Introduction
Colorectal cancer (CRC) is one of the most common
causes of cancer-associated mortality, accounting for >600,000
mortalities per year, worldwide (1).
Tumor recurrence and metastasis to distant organs are the
predominant contributing factors to the high mortality and poor
survival rates associated with this disease (2). Recently, a number of studies have
indicated that only a subpopulation of tumor cells, termed cancer
stem cells (CSC), are capable of regenerating the tumor (3–5). In
addition, CSCs may be involved in therapeutic resistance, tumor
relapse and metastasis (3). Thus, the
emergence of the CSC theory may have significant implications in
cancer therapy. CSCs are considered to originate from mutant
wild-type stem cells (6). In 2007,
Barker et al (7) identified
that leucine-rich repeat-containing G protein-coupled receptor 5
(LGR5) expression was restricted to cells in the crypt base of the
small and large intestines; in addition, LGR5 was considered to be
a stem cell marker. A subsequent study proposed that intestinal
epithelial tumors may originate from LGR5-positive stem cells
(8).
LGR5, also known as HG38 and G protein-coupled
receptor 49 (9,10), is a target gene of the Wnt/β-catenin
signaling pathway (11), acting as
receptor for the Wnt/β-catenin signaling agonist R-spondin
(12,13). This signaling pathway has a critical
role in normal development and the maintenance of adult stem cells
as well as in tumor pathogenesis and growth (14,15). In
healthy mucosa tissue, β-catenin is maintained at low cytoplasmic
levels due to degradation regulated by a destruction complex
composed of glycogen synthase kinase 3, Axin, adenomatous polyposis
coli (APC) and other factors (16).
In the progression of the majority of cases of CRC, the Wnt
signaling pathway is activated early via truncations of APC and,
less frequently, mutations of β-catenin (17). These mutations inhibit the activity of
the destruction complex, resulting in the accumulation and nuclear
translocation of β-catenin, ultimately resulting in transcriptional
activation of target genes (18,19).
Nuclear β-catenin is involved in two processes that are essential
for embryonic development: Epithelial-mesenchymal transition and
stem cell formation (20).
Accumulating data indicates that aberrant nuclear expression of
β-catenin may confer these two traits to tumor cells, therefore
driving malignant tumor progression (21,22).
It is generally accepted that upregulation of LGR5
is associated with activated Wnt/β-catenin signaling, resulting in
the overexpression of LGR5 in various types of cancer, including
hepatocellular carcinoma, ovarian cancer and CRC (23,24).
However, the underlying mechanisms for the role of LGR5 in
carcinogenesis and intracellular signaling are poorly understood.
Previous studies have identified that LGR5 and its homologs
function as receptors of the R-spondin family of stem cell factors
in order to enhance Wnt/β-catenin signaling (25). Furthermore, knockdown of LGR5 induced
cell death in adenoma and carcinoma cells (26); in addition, LGR5-positive stem cell
fractions were capable of forming tumors via activation of the
Wnt/β-catenin signaling pathway (8).
However, alternative studies propose that loss of LGR5 does not
affect the proliferation or migration of intestinal cells (27). The aims of the present study were to
further clarify the association between Lgr5, β-catenin and APC in
the Wnt/β-catenin signaling pathway and to identify a novel method
for the treatment of colorectal cancer.
Patients and methods
Patients and specimens
Specimens were collected from 20 patients with CRC
who underwent surgical resection at the Department of Colorectal
Surgery of Xin Hua Hospital Affiliated to Shanghai Jiaotong
University School of Medicine (Shanghai, China) between November
2010 and May 2013. Tumor and paired healthy adjacent colorectal
mucosa tissue samples were collected from each patient. All samples
were obtained from the surgically resected material, immediately
frozen in liquid nitrogen (Novobio Scientific, Shanghai, China) and
stored at −80°C. The current study was approved by the Ethics
Committee of Shanghai Jiaotong University School of Medicine and
all samples were obtained following receipt of written informed
consent from all patients.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA extraction was performed using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer's instructions. Complementary DNA was then synthesized
using SuperScript III Reverse Transcriptase (Invitrogen Life
Technologies). Subsequently, qPCR was performed on a CFX96TM
Real-Time System (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
using the following primers (Invitrogen Life Technologies): APC, F
5′-GCTCCAAGCCCAACCTTAA-3′ and R 5′-GTTTTCGCCATCCACCAG-3′;
β-catenin, F 5′-CATTCAGCAGAAGGTCCGA-3′ and R
5′-CTGGAAAACGCCATCACC-3′; and LGR5, F 5′-GTGGCAGCAAGTATGGCG-3′ and
R 5′-AGCAAAGGGAATTGAGCAAG-3′. Fold induction values were calculated
using the cycle threshold (Ct) method (2−ΔΔCt) (28). All experiments were performed in
triplicate and independently repeated a minimum of three times.
