Introduction
Osteosarcoma is a highly malignant bone cancer that
predominantly affects children and adolescents. It presents with
aggressive local growth and early metastasis (1). Neo-adjuvant chemotherapy and advanced
surgical techniques have improved long-term survival and quality of
life for patients with osteosarcoma (2,3). However,
20% of patients will eventually develop recurrence, and patients
with metastatic or recurrent disease have a poor prognosis
(4,5).
Targeting critical molecular signaling pathways involved in
osteosarcoma carcinogenesis may be the key to providing novel
treatment approaches for patients with recurrent disease.
When systemic chemotherapeutic agents are
administered to patients, the response of each cell type is
different (6). Various factors
contribute to this heterogeneic response, in which cell hierarchy
plays an important role. A number of reports have identified the
existence of osteosarcoma stem cells, a subpopulation of cells that
possess the capacity to self-renew and multi-differentiate
(7–9).
In addition, cancer stem cells participate in drug resistance, thus
contributing to treatment failure (10,11).
Therefore, elucidating the effect of conventional chemotherapeutic
agents on osteosarcoma is critical for understanding osteosarcoma
tumor biology.
The Notch signaling pathway is pivotal in a variety
of biological processes, including cell proliferation and
apoptosis, as well as stem cell maintenance and differentiation
(12–14). The pathway consists of Notch ligands,
receptors, negative and positive modifiers, and target
transcription factors. The Notch receptor undergoes two successive
proteolytic cleavages upon interaction with the ligand.
Subsequently, the intracellular domain of Notch is released,
translocates to the nucleus and forms a complex that activates the
transcription of specific target genes, including hairy/enhancer of
split (Hes) and Hes related with YRPW motif
(Hey) (15,16). Dysregulated Notch activity has been
reported in an increasing number of malignancies, such as colon
(17,18), pancreatic (19,20) and
cervical (21) cancer. Additionally,
dysregulated Notch activity has been reported to contribute to the
carcinogenesis of osteosarcoma (22–26).
However, the role of Notch in osteosarcoma chemoresistance remains
unclear. Therefore, in the present study, the effect of doxorubicin
on the activity of the Notch signaling pathway was evaluated in
143B osteosarcoma cell lines.
Materials and methods
Cell culture
The 143B human osteosarcoma cell line was purchased
from China Center for Type Culture Collection (Wuhan, China). All
the cells were cultured in RPMI-1640 medium containing 10% (v/v)
fetal bovine serum and 1% (v/v) penicillin/streptomycin (Invitrogen
Life Technologies, Carlsbad, CA, USA). The cells were propagated in
a humidified atmosphere with 5% CO2 at 37°C. Cell
viability was determined by trypan blue staining (Invitrogen Life
Technologies).
Cell cytotoxicity assay
Cells were added to 96-well culture plates at a
density of 5000 cells/well. The cells were treated with various
concentrations of doxorubicin dissolved in DMSO, to a total volume
of 100 µl per well; control cells were treated with DMSO only. The
cells were cultured (as previously described) for different time
periods, as indicated in Fig. 1A.
