Introduction
Obesity has been associated with an increased risk
of developing breast cancer (BC) and has been correlated with
inferior oncological outcomes at the time of BC diagnosis (1). Majed et al (2) analyzed 19 years of follow-up data from a
cohort of 14,709 patients with BC, and identified a significant
association between obesity and distant recurrence for estrogen
receptor (ER)-positive compared with ER-negative disease. However,
only 30% of the cohort received adjuvant chemotherapy and not all
patients were treated with endocrine therapy. Two subsequent
studies provided consistent results: de Azambuja et al
(3) reported worse outcomes for
ER-positive compared with ER-negative disease; and Sestak et
al (4) reported that
postmenopausal women with ER-positive disease and high baseline
body mass index (BMI) exhibited more distant recurrence.
Furthermore, Sparano et al (5)
reported the clinical outcomes from three clinical trials and
determined that obese patients (BMI, ≥30 kg/m2) had a
significantly higher risk of recurrence and mortality. In addition,
this was the first study to reveal that a poorer prognosis was
associated with hormone receptor (HR)-positive and human epidermal
growth factor receptor 2 (HER-2)-negative disease in obese patients
with BC. Menopausal status was not defined in this study; however,
a recent meta-analysis observed a poorer prognosis in obese
patients with premenopausal TNBC compared with non-obese patients
with BC, while no similar association was identified in
postmenopausal women with TNBC (6).
Ongoing trials are investigating possible
associations between obesity and poor prognosis in BC,
etiopathogenesis and other malignancies. There are a number of
theories regarding the association between obesity and BC. One is
that increased estrogen produced by adipocytes may trigger the
growth of ER-positive mammary cells (7,8). An
alternative theory is that obesity and metabolic syndromes induce
the production of insulin-like growth hormone, which has potent
mitogenic activity on the epithelium (9). Finally, pro-inflammatory cytokines, such
as interleukin-6 (IL-6) and tumor necrosis factor α (TNFα), which
are synthesized by paracrine glands, may induce malignant
phenotypes (10). However, these
three possible mechanisms do not extensively clarify why specific
subtypes of BC have poorer prognoses compared with others. In the
current study, a number of inflammatory signaling pathways, such as
the TNF pathway, were investigated. TNF-like weak inducer of
apoptosis (TWEAK) is a member of the TNF superfamily originally
identified in 1997, and is pivotal in various inflammatory
conditions. Recent studies have identified TWEAK expression in
human brain, lung, colon, liver and breast malignancies (11–14).
Additionally, TNF receptor-associated factor (TRAF) proteins are
involved in various intracellular signaling pathways. In
particular, TRAF6 is an important E3 ubiquitin ligase that is
involved in protein degradation (15,16), with
increased TRAF6 expression identified in the skeletal muscles of
mice during atrophy (17). Therefore,
we hypothesize that obesity may mask ongoing catabolism in obese
patients with cancer. Additionally, it is known that obese patients
with BC have poorer outcomes, particularly for HR-positive and
HER-2-negative disease (5).
Therefore, the aim of the present study was to investigate a
possible association between various subtypes of BC and novel
catabolic pathway parameters, such as serum TWEAK and TRAF6
expression levels.
Patients and methods
The present study cohort consisted of 74 patients
(48 with non-metastatic BC and 26 obese controls) and was approved
by the Bülent Ecevit University School of Medicine (Kozlu, Turkey)
and Local Ethics Committees. The inclusion criteria were as
follows: i) A diagnosis of histopathologically confirmed stage
I-III BC, according to tumor-node-metastasis staging (18); ii) an Eastern Cooperative Oncology
Group (ECOG) score (19) of 0–2; iii)
an age of 18–80 years; iv) a BMI of ≥30 kg/m2; and v)
eligibility to receive adjuvant chemotherapy. Furthermore, the
exclusion criteria were as follows: i) refusal to consent to be
included in the study; ii) chronic disease, such as hypertension,
chronic heart disease, renal failure and thyroid disorders; and
iii) an ECOG score of 3–4.
Pathological examination
Paraffin blocks were obtained from patients
diagnosed with breast cancer and immunohistochemical (IHC) analysis
for ER and PR expression was performed and scored based on staining
intensity and the percentage of tumor cells with nuclear staining
as well as Her-2 status. The results indicated that HER-2/neu
status by fluorescence in situ hybridization (FISH) should
be performed in all cases of breast tumour with a 2+ score by
IHC.