Small interfering RNA (siRNA)
To knockdown LGR5, short hairpin RNA (shRNA) of the
human LGR5 lentivirus gene transfer vector was constructed (Novobio
Scientific). This gene transfer vector encoded the RNA sequence for
green fluorescent protein (GFP). The following LGR5 siRNA sequence
was used to target nucleotides: 5′-GTCTGCAATCAGTTACCTA-3′. Titer
was measured by detecting GFP-positive HEK293T cells (Novobio
Scientific) using fluorescence microscopy (IX51 microscope, Olympus
Corporation, Tokyo, Japan), with the recombinant LGR5-targeting
siRNA lentivirus prepared and titered to a concentration of
2.5×109 transfection units/ml. A scramble siRNA (Novobio
Scientific) was used as a negative control (NC).
Detection of cell proliferation
NC-siRNA- and LGR5-siRNA-transfected cells were
seeded into 96-well plates at a density of 4×103
cells/well and incubated for 24 h. On days 1, 3, 5 and 7, 10 µl
Cell Counting Kit 8 (CCK8) solution (Dojindo Molecular
Technologies, Inc., Shanghai, China) was added to each well. Color
intensity was measured using an RT-2100C microplate reader (Rayto
Life and Analytical Sciences Co.,Ltd., Nanshen, China) an
absorbance of 450 nm to obtain cell growth curves. All experiments
were performed in triplicate and repeated independently three
times.
Statistical analysis
SAS software (version 8.5; SAS Institute, Inc.,
Cary, NC, USA) was used for all statistical analyses.
Kruskal-Wallis non-parametric tests were performed to analyze
differences in LGR5, APC and β-catenin mRNA expression between CRC