Next, 10 µl Cell Counting Kit-8 (Beyotime Institute of
Biotechnology, Shanghai, China) was added to each well and
incubated at 37°C for 2 h. The optical density of each well was
measured at a wavelength of 450 nm using a microplate reader
(Thermo Fisher Scientific, Waltham, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 143B cells using the
RNeasy Plus Mini Kit (Qiagen China Co., Ltd, Shanghai, China) and
the concentration and purity determined using an ND-1000
spectrophotometer (NanoDrop Technologies, Thermo Fisher Scientific,
Wilmington, DE, USA). Reverse transcription was performed using the
TaqMan Reverse Transcription Reagents (Applied Biosystems Life
Technologies, Foster City, CA, USA). RT-qPCR reactions were set up
in triplicate and performed on the 7900 PCR machine (Applied
Biosystems Life Technologies) using SYBR Green PCR Master Mix
(Applied Biosystems Life Technologies). Conditions used for
amplification of cDNA fragments were as follows: 95°C for 5 min, 40
cycles of amplification (95°C for 15 sec, 60°C for 1 min). The
expression levels were calculated using the 2−ΔΔCt
method as described previously (27)
and normalized to β-actin. The gene-specific primers used are
listed in Table I.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
sequence | Reverse primer
sequence |
|---|
| Hes1 |
5′-CAGATCAATGCCATGACCTACC-3′ |
5′-AGCCTCCAAACACCTTAGCC-3′ |
| Hes5 |
5′-AGCCCCAAAGAGAAAAACCGACTG-3′ |
5′-TGGAGCGTCAGGAACTGCACGG-3′ |
| Hey1 |
5′-CATGTCCCCAACTACATCTTCC-3′ |
5′-CCTTGCTCCATTACCTGCTTC-3′ |
| Hey2 |
5′-ACCTCTCTCTTGTCCCTCTCTG-3′ |
5′-GGTTTATTGTTTGTTCCACTGC-3′ |
| HeyL |
5′-ACCGCATCAACAGTAGCCTTTCT-3′ |
5′-GCATTTTCAAGTGATCCACCGTC-3′ |
| β-actin |
5′-GTCCACCGCAAATGCTTCTA-3′ |
5′-TGCTGTCACCTTCACCGTTC-3′ |
Western blot analysis
Proteins were extracted with Protein Lysis Buffer
(Sigma Aldrich, St. Louis, MO, USA). Lysates were then centrifuged
at 10000 × g for 10 min at 4°C, and supernatants were collected.
Protein concentrations were assessed using the Bicinchoninic acid
Protein Assay Kit (Sigma-Aldrich). Cell lysates containing 40 µg
protein were separated on a 10% SDS-PAGE gel and then were
transferred onto polyvinylidene difluoride membranes (Invitrogen
Life Technologies) using a Trans Blot Turbo (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Membranes were blocked in a solution of
Tris buffered saline with containing 0.05% Tween-20 and 5% skimmed
milk for 1 h at room temperature. Primary antibodies were incubated
overnight at 4°C. The following polyclonal rabbit anti-human
primary antibodies were used: anti-Hes1 (catalog no. ab71559;
Abcam, Cambridge, MA, USA; dilution, 1:500); anti-Hey1 (catalog no.
ab22614; Abcam; dilution, 1:500) and anti-β-actin (catalog no.
ab8227; Abcam; dilution, 1:2,000). Horseradish
peroxidase-conjugated secondary antibodies (Abcam; dilution,
1:5,000) were incubated for 2 h at room temperature. Finally, the
membranes were washed again and developed using an enhanced
chemiluminescence substrate (Sigma-Aldrich).
Statistical analysis
Statistical analyses were performed using the SPSS
13.0 statistical software package (SPSS Inc., Chicago, IL, USA).
Data are expressed as the mean ± standard deviation of three
independent experiments. The Student's t-test was used to
compare the means of the two groups. When more than three means
were compared, a one-way analysis of variance followed by multiple
comparisons among the means was used. P<0.05 was considered to
indicate a statistically significant difference.
