Introduction
Primary hepatocellular carcinoma (HCC) is the third
most common cause of cancer-associated death worldwide, with 85% of
cases occurring in developing countries. Primary HCC has also
become the fastest growing cause of cancer-associated death in the
United States of America (1,2). HCC is classified into four subtypes:
Massive, nodular, diffuse and small HCC, among which the massive
type (≥10 cm) is most common (3).
Surgery is the curative modality of treatment for patients
presenting with a solitary lesion without vascular invasion, and
with sufficient underlying liver function (4). However, due to the large volume of
massive HCC lesions, major blood vessels, including the portal
vein, hepatic artery and vena cava are frequently invaded (5). In addition, the majority of patients
with HCC suffer from cirrhosis or abnormal liver function, which
may also result in difficulties for surgical intervention (6). Thus, in the majority of massive HCC
cases, surgery is unfeasible due to the disease burden or location,
comorbidities or inadequate functional liver reserve (4).
Despite the fact that liver tumors are sensitive to
the effects of radiation, traditional radiotherapy has not had a
significant role in the treatment of HCC. This is primarily due to
the challenges associated with the delivery of a sufficient dose of
radiation to the target to be tumoricidal, without inducing
significant toxicity (7). Following
the development of modern stereotactic radiotherapy techniques, the
safe delivery of radiation to the liver has become more achievable,
and radiotherapy has had a growing role in the local treatment of
HCC (6,8,9). However,
due to the large volume of massive HCC lesions and the poor
radiation tolerance of normal liver tissue, increasing the
radiation dose has remained an issue and thus the curative effect
of radiotherapy is limited (10).
Therefore, the delivery of higher tumoricidal radiation doses to
focal HCC, with low rates of toxicity is a desirable development to
improve existing treatments (11). In
the present study, alternating hyperfraction radiotherapy was
compared with regular intensity modulated radiotherapy (IMRT)
treatment in the treatment of patients with massive HCC. The
results may provide evidence for the development of a novel
radiotherapy treatment strategy for massive HCC.
Materials and methods
Patient characteristics
The present prospective study of alternating
hyperfraction radiotherapy for patients with massive HCCs was
approved by the ethics committee of the Fifth Hospital of Wuhan
(Wuhan, Hubei, China) and was initiated in May 2008. Written
informed consent was obtained from the patients for participation
in the study. Massive HCCs were defined as those with a maximum
tumor diameter of ≥10 cm (12–14). The
72 patients, recruited between May 2008 and June 2011 at the Fifth
Hospital of Wuhan, comprised 40 males and 32 females, with an age
range of 38–65 years, and a mean age of 54.2 years. The tumor
sizes, determined using contrast-enhanced magnetic resonance
imaging (MRI; Achieva 1.5T, Philips Medical Systems, Best,
Netherlands) by the radiologist, were 10–20 cm in diameter. The
eligibility criteria for patients were: Karnofsky performance
status score, ≥70 (15); Child-Pugh
class, A or B (16); serum glutamic
pyruvic transaminase, <100 U/l; serum glutamic-oxaloacetic
aminotransferase, <100 U/l; serum total bilirubin, <170
µmol/l; ultrasonic examination without massive ascites and an
absence of intrahepatic metastasis or other distant metastasis.
Radiotherapy equipment
An Elekta electron linear precise accelerator
[configuration iView-GT portal imaging verification system, 40
pairs of electric MLC; Elekta AB (Publ), Stockholm, Sweden], Xinhua
SL-11 simulator (Shandong Xinhua Medical Instrument Co., Ltd,
Shandong, China), Philips 64-slice spiral computed tomography
scanner (Brilliance 64, Philips Medical Systems), Elekta Precise
three-dimensional (3D) treatment planning system [Elekta AB
(Publ)], immobilization vacuum pad (Shanghai Gerui Co., Ltd,
Shanghai, China) and an abdominal bandage (Hengshui Runde Medical
Instrument Co., Ltd, Hebei, China) were used in the present
study.
