Introduction
Osteosarcoma (OS) is the eighth most common
malignancy in children and young adolescents, and accounts for 2.4%
of all pediatric malignancies. Following diagnosis, the mean
survival rate of patients with OS is less than five years (1,2). In total,
~20% of patients are at the metastatic lung stage at the time of
diagnosis. Unsuccessful treatment strategies lead to mortality.
Therefore, an understanding of the molecular mechanisms that
underlie tumorigenesis is important. Previous studies concerning a
number of different cancers have identified the existence of a
small sub-population of cancer-initiating cells known as cancer
stem cells (CSCs), which exhibit stem cell-like properties and are
responsible for tumor metastasis, invasion and chemotherapy
resistance (3–8). The existence of CSCs has also been
demonstrated in human OS, which possesses characteristic features
of stem cells and expresses the octamer-binding transcription
factor (Oct)3/4A and Nanog stem cell surface markers (9,10). A
noteworthy feature of these cells is their ability to efflux
Hoechst 3342 dye and other chemotherapeutic drugs (10), which ultimately results in
chemoresistance and tumor recurrence.
CSCs can be isolated based upon the expression of
stem cell surface markers, such as cluster of differentiation
(CD)44 and CD133 (11), or by the
Hoechst 33342 dye exclusion technique by fluorescence-activated
cell sorting (FACS) (12). With FACS,
the cells that exclude Hoechst 33342 dye are named the side
population (SP) cells. Another technique is the sphere formation
assay, in which individual cancer cells form a spherical colony in
a defined serum-free medium (10). In
each of these methods, the isolated cancer stem-like cells exhibit
resistance to chemotherapy and apoptosis, a high level of
self-renewal and a higher level of stem cell surface markers.
However, the molecular and signaling pathways involved in these
functions are yet to be elucidated. The present study attempted to
isolate and characterize the stem-like cells from the OS-55 cell
line. The isolated cancer stem-like cells were further analyzed for
the expression of the stem cell surface markers, Oct-3/4A, CD44 and
Nanog.
Materials and methods
Cell lines and culture conditions
Samples of stage III aggressive primary human OS
with lung metastasis (OS-55 cells) were obtained from the American
Type Culture Collection (Manassas, VA, USA). Subsequent to
isolation, the samples were washed in phosphate-buffered saline
(PBS) containing antibiotics, and then incubated overnight in
Dulbecco's modified Eagle's medium (DMEM)/F12 (Gibco Life
Technologies, Carlsbad, CA, USA) containing 500 U/ml penicillin,
500 µg/ml streptomycin and 1.25 µg/ml amphotericin B (Gibco Life
Technologies). Enzymatic digestion was performed using 1.5 mg/ml
collagenase (Gibco Life Technologies) and 20 µg/ml hyaluronidase in
PBS for 1 h. Next, the cells were cultured in DMEM with 10% fetal
bovine serum (Sigma-Aldrich, St. Louis, MO, USA) supplemented with
antibiotics, and then maintained in T-75 flasks at 37°C in a
humidified 5% CO2 and 95% air atmosphere. The cells that
reached 90% confluency were removed from the culture flask using
Trypsin-EDTA (0.25% Trypsin/53 mM EDTA; Sigma-Aldrich), washed and
then suspended in 10% DMEM. The cell count was performed using a
hemocytometer.
FACS analysis
The experimental groups were as follows: i) A
control group consisting of cells treated with Hoechst 33342 dye
(n=7); and ii) a drug-treated group consisting of cells treated
with verapamil (G.D. Searle LLC Division of Pfizer Inc., Chicago,
IL, USA) and Hoechst 33342 dye (n=7). In total, ~106
cells/ml, which had been incubated with 10% DMEM, were labeled with
Hoechst 33342 stock containing 5 µl/ml bis-benzimide
(Sigma-Aldrich), alone or in combination with 0.8 µl/ml verapamil.
Next, the cells were resuspended in 500 µl Hank's balanced salt
solution containing 10 mM HEPES (Sigma-Aldrich) for FACS analysis.
Finally, the cells were counterstained with 2 µg/ml propidium
iodide (Sigma-Aldrich).
Sarcosphere formation assay
A sphere formation assay was performed as previously
described (10). Firstly, the cells
were plated at a density of 60,000 cells/well in ultra-low
attachment six-well plates containing serum-free DMEM/F12 medium
supplemented with N-2, 10 ng/ml epidermal growth factor and 10
ng/ml human basic fibroblast growth factor. The culture was then
analyzed for sphere formation each day until day seven. Images were
captured using an inverted phase contrast microscope (Eclipse
TS100; Nikon Corporation, Tokyo, Japan). Subsequent to seven days
of culturing, the total number of spheres (also known as
sarcospheres) were generated using FACS. The sorted SP and non-SP
cells were then quantified.