Results
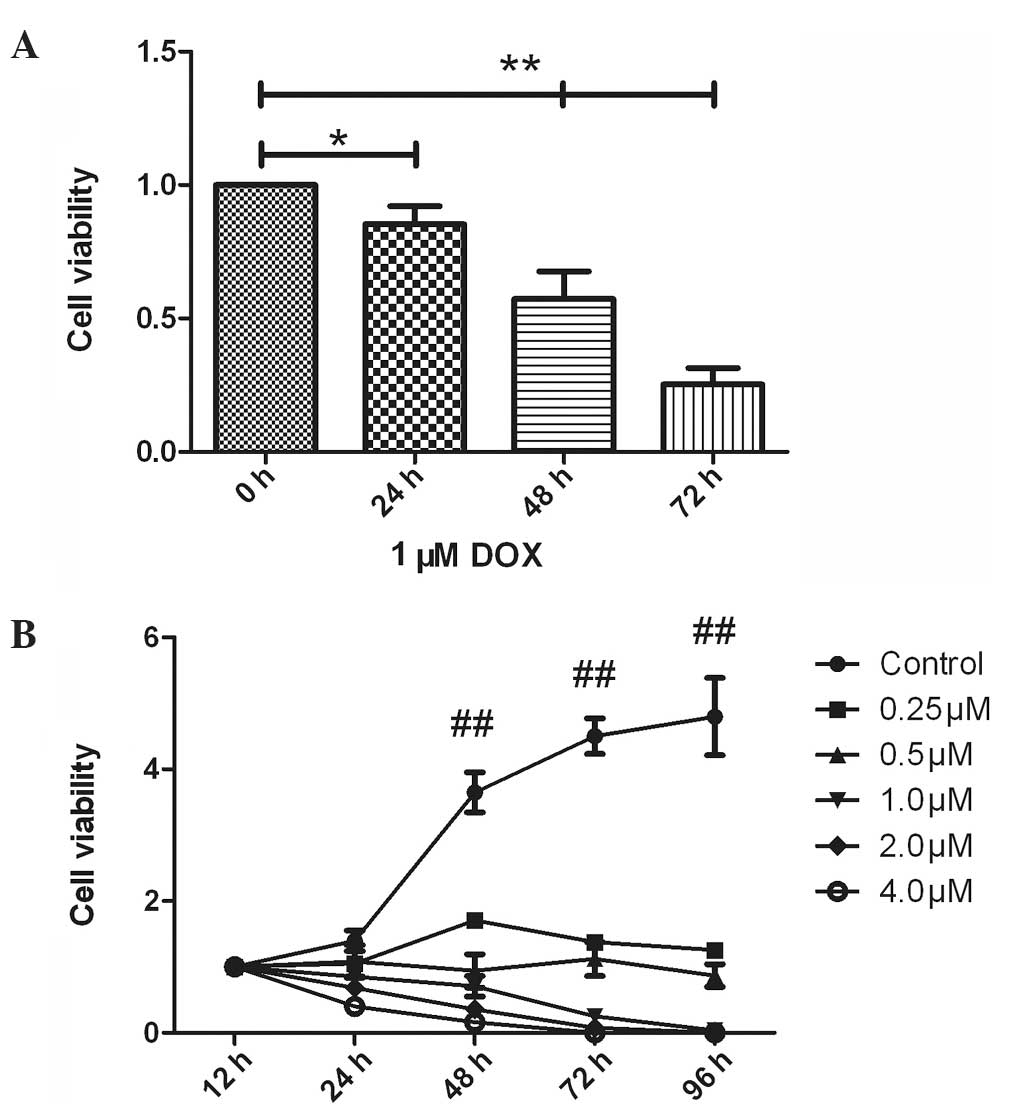
Optimizing the concentration of
doxorubicin treatment
To determine an optimum doxorubicin dose range for
subsequent studies, time- and dose-dependent cytotoxic assays were
performed. The data indicated that the treatment of osteosarcoma
cells with doxorubicin exhibited time and dose dependency. Toxicity
was significantly enhanced 48 h after exposure to doxorubicin
(P<0.01; Fig. 1A). In addition, a
concentration of ≥0.5 µM doxorubicin resulted in significantly
higher toxicity; however, a doxorubicin concentration of <0.5 µM
exhibited a cytostatic effect (Fig.
1B).