and corresponding healthy mucosal tissues. In addition, a paired
t-test was used to compare differences between the LGR5
knockdown group and negative control group. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
LGR5 and β-catenin expression is
elevated and APC expression is reduced in CRC tissues
RNA was extracted from 20 CRC and adjacent healthy
tissues samples, then subjected to RT-qPCR to determine the mRNA
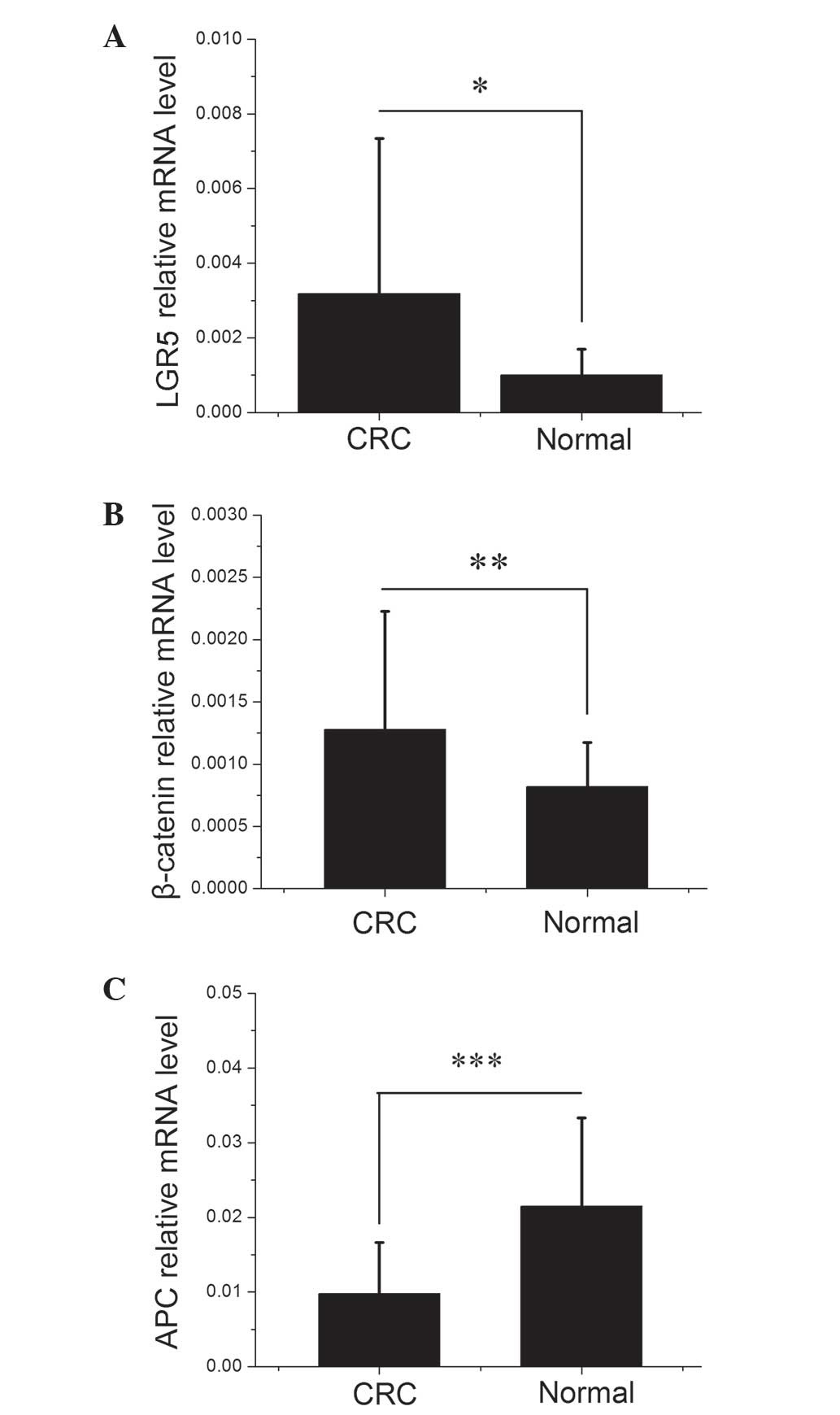
expression profiles of LGR5, β-catenin and APC. As demonstrated in
Fig. 1, there were significant
differences in LGR5, β-catenin and APC mRNA expression levels
between the CRC and healthy colorectal mucosa samples (P=0.0484,
0.0032 and 0.0006, respectively). As shown in Table I, LGR5, β-catenin and APC expression
in the CRC samples were divided by their expression in the matched
healthy mucosa samples to obtain the tumor/normal healthy tissue
expression (T/N) ratio. A T/N ratio of >1 indicated increased
expression in CRC. Of the 20 CRC samples investigated, 14 (70%)
exhibited elevated levels of LGR5 expression and 15 (75%)
demonstrated elevated β-catenin expression compared with their
corresponding healthy mucosa samples, with mean T/N ratios of 3.57
and 1.78, respectively. By contrast, APC mRNA expression was
decreased in 17 (85%) CRC samples with a mean T/N ratio of
0.87.
 | Table I.T/N ratio of LGR5, β-catenin and APC
mRNA expression. |
Table I.
T/N ratio of LGR5, β-catenin and APC
mRNA expression.