Immunofluorescence staining
The sorted OS-55 SP and non-SP cells were fixed in
Cytofix™ solution (BD Biosciences, Franklin Lakes, NJ, USA) and
incubated for 20 min at 4°C. Subsequent to blocking in donkey serum
(Sigma-Aldrich) for 20 min, the cells were incubated with a goat
anti-Oct3/4A antibody (dilution, 1:200; cat. no. sc-8628; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. Next,
the cells were incubated with a rhodamine red-conjugated donkey
anti-goat antibody (dilution, 1:200; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA). For the CD44 and Nanog
immunofluorescence analysis, the cells were stained with a
fluorescein isothiocyanate-conjugated anti-human Nanog or CD44
antibody (dilution, 1:5; eBioscience, Inc., San Diego, CA, USA).
Human embryonic stem cells were used as a positive control.
Finally, the cells were observed under a confocal fluorescence
microscope (LSM 150; Zeiss, Oberkochen, Germany), and images were
analyzed and processed using Adobe Photoshop CS4 (Adobe Systems,
Inc., San Jose, CA, USA).
Statistical analysis
A one-way analysis of variance and Student's t-test
were performed in order to identify any significant differences
between the treatment and control groups. P<0.01 was considered
to indicate a statistically significant difference.
Results
The present study analyzed the human OS-55 cell line
using Hoechst 33342 dye exclusion by FACS in order to identify and
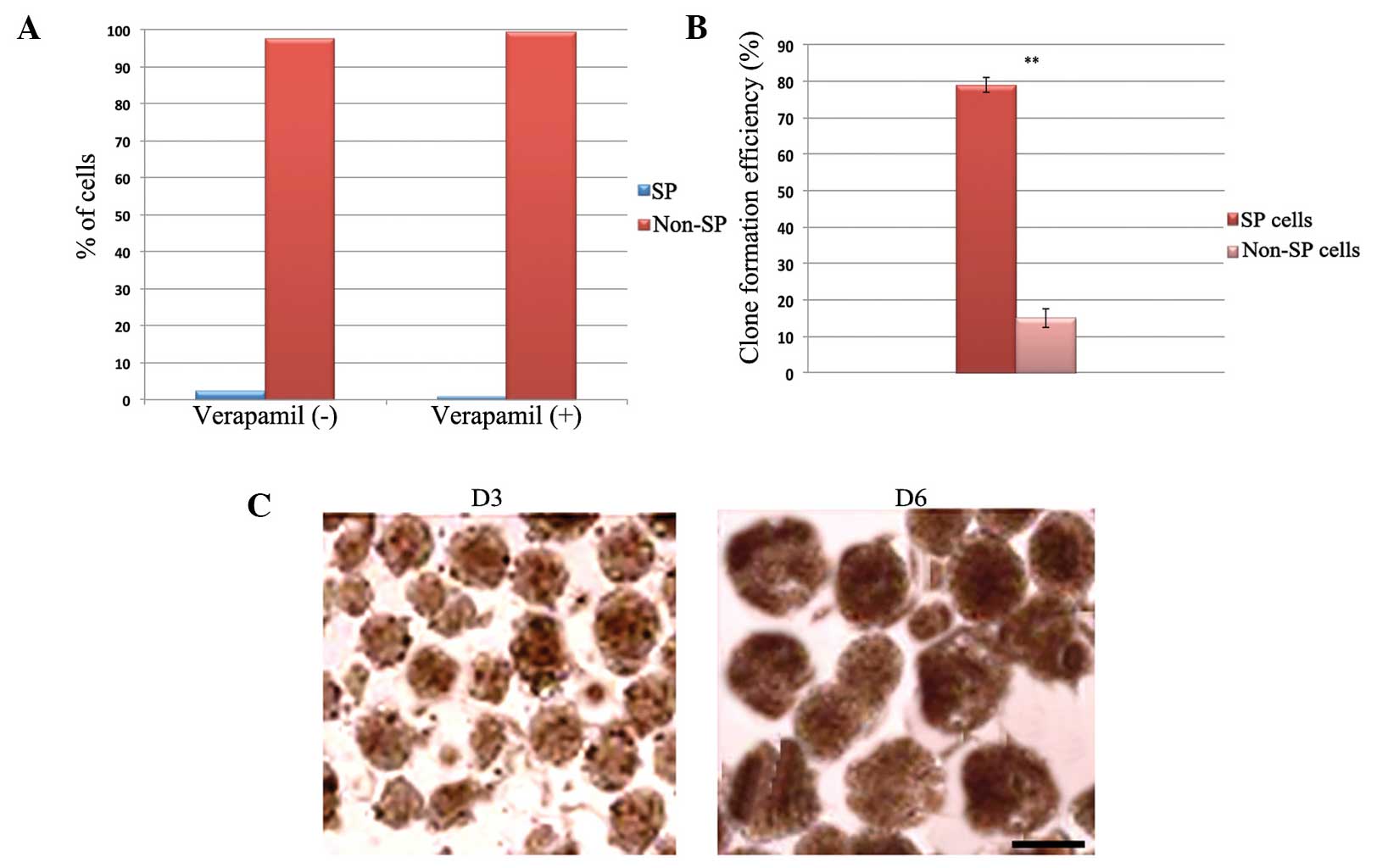