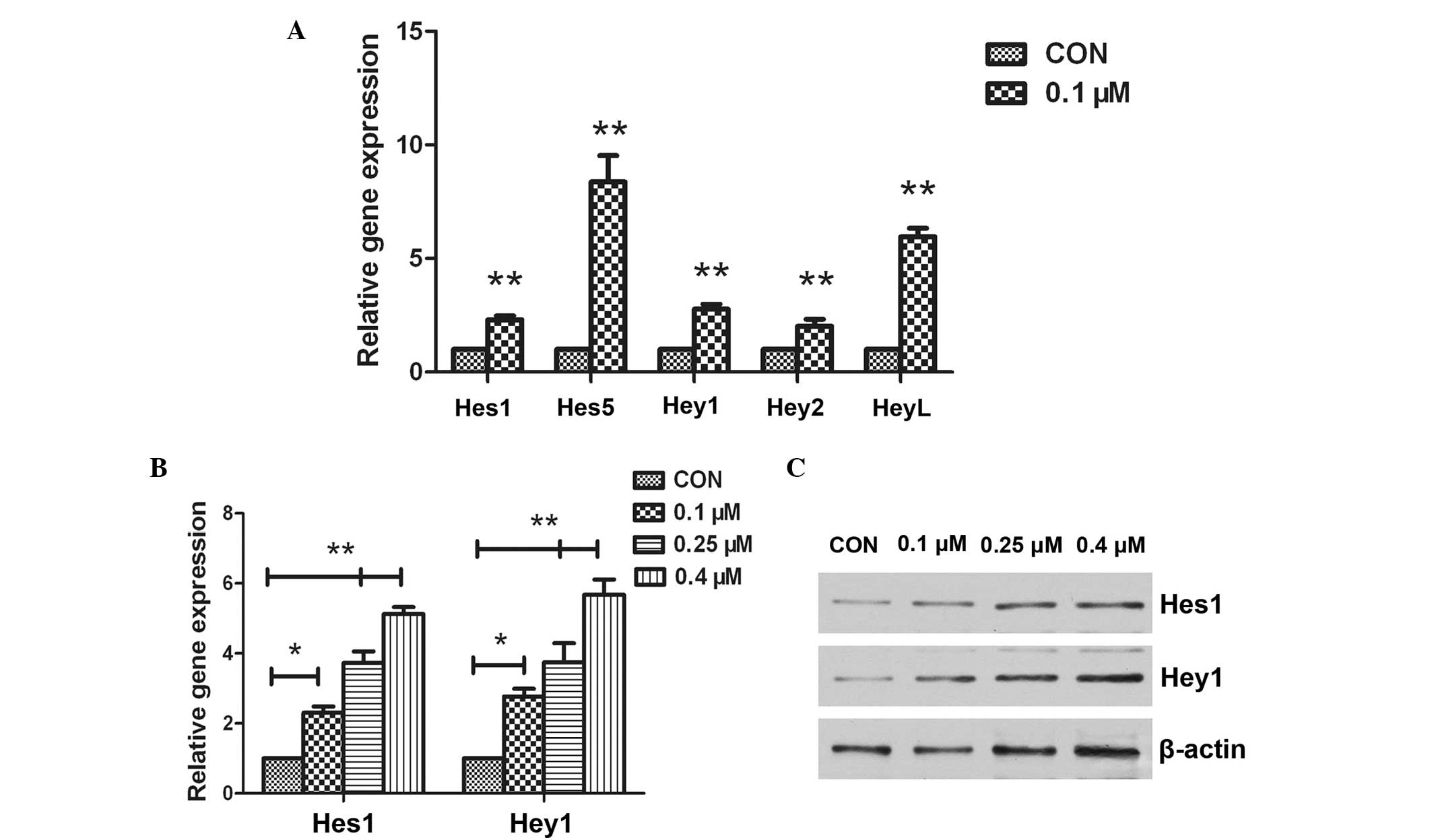
Doxorubicin increases Notch target
gene expression in osteosarcoma cells
To understand the molecular mechanism involved in
doxorubicin-induced stemness, changes in the Notch signaling
pathway were investigated. The expression levels of various Notch
target genes, including Hes1, Hes5, Hey1,
Hey2 and HeyL, were assessed in the 143B cells
treated with 0.1 µM doxorubicin for 48 h using RT-qPCR analysis. A
significant increase in Hes1, Hes5, Hey1,
Hey2 and HeyL mRNA expression levels was detected
following doxorubicin treatment (P<0.05; Fig. 2A). Additional analysis was performed
to determine whether the increase was dose-dependent. The 143B
cells wre treated with increasing concentrations of doxorubicin
(0.1, 0.25 and 0.4 µM) for 48 h and the results demonstrated that
Hes1 and Hey1 expression levels were upregulated in a
dose-dependent manner (Fig. 2B). In
order to confirm that Notch signaling was activated by doxorubicin,
the expression of Notch target genes were also detected using
western blotting. The results demonstrated that the expression
levels of Hes1 and Hey1 were significantly enhanced
by doxorubicin treatment (Fig.