Patient characteristics
Patient height and weight were recorded.
Subsequently, BMI was calculated as body weight divided by height
squared (kg/m2). In addition, patients included in the
present study were pathologically diagnosed with infiltrative
ductal carcinoma, one of the most common subtypes recorded at
Bülent Ecevit University School of Medicine. Written informed
consent was obtained from the patients prior to the study according
to institutional guidelines.
Blood samples were obtained from each subject prior
to food consumption, between 8.00 a.m. and 9.00 a.m., following
overnight bed rest. Routine biochemical parameters, such as glucose
and hemoglobin concentration, were determined immediately after
blood was drawn and plasma samples were stored at −80°C until TWEAK
and TRAF-6 analysis was performed.
Serum expression levels of TWEAK and TRAF-6 were
measured using commercially available ELISA kits from Cusabio
(Wuhan, China). Data were analyzed using a label study with an
ELx50 microplate washer and an ELx800 ELISA reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Statistical analyses
Descriptive and continuous variables are presented
as the mean ± standard deviation or the median (range). Categorical
variables are presented as the relative frequency proportions and
95% confidence intervals. Disease-free survival, defined as the
interval from the date of diagnosis to the date of progression or
final follow-up visit, was calculated using the Kaplan-Meier
method. In addition, appropriateness of data to normal ranges was
controlled by the Shapiro-Wilk test. The data were analyzed using
two-way analysis of variance and non-parametric tests. In all
cases, P<0.05 indicated a statistically significant difference
and analyses were performed using the SPSS software package
(version 17.0; SPSS, Inc., Chicago, IL, USA).
Results
The present study included 74 patients with a mean
age of 52.5 years (range, 35–78 years) and a median BMI of 33.5
kg/m2 (range, 30–47 kg/m2). The cohort
included 26 obese controls and 48 patients with diagnosed BC. The
characteristics of the two groups are summarized and compared in
Table I. There were no significant
differences in the majority of the investigated parameters between
the obese controls and obese patients with BC, including TWEAK and
TRAF6 expression, baseline liver functions and hematological
measurements. However, serum C reactive protein (CRP) concentration
was significantly higher in patients with BC compared with the
obese controls (P<0.001).
 | Table I.Comparison of characteristics between
the control group (n=26) and the patients with BC (n=48). |
Table I.
Comparison of characteristics between
the control group (n=26) and the patients with BC (n=48).
| Characteristic | Control group, n
(±SD/range) | BC group, n
(SD/range) | P-value |
|---|
| Age, years | 52.00 (±11) | 53.00 (±10) | 0.850 |
| BMI,
kg/m2 | 36.30 (±5.0) | 34.40 (±3.9) | 0.120 |
| Glucose, mg/dl | 99.00 (±11) | 105.00 (±16) | 0.230 |
| Hemoglobin, g/dl | 12.70 (±0.9) | 12.40 (±1.4) | 0.950 |
| Creatinine,
mg/dl | 1.00 (±0.3) | 0.93 (±0.15) | 0.790 |
| Albumin, g/dl | 4.00 (±0.45) | 4.40 (±0.3) | 0.850 |
| WBC,
mm3 | 7200.00
(±1900) | 8100.00
(±1600) | 0.064 |
| CRP, mg/dl | 2.00 (1–5) | 3.00 (1–23) | <0.001 |
| AST, IU/l | 21.00 (14–74) | 20.00 (13–64) | 0.590 |
| ALT, IU/l | 13.00 (5–62) | 17.00 (8–102) | 0.160 |
| TWEAK, pg/ml | 723.00
(400–1010) | 649.00
(380–1451) | 0.170 |
| TRAF6, ng/ml | 0.73
(0.5–1.14) | 0.70
(0.49–1.53) | 0.320 |
The characteristics of the patients with BC are
summarized in Table II. In the
current study population, 27.1% of BC patients were triple negative
and 70.8% were HR-positive (ER- and/or PR-positive). The majority
of patients were classified with tumor stages T2-3 and or lymph
node stage N0. Furthermore, 60% of the patients were
postmenopausal. The median follow-up period was 15 months (range,
4–48 months) and no recurrence occurred during the follow-up period
of the study.
 | Table II.Characteristics of patients with
breast cancer. |
Table II.