Radiotherapy treatment protocol
The patients were randomly divided into two groups
(n=36): group A and group B. For the alternating hyperfractionated
intensity modulated conformal radiotherapy treatment (group A), the
liver lesion was divided into two sublesions, gross tumor volume
(GTV)1 and GTV2, based on the anatomical features of the tumor. The
interval between the radiotherapy treatment of the GTV1 and GTV2
sublesions was ≥6 h, to avoid radiation hot spots. The average
radiotherapy dose of the sublesions was 2 Gy/fraction, once a day,
five times per week for 4–5 weeks. The GTV received a total dose of
40–50 Gy, and the clinical target volume (CTV) received a total
dose of 30–40 Gy. For the regular intensity modulated conformal
radiotherapy group (group B), the liver lesions were treated with
intensity modulated radiotherapy (IMRT) at a dose of 2 Gy/fraction,
once a day, five times per week for 4–5 weeks. The GTV was treated
with a total dose of 40–50 Gy, and the CTV received a total dose of
30–40 Gy. Radiotherapy was performed with the aforementioned Elekta
electron linear accelerator and precise treatment planning system.
The abdominal bandage was used to control breathing during
radiotherapy, in order to reduce tumor motion. Target volume
delineation was performed by the same doctor and medical physicist
for all patients. To perform palliative radiotherapy, an electronic
image treatment verification system [Elekta AB (Publ)] was used to
improve the precision. Based on the CTV, the crown sagittal axis of
the planning target area (PTV) was extended outwards by 3 mm and
the long axis of the body was extended outwards by 5 mm. In total,
7–9 irradiation fields were designed, and 85–90% of the isodose
curve covered the PTV. Protection was provided for the normal liver
and adjacent organs (stomach, duodenum, pancreas, kidneys) as much
as possible, using the Elekta Precise 3D treatment planning system.
During radiotherapy, the patients were treated with liver
protection drugs glutathione (1.2–1.8 g/day) and magnesium
isoglycyrrhizinate (0.1–0.3 g/day). Regular inspections of
peripheral blood and liver function were conducted during the
course of treatment.
Follow up
Patients were monitored over the 2–3 months
following treatment, and trimonthly thereafter. Regular blood
tests, liver function tests, measurements of serum α-fetoprotein
(AFP) and contrast-enhanced MRI (Achieva 1.5T, Philips Medical
Systems) of the liver were performed at every follow-up
appointment. Treatment responses were evaluated by MRI every 3
months, using the modified Response Evaluation Criteria in Solid
Tumors (17). The treatment responses
were defined as follows: Complete regression (CR), total
disappearance of the tumor; partial regression (PR), a decrease of
>50% of the tumor size; stable disease (SD), a decrease of
<50% of the tumor or no change in tumor volume; progressive
disease (PD), tumor progression (18). Adverse reactions to radiotherapy were
evaluated by the standard criteria of the American Radiation
Therapy Oncology Group (19).
Survival was evaluated from the date of commencement of the
treatment (20). The follow-up was
completed in June 2014.
Statistics analysis
SPSS 17.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Effective rates,
survival rates and the incidence of adverse reactions were analyzed
using the χ2 test. The Kaplan-Meier method was used to
estimate survival rates, and the log-rank test was used to compare
differences in survival. A two-sided P<0.05 was considered to
indicate a statistically significant difference.
Results
Treatment outcomes
One patient in group A was unable to complete the
radiotherapy treatment due to upper gastrointestinal hemorrhage.
Four patients in group B did not complete the radiotherapy course
as a result of severe reactions. The follow-up rate was 100%
amongst the remaining patients. The results of the follow-up 2–3
months subsequent to treatment revealed that the average values of
serum AFP for groups A and B were 286.62±114.81 and 299.67±115.03
µg/l, respectively, compared with 557.78±142.08 and 547.02±151.01
µg/l, respectively, prior to treatment. The changes in serum AFP of
the two groups were statistically significant (P=0.000; Table I). The total effective rate of
treatment was 82.9% (29/35) in group A, and the CR, PR, SD and PD
rates were 5.7 (2/35), 28.6 (10/35), 48.6 (17/35) and 17.1% (6/35),
respectively. In group B, the total effective rate was 81.3%
(26/32), and the CR, PR, SD and PD rates were 3.1 (1/32), 21.9
(7/32), 56.3 (18/32) and 18.7% (6/32), respectively. The overall
response rates of the two groups were equivalent (P=0.864; Table II).
 | Table I.The changes in serum AFP of groups A
(n=35) and B (n=32) to radiotherapy. |
Table I.