characterize the small stem-like population of cells. As shown in
Fig. 1A, it was identified that ~2.3%
of the OS-55 cells were cancer stem-like SP cells. The percentage
of SP cells decreased to ~0.7% following treatment with verapamil,
which blocks the action of adenosine triphosphate-binding cassette
(ABC) transporters. This confirms the property of drug exclusion by
the cancer stem cells, which, as with other solid tumors, express
ABC transporter proteins. The sorted OS-55 SP and non-SP cells were
also examined in order to investigate their sarcosphere-forming
ability. The total number of sarcospheres formed by the SP cells
was significantly higher than that of the non-SP cells (Fig. 1B). SP cells rapidly formed spheres on
day three, which increased in size over time (Fig. 1C). In addition, the sorted SP and
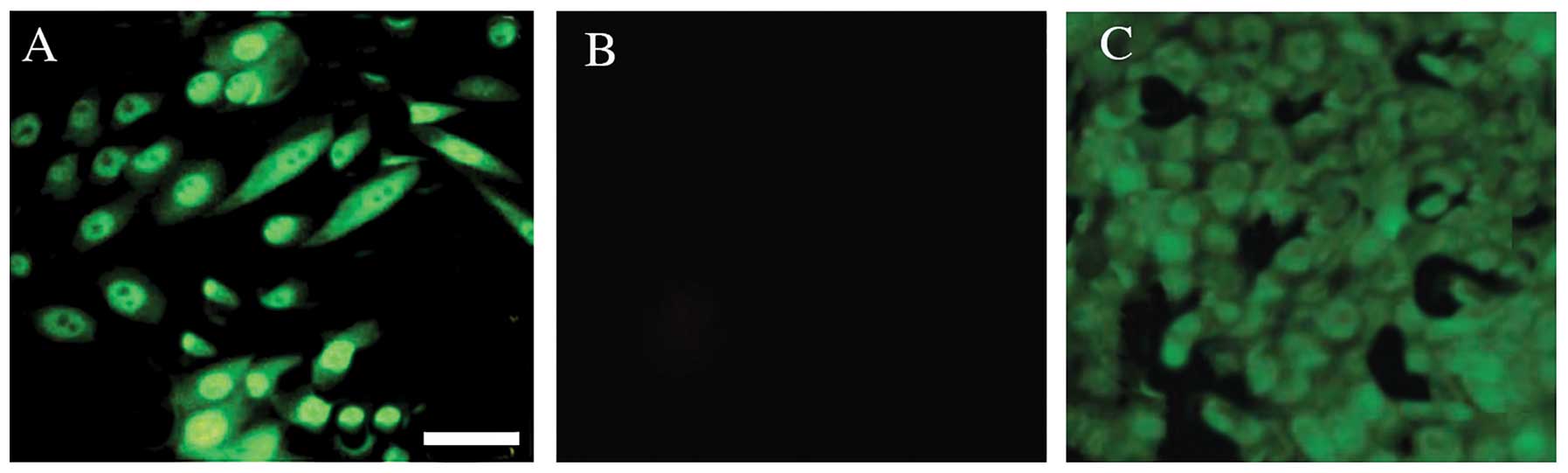
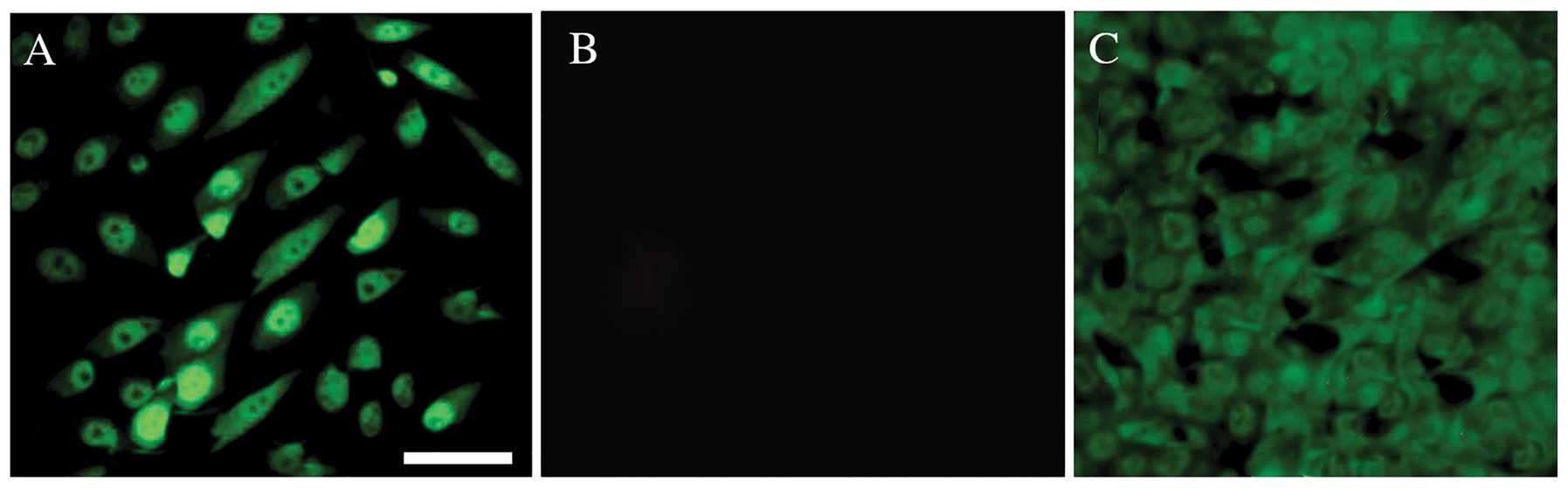
non-SP cells were subjected to immunocytochemical analysis in order
to examine the expression of stem cell surface markers. Using
confocal microscopy, the bright nuclear staining of Oct3/4A
(Fig. 2) and Nanog (Fig. 3) was detected in the SP cells.
Furthermore, cytoplasmic CD44 (Fig.
4), which was evident in the cancer stem-like SP cells, also
demonstrated high expression in the SP cells. However, very little
or null expression of the stem cell surface markers was observed in
the non-SP cells. Therefore, these data suggested that the human
OS-55 cell line contains a small population of cancer stem-like
cells, which demonstrate high levels of self-renewal and drug
resistance.
Discussion
A number of previous studies have identified the
presence of a small population of tumor-initiating cells known as
CSCs, which are able to initiate rapid tumor proliferation
(3,13,14). These
cells possess stem cell-like features and are responsible for
chemotherapy resistance and tumor recurrence following conventional
treatment strategies (8). In
addition, the overexpression of ABC transporters, such as ABCG2, in
CSCs actively contributes to multi-drug resistance and results in
treatment failure. Therefore, it is essential to identify the
characteristic features of CSCs in order to provide effective
treatment strategies. In the present study, it was revealed that
~2.3% of the human OS-55 cell population were cancer stem-like SP
cells. This decreased to 0.7% following treatment with verapamil,
which confirmed an overexpression of ABC transporters in the SP
cells. The sphere formation assay is able to determine the
self-renewal capacity of CSCs in several types of tumor (5). The results of the present study
demonstrated that the sorted SP cells rapidly formed sarcospheres
on day three, and that the size of the spheres increased with time.
Furthermore, the cancer stem-like SP cells generated a larger
number of sarcospheres than the non-SP cells. In accordance with
these findings, a previous study demonstrated that OS OS99-1 and
MG63 cells were able to form sarcospheres (15).