Characteristics of patients with
breast cancer.
| Characteristic | Frequency, n
(%) |
|---|
| Estrogen
receptor |
|
|
Positive | 25 (52.1) |
|
Negative | 23 (47.9) |
| Progesterone
receptor |
|
|
Positive | 31 (64.6) |
|
Negative | 17 (35.4) |
| Hormone
receptor |
|
|
Positive | 35 (70.8) |
|
Negative | 13 (29.2) |
| HER-2 |
|
|
Positive | 35 (70.8) |
|
Negative | 13 (29.2) |
| Triple
negative | 13 (27.1) |
| TNM stage |
|
| T1 | 9 (18.8) |
| T2 | 15 (31.3) |
| T3 | 24 (50.0) |
| T4 | 0 (0.0) |
| N0 | 28 (58.3) |
| N1 | 8 (16.7) |
| N2 | 6 (12.5) |
| N3 | 6 (12.5) |
| M0 | 48 (100.0) |
| I | 30 (62.5) |
| II | 11 (22.9) |
|
III | 7 (14.6) |
| Menopausal
status |
|
|
Premenopausal | 19 (39.6) |
|
Postmenopausal | 29 (60.4) |
ER status was negative in 23 patients and positive
in 25 patients. According to the ER status, BMI was higher in
ER-positive patients compared with ER-negative patients (36 vs. 31
kg/m2; P=0.003); however, there were no significant
differences in median TWEAK and TRAF6 expression levels, CRP
concentration or menopausal status (P=0.960, P=0.090, P=0.240 and
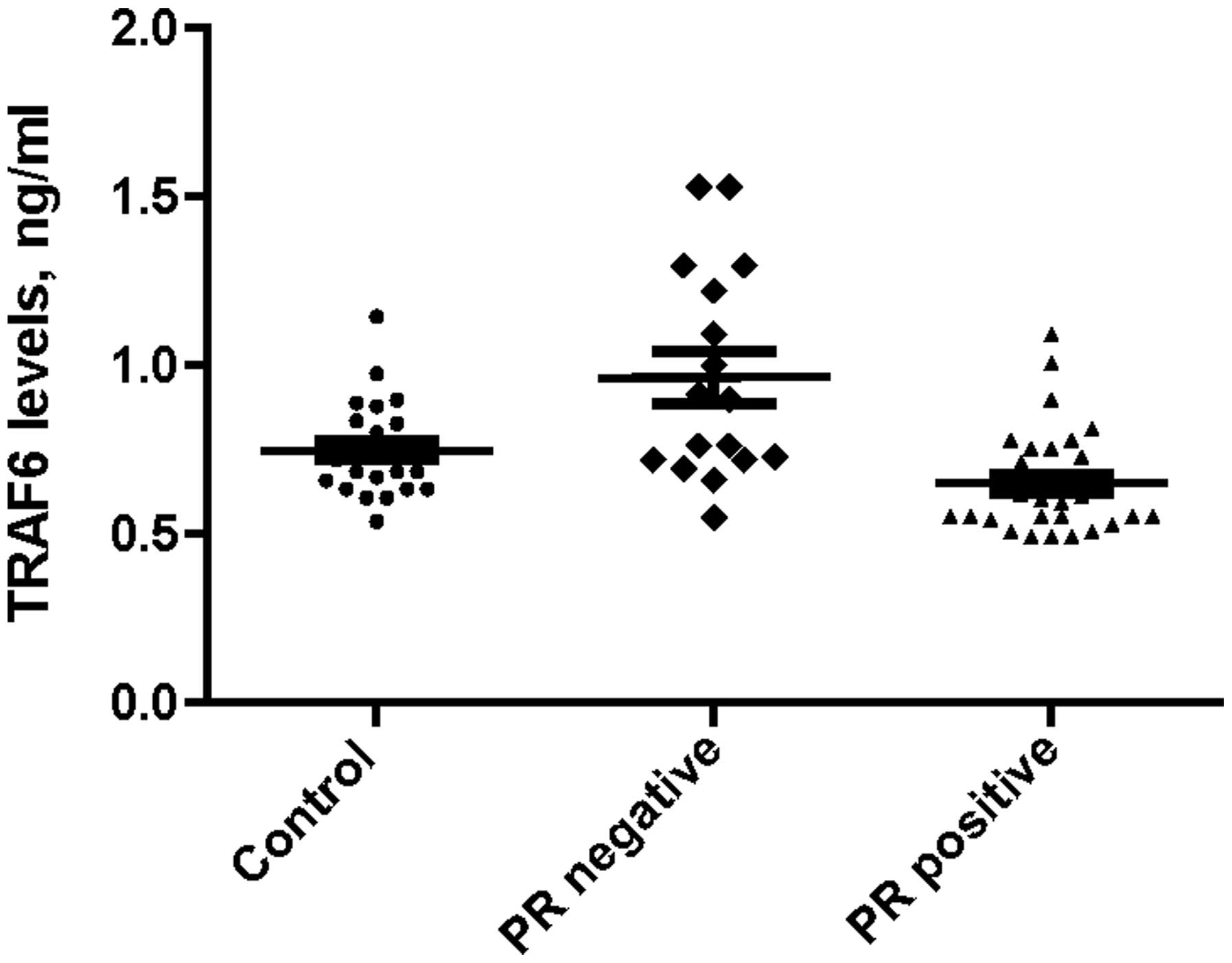
P=0.260, respectively). PR status was negative in 17 patients and
positive in 31 patients, with BMI significantly higher in
PR-positive patients compared with PR-negative patients (36 vs. 32
kg/m2; P=0.013). Furthermore, the median expression
levels of serum TRAF6 were 0.90 ng/ml (range, 0.55–1.53 ng/ml) in
PR-negative and 0.61 ng/ml (range, 0.49–1.40 ng/ml) in PR-positive
patients; this difference was statistically significant