The changes in serum AFP of groups A
(n=35) and B (n=32) to radiotherapy.
|
| Serum AFP (mean ±
SD), µg/l |
|
|---|
|
|
|
|
|---|
| Group | Before treatment | After treatment | P-values |
|---|
| A |
557.78±142.08 |
286.62±114.81 | <0.0001 |
| B |
547.02±151.01 |
299.67±115.03 | <0.0001 |
 | Table II.Treatment responses of groups A (n=35)
and B (n=32) to radiotherapy. |
Table II.
Treatment responses of groups A (n=35)
and B (n=32) to radiotherapy.
|
| Patients, % (n) |
|
|---|
|
|
|
|
|---|
| Treatment
response | Group A | Group B | P-value |
|---|
| CR | 5.7 (2) | 3.1 (1) |
|
| PR | 28.6 (10) | 21.9 (7) |
|
| SD | 48.6 (17) | 56.3 (18) |
|
| PD | 17.1 (6) | 18.7 (6) |
|
| CR+PR+SD | 82.9 (29) | 81.3 (26) | 0.864 |
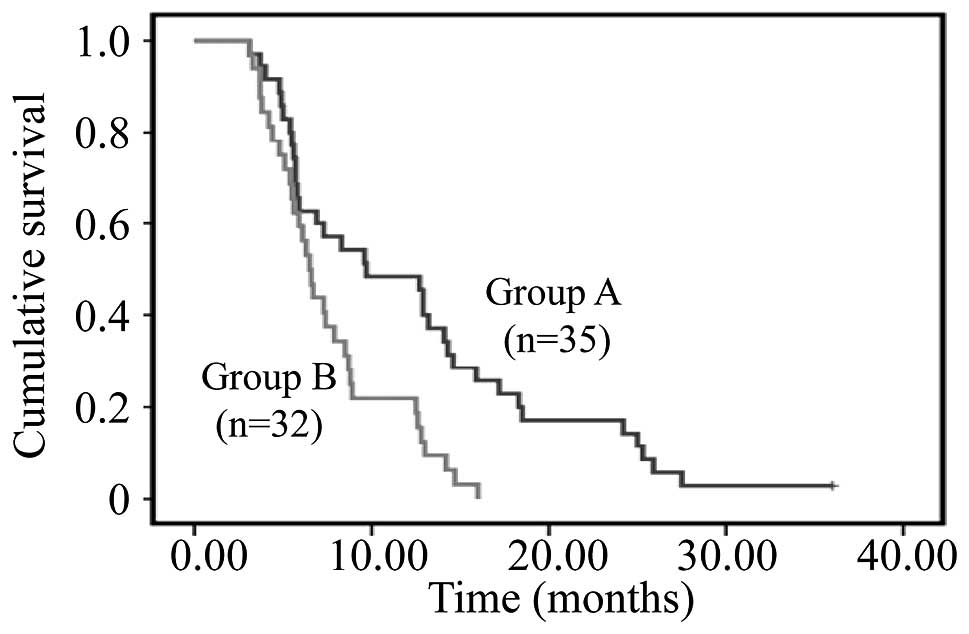
The follow-up ended in June 2014. The median
survival time of patients in group A was 9.7 months, compared with
6.5 months in those of group B. The survival time of group A was
significantly longer than that of group B (P=0.002; Fig. 1). The 6-month, 1-year, 2-year and
3-year overall survival rates of the two groups were 62.9 and 59.4%
(P=0.770), 48.6 and 21.9% (P=0.040), 17.1 and 0.0% (P=0.025) and
2.9 and 0.0% (P=1.000), respectively.
Treatment-associated toxicity
The major toxic effects of radiotherapy during the
treatment were gastrointestinal reactions, abnormal liver function
and myelosuppression. The I-II degree gastrointestinal reactions
and I-II degree myelosuppression of the two groups were similar
(Table III). However, the I-II
degree of abnormal liver function and radiation-induced liver
disease (RILD) of group B were significantly higher than that of
group A (P=0.021 and 0.046, respectively; Tables I and III). RILD was determined by an elevation
of alkaline phosphatase level of ≥2-fold and/or elevated
transaminases of ≥4-fold the upper limit of normal levels (18).
 | Table III.Adverse reactions of groups A (n=35)
and B (n=32) to radiotherapy. |
Table III.