| Case | LGR5 | β-catenin | APC |
|---|
| 1 | 2.73 | 6.37 | 0.58 |
| 2 | 1.25 | 0.89 | 0.25 |
| 3 | 0.76 | 1.65 | 3.32 |
| 4 | 1.69 | 1.10 | 0.15 |
| 5 | 3.10 | 1.16 | 0.46 |
| 6 | 2.61 | 2.58 | 0.38 |
| 7 | 2.03 | 0.98 | 1.07 |
| 8 | 1.09 | 1.26 | 0.20 |
| 9 | 20.47 | 0.99 | 0.32 |
| 10 | 0.77 | 1.99 | 0.47 |
| 11 | 5.53 | 1.86 | 0.31 |
| 12 | 0.01 | 1.07 | 7.06 |
| 13 | 0.03 | 2.86 | 0.28 |
| 14 | 0.19 | 1.61 | 0.55 |
| 15 | 9.64 | 0.86 | 0.49 |
| 16 | 11.23 | 2.73 | 0.61 |
| 17 | 4.03 | 0.66 | 0.48 |
| 18 | 0.29 | 1.96 | 0.34 |
| 19 | 1.38 | 1.46 | 0.06 |
| 20 | 2.56 | 1.50 | 0.11 |
siRNA-mediated knockdown of LGR5
inhibits the expression of APC and β-catenin
To investigate the functional relevance of LGR5
expression in CRC cell lines, the expression of LGR5 was knocked
down in the HT-29 CRC cell line. Specific siRNA was constructed for
LGR5 and its ability to knock down LGR5 mRNA was evaluated. RT-qPCR
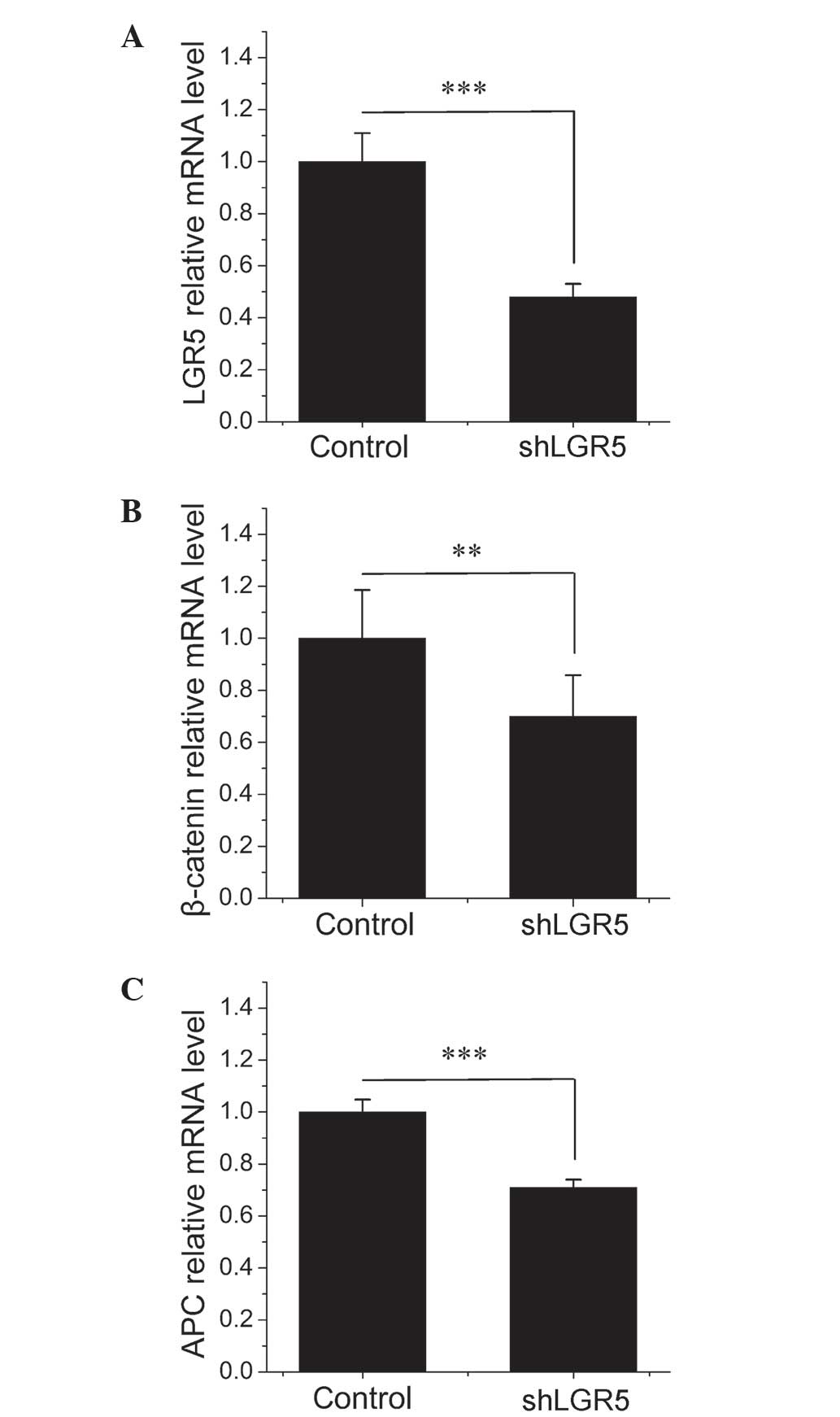
identified that the treatment of HT-29 cells with LGR5-siRNA
resulted in a significant 52% decrease in LGR5 mRNA expression
(P=0.0003) compared with the empty vector NC cells (Fig. 2A), indicating that the depletion of
LGR5 using the siRNA method was effective. As illustrated in
Fig. 2B and C, knockdown of LGR5
significantly decreased the expression of APC and β-catenin mRNA by
29% (P=0.0003) and 30% (P=0.001), respectively, compared with cells
transfected with NC-siRNA at 48 h post-transfection. As APC is
known to antagonize the transcriptional activity of β-catenin by
promoting its nuclear export and its proteasomal destruction in the
cytoplasm, decreasing the expression of APC may enhance
Wnt/β-catenin signaling (17–19). These results indicated that knockdown
of LGR5 may inhibit the expression of β-catenin as well as promote
β-catenin accumulation and nuclear translocation by downregulating
APC.