2C).
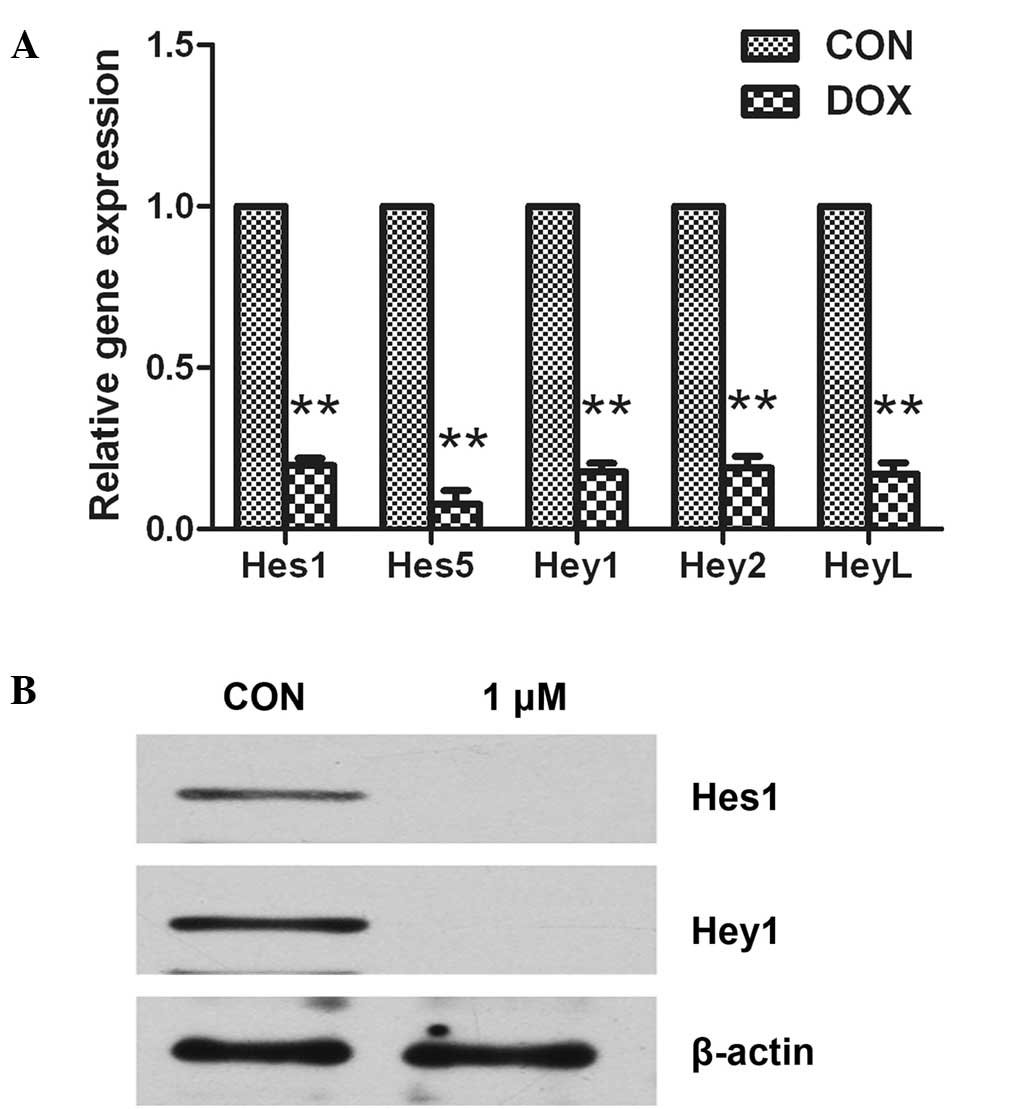
High-dose doxorubicin decreases the
expression of Notch target genes
To examine the effect of toxic doxorubicin on Notch
target genes in osteosarcoma, 143B cells were treated with 1 µM
doxorubicin. The Notch target genes, including Hes1,
Hes5, Hey1, Hey2 and HeyL, were found
to be significantly suppressed by doxorubicin treatment (P<0.05;
Fig. 3A). The results were confirmed
using western blot analysis, and the findings were in agreement
with the RT-qPCR data, as Hes1 and Hey1 were markedly
downregulated following treatment with high-dose doxorubicin
(Fig. 3B).