(P<0.001). According to the serum TWEAK expression levels, CRP
concentration and menopausal status, there were no statistically
significant differences between PR-positive and PR-negative
patients (P=0.800, P=0.580 and P=0.160, respectively).
The median serum TRAF6 expression levels were 0.90
ng/ml (range, 0.55–1.53 ng/ml) in the 13 TNBC patients and 0.63
ng/ml (range, 0.49–1.22 ng/ml) in the 35 HR-positive BC patients.
This difference was statistically significant (P=0.010). However,
there were no significant differences in the median expression
levels of serum TWEAK, CRP concentration, BMI and menopausal status
(P=0.580, P=0.490, P=0.070 and P=0.220, respectively) between the
TNBC and HR-positive patients. HR expression was negative in 14
patients and positive in 34 patients. Median serum TRAF6 expression
levels were significantly higher in HR-negative patients compared
with HR-positive patients (0.83 vs. 0.62 ng/ml; P=0.002). By
contrast, BMI was significantly higher in HR-positive patients
compared with HR-negative patients (36 vs. 31.5 kg/m2;
P=0.005). Additionally, there were no significant differences
between the groups according to the median serum TWEAK expression
levels, CRP concentration and menopausal status (P=0.400, P=0.600
and P=0.350, respectively).
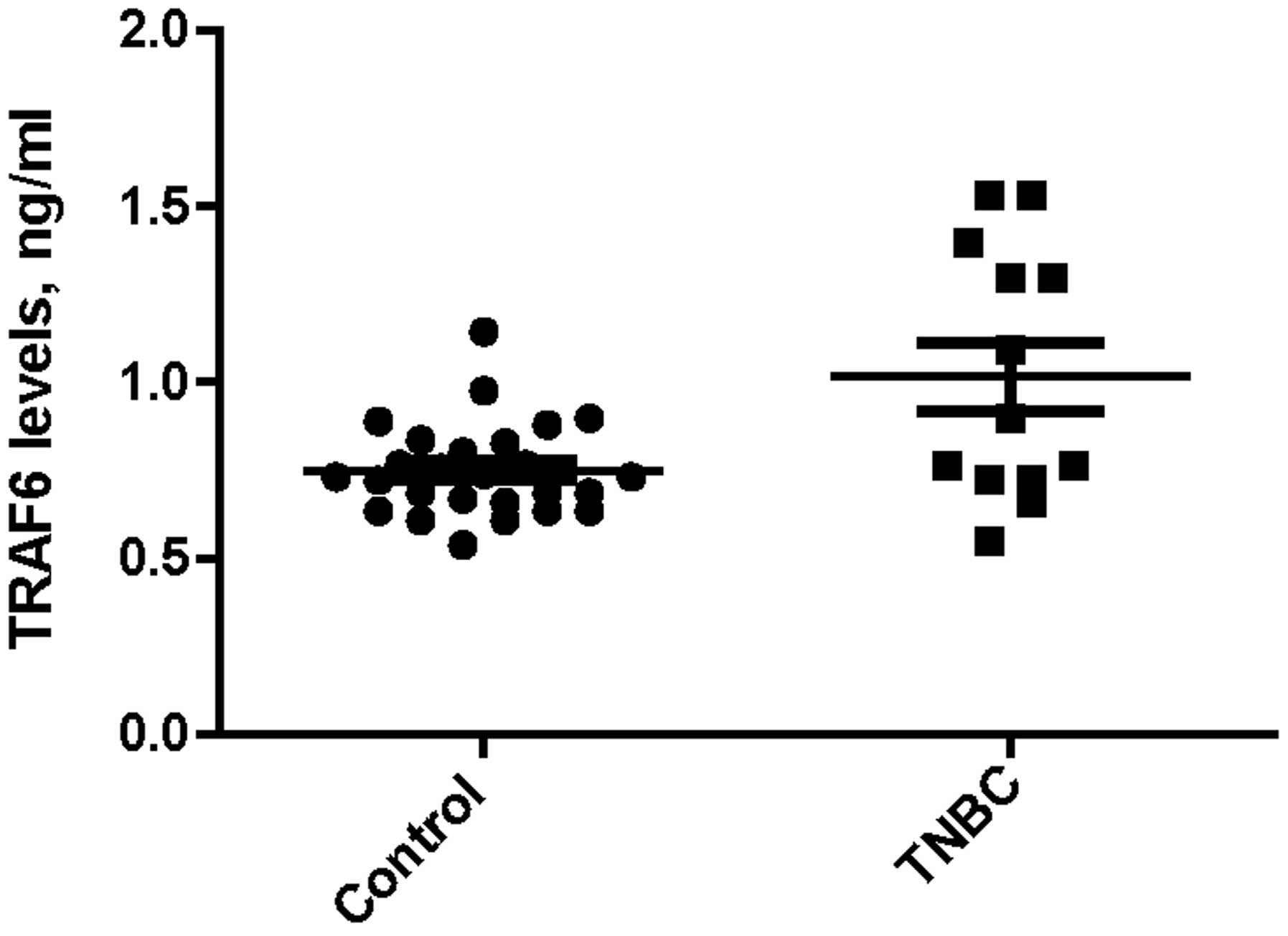
Median serum TRAF6 expression levels were
significantly higher in the TNBC group compared with the obese
control group (0.90 vs. 0.73 ng/ml; P=0.033), as indicated in
Fig. 1. Additionally, there was a
similar difference in median serum TRAF6 expression levels between