Knockdown of LGR5 inhibits CRC cell
proliferation
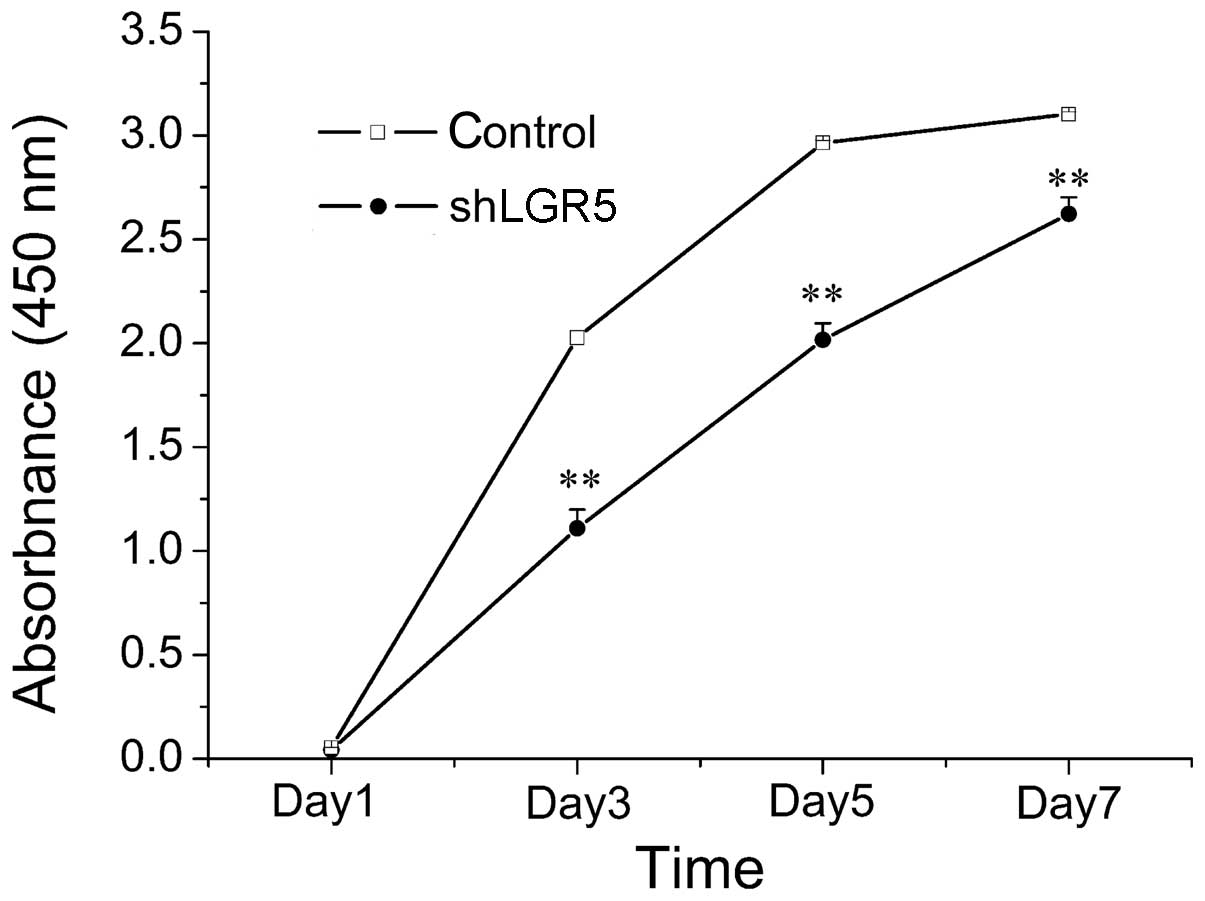
To investigate the effect of LGR5 on CRC cells
viability, a viability curve of LGR5-knockdown HT29 cells was
constructed by performing a CCK8 assay. As indicated in Fig. 3, HT29 cell growth was significantly
inhibited following LGR5 knockdown compared with the growth of
control group cells (P<0.001). This therefore indicated that
downregulation of LGR5 expression using siRNA significantly
inhibited the growth of HT-29 cells.
Discussion
LGR5, which has been established as a stem cell
marker in the small intestine and colon, has also been identified
as a downstream target gene of the Wnt signaling pathway (7). The current study demonstrated that LGR5
was significantly upregulated in CRC compared with healthy mucosa,
which is comparable with the results of a number of previous
studies (24,29,30). The
Wnt signaling pathway is comprised of a vast number of proteins,
including APC and β-catenin, two proteins that are critical in CRC
tumorigenesis (18). Therefore, the
present study aimed to evaluate the association between LGR5, APC
and β-catenin expression and CRC, as well as identify the role of
LGR5 in Wnt signaling.
The mRNA expression levels of LGR5, APC and
β-catenin were detected in 20 CRC tissue samples and their
corresponding healthy mucosa samples. The results demonstrated
significant differences in LGR5, β-catenin and APC mRNA expression
between CRC and healthy colorectal mucosa, indicating that CRC may
be associated with aberrant activation of the Wnt/β-catenin
signaling pathway. In CRC, mutations in β-catenin, Axin and certain
signaling pathways may result in the accumulation of β-catenin and
enhance Wnt signaling activation (31). However, APC mutations, which result in
aberrant activation of the Wnt signaling pathway, occur most
frequently in CRCs (17).