Discussion
The acceptance of chemotherapy as an integral and
essential component of the treatment of osteosarcoma marked a new
era for this disease. Doxorubicin was introduced for the treatment
of osteosarcoma in the early 1970s (1). Although it is widely recognized that the
agent intercalates into DNA and generates free radicals, the
precise effect of doxorubicin on cancer cells requires further
investigation (28).
Dysregulated Notch activity has been reported to
contribute to the carcinogenesis of osteosarcoma (22), with Notch1 activity appearing
to be crucial for the invasion and metastasis of osteosarcoma
(25). Furthermore, inhibition of the
Notch signaling pathway suppressed osteosarcoma growth in
vitro and in vivo (26). A
number of studies have assessed the effect of conventional
chemotherapeutic agents on the Notch signaling pathway. For
instance, cisplatin has been reported to activate Notch signaling,
as determined by increased expression levels of cleaved
Notch1 (29). However, the
effect of doxorubicin on the Notch signaling pathway remains
unclear. The present study demonstrated that doxorubicin elicits a
dynamic and concentration-dependent effect on the Notch signaling
pathway in osteosarcoma cells.
Cells may survive when they are exposed to a
sublethal dose of therapeutic agent; however, the specific effects
of conventional sublethal agents on osteosarcoma is critically
important. Liu et al (29)
identified that the administration of a low concentration of
cisplatin enriched the population of multidrug resistant
CD133+ cells in lung adenocarcinoma. By contrast,
blocking the Notch signaling pathway sensitizes cancer cells to
chemotherapy (29,30). Therefore, the present study aimed to
identify an effective but nontoxic optimum dose of doxorubicin for
use in subsequent studies. The effect of doxorubicin was found to
be dose- and time-dependent, and a period of 48 h was required for
the agent to exert its effect. Furthermore, the current data
demonstrated that the treatment of osteosarcoma cells with ≥0.5 µM
doxorubicin for ≥48 h resulted in significant toxicity. Thus,
subsequent investigations were conducted using concentrations
limited to 0.5 µM. It was identified that the expression levels of
various Notch target genes, including Hes1, Hes5,
Hey1, Hey2 and HeyL, were significantly
increased in osteosarcoma cells following treatment with
doxorubicin.
The observed enhancement in Notch signaling may be
simply explained by the resistance of Notch-active cells to
doxorubicin and doxorubicin treatment enriching this resistant cell
population. In accordance with this hypothesis, recent studies have
demonstrated that Notch appears to be involved in the mechanisms of
cisplatin resistance (31–35). Notch1 expression is negatively
correlated with chemosensitivity; therefore, enhanced
chemotherapeutic sensitivity may be obtained by blocking Notch
signaling, in which case Notch gene expression should not be
altered with different concentrations of doxorubicin. In the
current study, treatment with a cytostatic concentration of
doxorubicin appeared to directly activate the Notch signaling
pathway in a dose-dependent manner. However, the underlying
mechanism of this process requires further investigation.