the PR-negative and obese control groups (0.92 vs. 0.72 ng/ml;
P=0.023), as indicated in Fig. 2.
Discussion
In the present study, TRAF6, which is central in the
regulation of TNF pathway functions, was demonstrated to exhibit
increased expression levels in TNBC and HR-negative non-metastatic
BC patients compared with HR-positive and HER-2-positive patients
or the obese control group. Excluding TRAF6 expression levels, no
significant differences were identified in the serum TWEAK, CRP and
biochemical parameters between any hormonal or HER-2 status of
BC.
In a healthy population, body muscle mass is
sustained by physical activity and nutritional status, with an
ongoing balance between protein degradation and synthesis. If one
of these processes becomes dominant, muscle hypertrophy or atrophy
will result. Small changes in protein synthesis or degradation may
cause large protein deficits and continuous protein turnover.
However, obesity can mask these vitally important catabolic changes
in healthy individuals and cancer patients, even if the individual
has progressed to cachexia (20,21).
Inflammatory-associated signaling pathways may limit muscle protein
synthesis via numerous mechanisms (20). For example, TNFα is able to activate
the transcription factor nuclear factor κB (NF-κB), a molecule that
inhibits the synthesis of the muscle-specific transcription factor
MyoD, as well as cellular differentiation (22). In addition, members of the TGF-β
family induce muscle wasting downstream of mothers against
decapentaplegic activation (23).