In the present study, to understand the effects of
LGR5 on APC and β-catenin expression, which are two key components
of Wnt signaling, LGR5 expression was silenced in the HT-29 CRC
cell line using siRNA. A decrease in APC and β-catenin mRNA
expression was observed following knockdown of LGR5. These results
indicated that LGR5 may be involved in regulating Wnt/β-catenin
signaling via modulation of the expression of APC and β-catenin.
The role of APC and β-catenin in CRC tumorigenesis has been well
studied. It was reported that >90% of cases of CRC exhibit
cytoplasmic accumulation of β-catenin (32). When activated and accumulated in the
cytoplasm, β-catenin is transferred to the nucleus, where it
activates numerous nuclear transcription factors, such as
transcription factor (TCF)/lymphoid enhancer-binding factor, which
results in the activation of downstream target molecules. Abnormal
expression of these molecules may result in abnormal proliferation
and tumorigenesis (33). APC is an
important tumor suppressor that downregulates the transcriptional
activity of β-catenin by the following three mechanisms: i)
Reducing the levels of cytoplasmic β-catenin by binding to Axin;
ii) promoting the export of nuclear β-catenin; and iii)
sequestering β-catenin, preventing it from binding to TCF (34). The simultaneous decrease in APC and
β-catenin expression observed in the present study provided
evidence that LGR5 may mediate bidirectional regulation in the
Wnt/β-catenin signaling pathway (β-catenin- or APC-directed
signaling). Accumulating data has demonstrated that the silencing
of LGR5 influences the functional and molecular outcome of CRC
cells; for example, previous reports have indicated that knocking
down endogenous LGR5 in cultured CRC cell lines reduced their
proliferation, migration, growth rates and colony formation
capability (24,35,36).
