell lung cancer. Exp
Physiol. 99:974–984. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen JS, Li HS, Huang JQ, Zhang LJ, Chen
XL, Wang Q, Lei J, Feng JT, Liu Q and Huang XH: Down-regulation of
Gli-1 inhibits hepatocellular carcinoma cell migration and
invasion. Mol Cell Biochem. 393:283–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Shen L, Yang L, Hu S, Xu L and Wu
S: High expression of β3GnT8 is associated with the metastatic
potential of human glioma. Int J Mol Med. 33:1459–1468.
2014.PubMed/NCBI
|
|
22
|
Wen X, Huang A, Liu Z, Liu Y, Hu J, Liu J
and Shuai X: Downregulation of ROCK2 through nanocomplex sensitizes
the cytotoxic effect of temozolomide in U251 glioma cells. PLoS
One. 9:e920502014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang HS, Zhong YH, Luo ZJ, Huang Y, Lin
HD, Luo M, S-Zhan, Su HX, Zhou SB and Xie KQ: Comparative analysis
of protein expression in differentiated thyroid tumours: a
multicentre study. J Int Med Res. 37:927–938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang H, Zhong Y, Luo Z, Huang Y, Lin H,
Luo M, Zhan S, Xie K, Ma Y and Li QQ: Assessment of biomarkers for
clinical diagnosis of papillary thyroid carcinoma with distant
metastasis. Int J Biol Markers. 25:38–45. 2010.PubMed/NCBI
|
|
25
|
Malik MT and Kakar SS: Regulation of
angiogenesis and invasion by human pituitary tumor transforming
gene (PTTG) through increased expression and secretion of matrix
metalloproteinase-2 (MMP-2). Mol Cancer. 5:612006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia YH, Li M, Fu DD, Xu SL, Li ZG, Liu D
and Tian ZW: Effects of PTTG down-regulation on proliferation and
metastasis of the SCL-1 cutaneous squamous cell carcinoma cell
line. Asian Pac J Cancer Prev. 14:6245–6248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wieczorek E, Jablonska E, Wasowicz W and
Reszka E: Matrix metalloproteinases and genetic mouse models in
cancer research: a mini-review. Tumour Biol. 36:163–175. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Yin C, Zhang B, et al: PTTG1
promotes migration and invasion of human non-small cell lung cancer
cells and is modulated by miR-186. Carcinogenesis. 34:2145–2155.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li XX, Huang LY, Peng JJ, Liang L, Shi DB,
Zheng HT and Cai SJ: Klotho suppresses growth and invasion of colon
cancer cells through inhibition of IGF1R-mediated PI3K/AKT pathway.
Int J Oncol. 45:611–618. 2014.PubMed/NCBI
|
|
30
|
Salehi F, Scheithauer BW, Sharma S, Kovacs
K, Lloyd RV, Cusimano MD and Munoz DG: Immunohistochemical
expression of PTTG in brain tumors. Anticancer Res. 33:119–122.
2013.PubMed/NCBI
|
|
31
|
Zhang J, Yang Y, Chen L, Zheng D and Ma J:
Overexpression of pituitary tumor transforming gene (PTTG) is
associated with tumor progression and poor prognosis in patients
with esophageal squamous cell carcinoma. Acta Histochem.
116:435–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zatelli MC, Tagliati F, Amodio V, Buratto
M, Pelizzo M, Pansini G, Bondanelli M, Ambrosio MR and Degli Uberti
EC: Role of pituitary tumour transforming gene 1 in medullary
thyroid carcinoma. Anal Cell Pathol (Amst). 33:207–216. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JW, Song JY, Lee JM, Lee JK, Lee NW,
Yeom BW and Lee KW: Expression of pituitary tumor-transforming gene
in endometrial cancer as a prognostic marker. Int J Gynecol Cancer.
18:1352–1359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jia W, Lu R, Jia G, Ni M and Xu Z:
Expression of pituitary tumor transforming gene (PTTG) in human
pituitary macroadenomas. Tumour Biol. 34:1559–1567. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan S, Zhou C, Lou X, Xiao Z, Zhu H, Wang
Q, Wang Y, Lu N, He S, Zhan Q, Liu S and Xu N: PTTG overexpression
promotes lymph node metastasis in human esophageal squamous cell
carcinoma. Cancer Res. 69:3283–3290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pan SJ, Zhan SK, Pei BG, Sun QF, Bian LG
and Sun BM: MicroRNA-149 inhibits proliferation and invasion of
glioma cells via blockade of AKT1 signaling. Int J Immunopathol
Pharmacol. 25:871–881. 2012.PubMed/NCBI
|
|
37
|
Nakabayashi H and Shimizu K: Involvement
of Akt/NF-κB pathway in antitumor effects of parthenolide on
glioblastoma cells in vitro and in vivo. BMC Cancer. 12:4532012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dudley A, Sater M, Le PU, Trinh G, Sadr
MS, Bergeron J, Deleavey GF, Bedell B, Damha MJ and Petrecca K: DRR
regulates AKT activation to drive brain cancer invasion. Oncogene.
33:4952–4960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thompson AD III and Kakar SS: Insulin and
IGF-1 regulate the expression of the pituitary tumor transforming
gene (PTTG) in breast tumor cells. FEBS Lett. 579:3195–3200. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pan H, Gao F, Papageorgis P, Abdolmaleky
HM, Faller DV and Thiagalingam S: Aberrant activation of
gamma-catenin promotes genomic instability and oncogenic effects
during tumor progression. Cancer Biol Ther. 6:1638–1643. 2007.
View Article : Google Scholar : PubMed/NCBI
|