Finally, IL-6 activates signaling by binding to ligand-specific
receptors, in soluble or membrane-bound forms, to induce the signal
transducers and activators of transcription-1 and −3 (STAT1/3),
extracellular signal-regulated kinase (ERK) and
phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways
(20,24,25). It is
well known that the ERK pathway has a function in cancer cachexia
and muscle wasting (26). In
addition, the central role of the ubiquitin proteasome proteolysis
pathway in muscle atrophy has been demonstrated in various animal
models (27–29).
TRAF6 is an important E3 ubiquitin ligase that
targets proteins for degradation (30). In a number of previous studies, it was
determined that TRAF6 may activate the NF-κB, MAPK and PI3K/Akt
signaling pathways (31,32). In addition, increased TRAF6 expression
and autoubiquitination were observed in the skeletal muscle of mice
during atrophy (17). To the best of
our knowledge, the current study is the first to measure serum
TRAF6 and TWEAK expression levels in patients with BC. TRAF6
expression levels were significantly higher in TNBC and HR-negative
patients with BC compared with the obese control subjects. These
elevated TRAF expression levels may be a marker of upcoming
catabolism in BC patients, particularly in those with specific BC
subtypes. Yamamoto et al (33)
reported that NF-κB-inducing kinase (NIK) is highly expressed in
basal-like (ER-, PR- or HER-2-negative) subtypes of BC specimens
and cell cultures. Thus, the study proposed that suppressing NIK
expression may be a potential treatment strategy for patients with
TNBC. The current findings are consistent with this result, as
TRAF6 has a regulatory function in the NF-κB signaling pathway. To
date, it is unknown why TRAF6 expression levels are higher in TNBC
and HR-negative patients; however, it may be associated with the
numerous signaling pathways involved in BC, including the PI3K/Akt
pathway. The PI3K/Akt signaling pathway has an important prognostic
role in the luminal type of BC. Furthermore, Arsenic et al
(34) recently demonstrated that PI3K
catalytic subunit α mutations are more frequent in
HR-positive/HER-2-negative patients and that the H1047R mutation in
particular resulted in worse outcomes. Therefore, it is possible
that luminal types of BC may be affected by factors that have yet
to be identified.
No significant differences were found in the serum
TWEAK expression levels between BC subtypes. Previous studies have
indicated that the interaction between TWEAK and its receptor
(fibroblast growth factor-inducible 14) causes cell proliferation,
differentiation, migration, apoptosis, inflammation, angiogenesis
and malignancies in various organs, such as the lungs, brain,
liver, pancreas, breast and esophagus (35,36). Chao
et al (37) demonstrated that
the TWEAK receptor was highly expressed in all subtypes of invasive
ductal breast carcinoma. In an alternative study, TWEAK mRNA
expression levels were not significantly different between lean and
obese subjects. However, TWEAK receptor expression was only
quantifiable in subjects with a BMI of >38 kg/m2
(38). In the present study, no
significant difference in the serum TWEAK expression levels was
identified between obese control and BC patients. However, the
total current study population was not severely obese (>38
kg/m2) and TWEAK mRNA expression determined from tissue
samples may not reflect its expression in plasma.
To date, there is no proven targeted therapy for the
treatment of patients with TNBC; however, TRAF6 may be a good
target in this population. The NF-κB signaling pathway is activated
by TRAF6 (39), and certain targeted
therapies against the pathway have been studied to determine their
efficacy. For example, in a study investigating multiple myeloma,
bortezomib treatment was observed to downregulate TRAF6 expression
at the protein and mRNA levels, resulting in a reduction in
osteoclast formation (40).
Similarly, Tseng et al (41)
investigated the efficacy of bortezomib treatment in vitro
and identified that TNBC cells were sensitive to its cytotoxic
activity. The aforementioned data supports the results of the
current study; therefore, it is proposed that elevated TRAF6 may be
a novel therapeutic target for patients with TNBC. In addition,
combining a targeted therapy with systemic chemotherapy may reduce
the poor prognosis of patients with TNBC. Furthermore, we
hypothesize that PR negativity may be a more useful marker of a
poor prognosis compared with ER negativity, as TRAF6 expression
levels are higher in PR-negative patients compared with ER-negative
patients.
There were a number of limitations to the present
study. For example, due to the short follow-up period, the
association between TRAF6 expression levels, and the overall
survival of TNBC and non-TNBC patients could not be established.