Oct3/4A, CD44 and Nanog are markers associated with
stem cell-like properties, including self-renewal, pluripotency,
tumorigenesis and tumor invasion (14,16,17). An
overexpression of these factors appears to increase the
self-renewal capacity of cells. The significance of these proteins
has been well documented in a number of human cancers (18,19). A
previous study reported the elevated expression of Oct3/4A, Oct3/4B
and Nanog mRNA in the human OS99-1 cell line compared with three
other cell lines that were analyzed (15). The positive expression of Oct3/4A,
Nanong and CD44 can be used as a specific marker for the diagnosis
of cancer metastasis (20). It was
also identified that Oct3/4A and Nanog are highly expressed in OS
CSCs (15) and are involved in tumor
metastasis. In accordance with these findings, the present study
demonstrated that human OS-55 cells have enhanced Oct3/4A and Nanog
expression in the nuclei of the cancer stem-like SP cells compared
with the non-SP cells. CD44 is a member of a family of cell surface
proteoglycans and glycoproteins, which have roles in tumor
invasion, metastasis and resistance to chemotherapy and
radiotherapy (9,21–23). A
previous study that investigated nasopharyngeal carcinoma
demonstrated an increased expression of CD44-positive cells that
may have initiated increased rates of tumorigenesis and metastasis
(11). In accordance with these
findings, the present study revealed that the cancer stem-like SP
cells exhibited an increased level of CD44 expression compared with
the non-SP cells. Alongside ABCG2, CD44 may also contribute to
chemotherapy resistance, tumorigenesis and the invasion of OS-55
cells. The data from the present study demonstrated the existence
of cancer stem-like cells in the human OS-55 cell line. These cells
possess stem cell features and high levels of self-renewal, and
therefore, may be involved in tumor recurrence and metastasis.
However, the functional interaction between the stem cell surface
proteins, anti-apoptotic factors and ABC transporter proteins
requires further investigation.
References
|
1
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Damron TA, Ward WG and Stewart A:
Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National Cancer
Database report. Clin Orthop Relat Res. 459:40–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
et al: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hemmati HD, Nakano I, Lazareff JA,
Masterman-Smith M, et al: Cancerous stem cells can arise from
pediatric brain tumors. In: Proc Natl Acad Sci USA. 100. pp.
15178–15183. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. In: Proc Natl Acad Sci USA. 100.
pp. 3983–3988. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li C, Heidt DG, Dalerba P, et al:
Identification of pancreatic cancer stem cells. Cancer Res.
67:1030–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. In: Proc Natl Acad Sci USA. 101. pp.
781–786. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu C, Wei Q, Utomo V, Nadesan P, et al:
Side population cells isolated from mesenchymal neoplasms have
tumor initiating potential. Cancer Res. 67:8216–8222. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gibbs CP, Kukekov VG, Reith JD, et al:
Stem-like cells in bone sarcomas: implications for tumorigenesis.
Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su J, Xu XH, Huang Q, et al:
Identification of cancer stem-like CD44+ cells in human
nasopharyngeal carcinoma cell line. Arch Med Res. 42:15–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goodell MA, Brose K, Paradis G, et al:
Isolation and functional properties of murine hematopoietic stem
cells that are replicating in vivo. J Exp Med. 183:1797–1806. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pardal R, Clarke MF and Morrison SJ:
Applying the principles of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang CC: Recent translational research:
stem cells as the roots of breast cancer. Breast Cancer Res.
8:1032006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Park P and Lin CY:
Characterization of stem cell attributes in human osteosarcoma cell
lines. Cancer Biol Ther. 8:543–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee J, Kim HK, Rho JY, Han YM and Kim J:
The human OCT-4 isoforms differ in their ability to confer
self-renewal. J Biol Chem. 281:33554–33565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okita K, Ichisaka T and Yamanaka S:
Generation of germline-competent induced pluripotent stem cells.
Nature. 448:313–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ezeh UI, Turek PJ, Reijo RA and Clark AT:
Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are
expressed in both seminoma and breast carcinoma. Cancer.
104:2255–2265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Looijenga LH, Stoop H, de Leeuw HP, et al:
POU5F1 (OCT3/4) identifies cells with pluripotent potential in
human germ cell tumors. Cancer Res. 63:2244–2250. 2003.PubMed/NCBI
|
|
20
|
Cheng L: Establishing a germ cell origin
for metastatic tumors using OCT4 immunohistochemistry. Cancer.
101:2006–2010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Setoguchi T, Taga T and Kondo T: Cancer
stem cells persist in many cancer cell lines. Cell Cycle.
3:414–415. 2004.PubMed/NCBI
|
|
22
|
Pesce M and Schöler HR: Oct-4: gatekeeper
in the beginnings of mammalian development. Stem Cells. 19:271–278.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodda DJ, Chew JL, Lim LH, et al:
Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem.
280:24731–24737. 2005. View Article : Google Scholar : PubMed/NCBI
|