In the present study, the expression of Notch genes
following exposure to high-dose doxorubicin was significantly
inhibited. Considering that the Notch signaling pathway appears to
be crucial in the development of osteosarcoma, the authors of the
present study propose that a high concentration doxorubicin
partially exerts its cytotoxic effect via inhibition of the Notch
signaling pathway. Alternatively, this observation may only be a
side effect of early apoptosis.
In conclusion, doxorubicin activates the Notch
signaling pathway at a sublethal dose and inhibits the Notch
signaling pathway at a toxic dose. Along with the results of
previous studies observing that cisplatin activates the Notch
signaling pathway (29,30), the present study supports the
combination treatment of recurrent osteosarcoma with a Notch
inhibitor. In addition, the current results support the use of
intensive chemotherapy to inhibit chemoresistance, since
doxorubicin exerts its chemoresistant effect in a
concentration-dependent manner.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81341078) and
Hubei Natural Science Foundation of China (no. 2011CHB039).
References
|
1
|
Jaffe N: Osteosarcoma: review of the past,
impact on the future. The American experience. Cancer Treat Res.
152:239–262. 2009.PubMed/NCBI
|
|
2
|
Hansen AR, Hughes BG, Paul S, et al:
Single institution retrospective review of perioperative
chemotherapy in adult and adolescent patients with operable
osteosarcoma. Asia Pac J Clin Oncol. Feb 20–2014.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haddox CL, Han G, Anijar L, et al:
Osteosarcoma in pediatric patients and young adults: a single
institution retrospective review of presentation, therapy and
outcome. Sarcoma. 2014:4025092014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gelderblom H, Jinks RC, Sydes M, et al:
European Osteosarcoma Intergroup: Survival after recurrent
osteosarcoma: data from 3 European Osteosarcoma Intergroup (EOI)
randomized controlled trials. Eur J Cancer. 47:895–902. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bacci G, Briccoli A, Longhi A, et al:
Treatment and outcome of recurrent osteosarcoma: experience at
Rizzoli in 235 patients initially treated with neoadjuvant
chemotherapy. Acta Oncol. 44:748–755. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014.PubMed/NCBI
|
|
7
|
Martins-Neves SR, Lopes AO, do Carmo A, et
al: Therapeutic implications of an enriched cancer stem-like cell
population in a human osteosarcoma cell line. BMC Cancer.
12:1392012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gibbs CP Jr, Levings PP and Ghivizzani SC:
Evidence for the osteosarcoma stem cell. Curr Orthop Pract.
22:322–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siclari VA and Qin L: Targeting the
osteosarcoma cancer stem cell. J Orthop Surg. 5:782010. View Article : Google Scholar
|
|
10
|
Yu L, Liu S, Zhang C, et al: Enrichment of
human osteosarcoma stem cells based on hTERT transcriptional
activity. Oncotarget. 4:2326–2338. 2013.PubMed/NCBI
|
|
11
|
Adhikari AS, Agarwal N, Wood BM, et al:
CD117 and Stro-1 identify osteosarcoma tumor-initiating cells
associated with metastasis and drug resistance. Cancer Res.
70:4602–4612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guruharsha KG, Kankel MW and
Artavanis-Tsakonas S: The Notch signalling system: recent insights
into the complexity of a conserved pathway. Nat Rev Genet.
13:654–666. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bianchi S, Dotti MT and Federico A:
Physiology and pathology of notch signalling system. J Cell
Physiol. 207:300–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fortini ME: Notch signaling: the core
pathway and its posttranslational regulation. Dev Cell. 16:633–647.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davis RL and Turner DL: Vertebrate hairy
and Enhancer of split related proteins: transcriptional repressors
regulating cellular differentiation and embryonic patterning.
Oncogene. 20:8342–8357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aranguren XL, Agirre X, Beerens M, et al:
Unraveling a novel transcription factor code determining the human
arterial-specific endothelial cell signature. Blood. 122:3982–3992.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin HY, Zhang HY, Wang X, Xu J and Ding Y:
Expression and clinical significance of Notch signaling genes in
colorectal cancer. Tumour Biol. 33:817–824. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ungerbӓck J, Elander N, Grünberg J,
Sigvardsson M and Söderkvist P: The Notch-2 gene is regulated by
Wnt signaling in cultured colorectal cancer cells. PLoS One.