However, despite the limited number of patients (13 TNBC and 35
non-TNBC patients) and the preliminary nature of the current study,
TRAF6 expression levels were identified as a possible prognostic
factor in TNBC patients. Furthermore, due to the limited number of
ELISA kits available, only an obese healthy control was selected
rather than using non-obese and obese healthy control groups.
In conclusion, elevated TRAF6 expression levels may
indicate a poor prognosis in patients with BC, particularly in
triple-negative and HR-negative patient subtypes. Furthermore,
therapies targeted to the PI3K-Akt and NF-κB signaling pathways may
be more effective in the treatment of TNBC patients with elevated
TRAF6 expression levels at the time of diagnosis.
References
|
1
|
Rose DP, Gilhooly EM and Nixon DW: Adverse
effects of obesity on breast cancer prognosis and the biological
actions of leptin (Review). Int J Oncol. 21:1285–1292.
2002.PubMed/NCBI
|
|
2
|
Majed B, Moreau T, Senouci K, et al: Is
obesity an independent prognosis factor in woman breast cancer?
Breast Cancer Res Treat. 111:329–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Azambuja E, McCaskill-Stevens W,
Francis P, et al: The effect of body mass index on overall and
disease-free survival in node-positive breast cancer patients
treated with docetaxel and doxorubicincontaining adjuvant
chemotherapy: The experience of the BIG 02–98 trial. Breast Cancer
Res Treat. 119:145–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sestak I, Distler W, Forbes JF, et al:
Effect of body mass index on recurrences in tamoxifen and
anastrozole treated women: an exploratory analysis from the ATAC
trial. J Clin Oncol. 28:3411–3415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sparano JA, Wang M, Zhao F, et al: Obesity
at diagnosis is associated with inferior outcomes in hormone
receptor-positive operable breast cancer. Cancer. 118:5937–5946.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pierobon M and Frankenfeld CL: Obesity as
a risk factor for triple-negative breast cancers: a systematic
review and meta-analysis. Breast Cancer Res Treat. 137:307–314.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vona-Davis L and Rose DP: Adipokines as
endocrine, paracrine and autocrine factors in breast cancer risk
and progression. Endocr Relat Cancer. 14:189–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schäffler A, Schölmerich J and Buechler C:
Mechanisms of disease: adipokines and breast cancer - endocrine and
paracrine mechanisms that connect adiposity and breast cancer. Nat
Clin Pract Endocrinol Metab. 3:345–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stephenson GD and Rose DP: Breast cancer
and obesity: an update. Nutr Cancer. 45:1–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weichhaus M, Broom I and Bermano G: The
molecular contribution of TNF-α in the link between obesity and
breast cancer. Oncol Rep. 25:477–483. 2011.PubMed/NCBI
|
|
11
|
Chicheportiche Y, Bourdon PR, Xu H, et al:
TWEAK, a new secreted ligand in the tumor necrosis factor family
that weakly induces apoptosis. J Biol Chem. 272:32401–32410. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Winkles JA: The TWEAK-Fn14
cytokine-receptor axis: discovery, biology and therapeutic
targeting. Nat Rev Drug Discov. 7:411–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawakita T, Shiraki K, Yamanaka Y, et al:
Functional expression of TWEAK in human colonic adenocarcinoma
cells. Int J Oncol. 26:87–93. 2005.PubMed/NCBI
|
|
14
|
Kawakita T, Shiraki K, Yamanaka Y, et al:
Functional expression of TWEAK in human hepatocellular carcinoma:
possible implication in cell proliferation and tumor angiogenesis.
Biochem Biophys Res Commun. 318:726–733. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pickart CM: Mechanisms underlying
ubiquitination. Annu Rev Biochem. 70:503–533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mukhopadhyay D and Riezman H:
Proteasome-independent functions of ubiquitin in endocytosis and
signaling. Science. 315:201–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paul PK, Gupta SK, Bhatnagar S, et al:
Targeted ablation of TRAF6 inhibits skeletal muscle wasting in
mice. J Cell Biol. 191:1395–1411. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tavassoli FA and Devilee P: Tumours of the
breastWorld Health Organization Classification of Tumours.