However, Walker et al (37)
reported that the ablation of LGR5 increased invasion, induced
anchorage-independent growth and enhanced tumorigenicity in a
xenograft model. Based on these controversial results, the current
authors proposed that LGR5 may act as a positive regulator of tumor
growth when the β-catenin signaling pathway is predominant and acts
as a negative regulator when the APC signaling pathway is
predominant.
In the latter experiments of the present study, LGR5
downregulation resulted in the attenuation of HT29 cell
proliferation. This data indicated that LGR5 may have a role in the
regulation of CRC cell growth and proliferation, which is
consistent with the results of previous investigations into basal
cell carcinoma (15), Ewing sarcoma
(24) and glioma (38). Thus, LGR5 may have the potential to
serve as a therapeutic target in patients with CRC. However, future
studies treating LGR5 as a therapeutic target should consider the
bidirectional regulation of LGR5. Additionally, the current authors
proposed that the blocking effect of LGR5 on APC may improve
treatment efficiency.
In conclusion, the current results demonstrated that
the majority of cases of CRC were associated with abnormal
expression of LGR5, β-catenin and APC. Furthermore, knockdown of
LGR5 significantly decreased the expression of β-catenin and APC.
Due to the critical role of APC and β-catenin in colorectal tumor
initiation and growth via the Wnt signaling pathway, LGR5 may be a
potential therapeutic target for patients with CRC. However, the
role of LGR5 in Wnt/β-catenin signaling requires further
investigation.
Acknowledgements
The present study was supported by grants from the
Program of Shanghai's Subject Chief Scientist from Shanghai
Municipal Health Bureau (no. XBR2011032), the Biomedical
Engineering Research Funds of Shanghai Jiaotong University (no.
YG2011MS32), the Special Fund for Outstanding Young Teachers of
Shanghai Municipal Education Commission (no. JDY10112), the
Ministry of Health (no. W2011JZC27) and Xinhua Hospital Affiliated
to Shanghai Jiaotong University School of Medicine (no.
11YJ005).
References
|
1
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weitz J, Koch M, Debus J, Höhler T, Galle
PR and Büchler MW: Colorectal cancer. Lancet. 365:153–165. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Odoux C, Fohrer H, Hoppo T, et al: A
stochastic model for cancer stem cell origin in metastatic colon
cancer. Cancer Res. 68:6932–6941. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu XS, Xi HQ and Chen L: Lgr5 is a
potential marker of colorectal carcinoma stem cells that correlates
with patient survival. World J Surg Oncol. 10:2442012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lobo NA, Shimono Y, Qian D and Clarke MF:
The biology of cancer stem cells. Annu Rev Cell Dev Biol.
23:675–699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barker N, van Es JH, Kuipers J, et al:
Identification of stem cells in small intestine and colon by marker
gene Lgr5. Nature. 449:1003–1007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barker N, Ridgway RA, van Es JH, et al:
Crypt stem cells as the cells-of-origin of intestinal cancer.
Nature. 457:608–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McDonald T, Wang R, Bailey W, et al:
Identification and cloning of an orphan G protein-coupled receptor
of the glycoprotein hormone receptor subfamily. Biochem Biophys Res
Commun. 247:266–270. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu SY, Liang SG and Hsueh AJ:
Characterization of two LGR genes homologous to gonadotropin and
thyrotropin receptors with extracellular leucine-rich repeats and a
G protein-coupled, seven-transmembrane region. Mol Endocrinol.
12:1830–1845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van der Flier LG, Sabates-Bellver J, Oving
I, et al: The intestinal Wnt/TCF signature. Gastroenterology.