6:e179572011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mysliwiec P and Boucher MJ: Targeting
Notch signaling in pancreatic cancer patients - rationale for new
therapy. Adv Med Sci. 54:136–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Avila JL and Kissil JL: Notch signaling in
pancreatic cancer: oncogene or tumor suppressor? Trends Mol Med.
19:320–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maliekal TT, Bajaj J, Giri V, Subramanyam
D and Krishna S: The role of Notch signaling in human cervical
cancer: implications for solid tumors. Oncogene. 27:5110–5114.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McManus MM, Weiss KR and Hughes DP:
Understanding the role of notch in osteosarcoma. Adv Exp Med Biol.
804:67–92. 2014.PubMed/NCBI
|
|
23
|
Mu X, Isaac C, Greco N, Huard J and Weiss
K: Notch signaling is associated with ALDH activity and an
aggressive metastatic phenotype in murine osteosarcoma cells. Front
Oncol. 3:1432013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma Y, Ren Y, Han EQ, et al: Inhibition of
the Wnt-β-catenin and Notch signaling pathways sensitizes
osteosarcoma cells to chemotherapy. Biochem Biophys Res Commun.
431:274–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hughes DP: How the NOTCH pathway
contributes to the ability of osteosarcoma cells to metastasize.
Cancer Treat Res. 152:479–496. 2009.PubMed/NCBI
|
|
26
|
Tanaka M, Setoguchi T, Hirotsu M, et al:
Inhibition of Notch pathway prevents osteosarcoma growth by cell
cycle regulation. Br J Cancer. 100:1957–1965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu L, Liu S, Guo W, et al: hTERT promoter
activity identifies osteosarcoma cells with increased EMT
characteristics. Oncol Lett. 7:239–244. 2014.PubMed/NCBI
|
|
28
|
Thorn CF, Oshiro C, Marsh S, et al:
Doxorubicin pathways: pharmacodynamics and adverse effects.
Pharmacogenet Genomics. 21:440–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu YP, Yang CJ, Huang MS, et al:
Cisplatin selects for multidrug-resistant CD133+ cells in lung
adenocarcinoma by activating Notch signaling. Cancer Res.
73:406–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J and Mao Z, Huang J, Xie S, Liu T and
Mao Z: Blocking the NOTCH pathway can inhibit the growth of
CD133-positive A549 cells and sensitize to chemotherapy. Biochem
Biophys Res Commun. 444:670–675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang M, Ma X, Wang J, Wang L and Wang Y:
Pretreatment with the γ-secretase inhibitor DAPT sensitizes
drug-resistant ovarian cancer cells to cisplatin by downregulation
of Notch signaling. Int J Oncol. 44:1401–1409. 2014.PubMed/NCBI
|
|
32
|
Zhou JX, Han JB, Chen SM, et al:
γ-secretase inhibition combined with cisplatin enhances apoptosis
of nasopharyngeal carcinoma cells. Exp Ther Med. 3:357–361.
2012.PubMed/NCBI
|
|
33
|
Zang S, Chen F, Dai J, et al:
RNAi-mediated knockdown of Notch-1 leads to cell growth inhibition
and enhanced chemosensitivity in human breast cancer. Oncol Rep.
23:893–899. 2010.PubMed/NCBI
|
|
34
|
Nefedova Y, Sullivan DM, Bolick SC, Dalton
WS and Gabrilovich DI: Inhibition of Notch signaling induces
apoptosis of myeloma cells and enhances sensitivity to
chemotherapy. Blood. 111:2220–2229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang ZP, Sun YL, Fu L, Gu F, Zhang L and
Hao XS: Correlation of Notch1 expression and activation to
cisplatin-sensitivity of head and neck squamous cell carcinoma. Ai
Zheng. 28:100–103. 2009.PubMed/NCBI
|