Pathology and Genetics of Tumours of the Breast and Female Genital
Organs. IARC Press; Lyon: pp. 13–59. 2003
|
|
19
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johns N, Stephens NA and Preston T: Muscle
protein kinetics in cancer cachexia. Curr Opin Support Palliat
Care. 6:417–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cohen S, Brault JJ, Gygi SP, et al: During
muscle atrophy, thick, but not thin, filament components are
degraded by MuRF1-dependent ubiquitylation. J Cell Biol.
185:1083–1095. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Acharyya S and Guttridge DC: Cancer
cachexia signaling pathways continue to emerge yet much still
points to the proteasome. Clin Cancer Res. 13:1356–1361. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SJ and Glass DJ: Treating cancer
cachexia to treat cancer. Skelet Muscle. 1:22011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fischer P and Hilfiker-Kleiner D: Survival
pathways in hypertrophy and heart failure: the gp130-STAT3 axis.
Basic Res Cardiol. 102:279–297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones SA, Scheller J and Rose-John S:
Therapeutic strategies for the clinical blockade of IL-6/gp130
signaling. J Clin Invest. 121:3375–3383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Penna F, Costamagna D, Fanzani A, Bonelli
G, Baccino FM and Costelli P: Muscle wasting and impaired
myogenesis in tumor bearing mice are prevented by ERK inhibition.
PLoS One. 5:136042010. View Article : Google Scholar
|
|
27
|
Jagoe RT and Goldberg AL: What do we
really know about the ubiquitin-proteasome pathway in muscle
atrophy? Curr Opin Clin Nutr Metab Care. 4:183–190. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lecker SH, Solomon V, Mitch WE and
Goldberg AL: Muscle protein breakdown and the critical role of the
ubiquitin-proteasome pathway in normal and disease states. J Nutr.
(Suppl):129:227S–237S. 1999.PubMed/NCBI
|
|
29
|
Bodine SC, Latres E, Baumhueter S, et al:
Identification of ubiquitin ligases required for skeletal muscle
atrophy. Science. 294:1704–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pickart CM: Mechanisms underlying
ubiquitination. Annu Rev Biochem. 70:503–533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang WL, Wang J, Chan CH, et al: The E3
ligase TRAF6 regulates Akt ubiquitination and activation. Science.
325:1134–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamashita M, Fatyol K, Jin C, Wang X, Liu
Z and Zhang YE: TRAF6 mediates Smad-independent activation of JNK
and p38 by TGF-beta. Mol Cell. 31:918–924. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto M, Ito T, Shimizu T, et al:
Epigenetic alteration of the NF-κB-inducing kinase (NIK) gene is
involved in enhanced NIK expression in basal-like breast cancer.
Cancer Sci. 101:2391–2397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arsenic R, Lehmann A, Budczies J, et al:
Analysis of PIK3CA mutations in breast cancer subtypes. Appl
Immunohistochem Mol Morphol. 22:50–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Polek TC, Talpaz M, Darnay BG and
Spivak-Kroizman T: TWEAK mediates signal transduction and
differentiation of RAW264.7 cells in the absence of Fn14/TweakR.
Evidence for a second TWEAK receptor. J Biol Chem. 278:32317–32323.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoriki R, Akashi S, Sho M, et al:
Therapeutic potential of the TWEAK/Fn14 pathway in intractable
gastrointestinal cancer. Exp Ther Med. 2:103–108. 2011.PubMed/NCBI
|
|
37
|
Chao DT, Su M, Tanlimco S, et al:
Expression of TweakR in breast cancer and preclinical activity of
enavatuzumab, a humanized anti-TweakR mAb. J Cancer Res Clin Oncol.
139:315–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chacón MR, Richart C, Gómez JM, et al:
Expression of TWEAK and its receptor Fn14 in human subcutaneous
adipose tissue. Relationship with other inflammatory cytokines in
obesity. Cytokine. 33:129–137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu H, Tamashiro S, Baritaki S, et al:
TRAF6 activation in multiple myeloma: a potential therapeutic
target. Clin Lymphoma Myeloma Leuk. 12:155–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hongming H and Jian H: Bortezomib inhibits
maturation and function of osteoclasts from PBMCs of patients with
multiple myeloma by downregulating TRAF6. Leuk Res. 33:115–122.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tseng LM, Liu CY, Chang KC, Chu PY, Shiau
CW and Chen KF: CIP2A is a target of bortezomib in human triple
negative breast cancer cells. Breast Cancer Res. 14:R682012.
View Article : Google Scholar : PubMed/NCBI
|