132:628–632. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carmon KS, Gong X, Lin Q, et al:
R-spondins function as ligands of the orphan receptors LGR4 and
LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci
USA. 108:11452–11457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Lau W, Barker N, Low TY, et al: Lgr5
homologues associate with Wnt receptors and mediate R-spondin
signalling. Nature. 476:293–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Early DS, Fontana L and Davidson NO:
Translational approaches to addressing complex genetic pathways in
colorectal cancer. Transl Res. 151:10–16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cadigan KM and Peifer M: Wnt signaling
from development to disease: Insights from model systems. Cold
Spring Harb Perspect Biol. 1:a0028812009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schneikert J and Behrens J: The canonical
Wnt signalling pathway and its APC partner in colon cancer
development. Gut. 56:417–425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Metcalfe C and Bienz M: Inhibition of GSK3
by Wnt signalling - two contrasting models. J Cell Sci.
124:3537–3544. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brabletz T, Hlubek F, Spaderna S, et al:
Invasion and metastasis in colorectal cancer:
epithelial-mesenchymal transition, mesenchymal: Epithelial
transition, stem cells and β-catenin. Cells Tissues Organs.
179:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morin PJ and Weeraratna AT: Wnt signaling
in human cancer. Cancer Treat Res. 115:169–187. 2003.PubMed/NCBI
|
|
22
|
Taketo MM: Shutting down Wnt
signal-activated cancer. Nat Genet. 36:320–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamamoto Y, Sakamoto M, Fujii G, et al:
Overexpression of orphan G-protein-coupled receptor, Gpr49, in
human hepatocellular carcinomas with beta-catenin mutations.
Hepatology. 37:528–533. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McClanahan T, Koseoglu S, Smith K, et al:
Identification of overexpression of orphan G protein-coupled
receptor GPR49 in human colon and ovarian primary tumors. Cancer
Biol Ther. 5:419–426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carmon KS, Lin Q, Gong X, et al: LGR5
interacts and cointernalizes with Wnt receptors to modulate
Wnt/β-catenin signaling. Mol Cell Biol. 32:2054–2064. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al-Kharusi MR, Smartt HJ, Greenhough A, et
al: LGR5 promotes survival in human colorectal adenoma cells and is
upregulated by PGE2: Implications for targeting adenoma stem cells
with NSAIDs. Carcinogenesis. 34:1150–1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garcia MI, Ghiani M, Lefort A, et al: LGR5
deficiency deregulates Wnt signaling and leads to precocious Paneth
cell differentiation in the fetal intestine. Dev Biol. 331:58–67.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uchida H, Yamazaki K, Fukuma M, et al:
Overexpression of leucine-rich repeat-containing G protein-coupled
receptor 5 in colorectal cancer. Cancer Sci. 101:1731–1737. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi H, Ishii H, Nishida N, et al:
Significance of Lgr5(+ve) cancer stem cells in the colon and
rectum. Ann Surg Oncol. 18:1166–1174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang M, Zhong WW and Srivastava N: G
protein-coupled lysophosphatidic acid receptors stimulate
proliferation of colon cancer cells through the {beta}-catenin
pathway. Proc Natl Acad Sci USA. 102:6027–6032. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu HC, Liu YS, Tseng KC, et al:
Overexpression of Lgr5 correlates with resistance to 5-FU-based
chemotherapy in colorectal cancer. Int J Colorectal Dis.
28:1535–1546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hirsch D, Barker N, McNeil N, et al: LGR5
positivity defines stem-like cells in colorectal cancer.
Carcinogenesis. 35:849–858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Walker F, Zhang HH, Odorizzi A and Burgess
AW: LGR5 is a negative regulator of tumourigenicity, antagonizes
Wnt signalling and regulates cell adhesion in colorectal cancer
cell lines. PLoS One. 6:e227332011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanese K, Fukuma M, Yamada T, et al:
G-protein-coupled receptor GPR49 is up-regulated in basal cell
carcinoma and promotes cell proliferation and tumor formation. Am J
Pathol. 173:835–843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan XS, Wu HY, Yu HP, et al: Expression of
Lgr5 in human colorectal carcinogenesis and its potential
correlation with beta-catenin. Int J Colorectal Dis. 25:583–590.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Scannell CA, Pedersen EA, Mosher JT, et
al: LGR5 is expressed by Ewing sarcoma and potentiates
Wnt/β-catenin signaling. Front Oncol. 3:812013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang D, Zhou J, Fan C, et al: Knockdown of
LGR5 suppresses the proliferation of glioma cells in vitro and in
vivo. Oncol Rep. 31:41–49. 2014.PubMed/NCBI
|