Adverse reactions of groups A (n=35)
and B (n=32) to radiotherapy.
| Adverse
reactiona | Group A, % (n) | Group B, % (n) | P-values |
|---|
| I-II degree
gastrointestinal reactions | 82.9 (29) | 81.3 (26) | 0.864 |
| I-II degree abnormal
liver function | 62.9 (22) | 87.5 (28) | 0.021 |
| I-II degree
myelosuppression | 65.7 (23) | 71.9 (23) | 0.587 |
| Radiation-induced
liver disease | 11.4 (4) | 31.3 (10) | 0.046 |
Discussion
HCC is one of the most common types of cancer
worldwide, and it is frequently not diagnosed until an advanced
stage when the majority of potentially curative therapies, for
example resection, transplantation or transarterial interventions,
are of limited efficacy (21,22). Previously, due to the low tolerance of
normal liver tissues to radiation, the use of radiotherapy for the
treatment of HCC has been limited (23). Following the development of modern
computers and medical imaging technology, radiation therapy of
tumors has entered a novel era of 3D conformal radiotherapy
(3D-CRT). 3D-CRT ensures that the target receives identical
high-dose conformal irradiation, so that the radiation dose to the
surrounding normal tissue is markedly reduced, thereby generating
the conditions for the incremental target area (24). It was demonstrated that there was a
dose-response association in local radiotherapy for primary HCC,
and thus 3D-CRT may potentially be used for the treatment of
primary HCC (25,26). In addition, stereotactic body
radiotherapy has emerged as a viable treatment option for patients
with liver tumors unsuitable for surgery, liver transplantation, or
radiofrequency ablation (20).
Recently, radiotherapy technology has evolved from
3D-CRT to a more advanced form, termed IMRT, which facilitates the
application of a substantial dose of radiation to a tumor whilst
avoiding damage to local radiosensitive organs (27). IMRT may enhance the quality of
radiation plans by utilizing an inverse planning algorithm to
generate complex spatial dose distributions and, therefore, conform
more closely to the target volume (27). It was demonstrated that IMRT may be an
effective treatment for locally advanced HCC, which provides
survival benefit without increasing severe toxicity (25). IMRT achieved a significantly higher
conformal index and lower hot spot values than those of 3D-CRT
(26). However, it was reported that,
for tumors of diameter >8 cm, the value of mean dose for 3D-CRT
was lower than that of IMRT, indicating that 3D-CRT was a more
suitable strategy for the treatment of larger tumors (28). For massive HCC, a larger volume of
normal liver is irradiated, and thus the majority of patients
suffer from cirrhosis and abnormal liver function. This makes it
more difficult to deliver high-dose radiation to the localized
tumor area without significantly damaging the surrounding normal
liver tissue. Thus, dose and volume reduction for the normal liver
is required in order to improve the effects of radiotherapy for
massive HCC. In addition, HCC is an early response tissue, whereas
normal liver is a late response tissue (29). According to the principle of
radiobiology, in order to reduce the radiation damage to the normal
liver, the split dose should generally be ≤2 Gy (30).
In the present study, the massive liver lesions of
group A were divided into sublesions, GTV1 and GTV2, and
alternating hyperfraction IMRT was designed to treat massive HCC.
The interval of the radiotherapy to the sublesions was ≥6 h. The
average radiotherapy dose to the sublesions was 2 Gy/fraction, once
a day, five times per week, with a total dose of 30–40 Gy. The
results revealed that the alternating hyperfraction radiotherapy
treatment strategy resulted in enhanced survival rates and reduced
risk of I-II degree of abnormal liver function and RILD. The
alternating hyperfraction IMRT protocol may alleviate the overall
damage to the normal liver by reducing the radiation exposure and
providing a greater period of time for repair, whilst ensuring the
successful completion of radiotherapy for the tumor. In addition,
an abdominal bandage was used to control breathing during
radiotherapy in order to reduce tumor motion, thereby circumventing
additional radiation-induced normal liver injury. Therefore,
alternating hyperfraction provides an improved radiation pattern
for the treatment of massive HCC. RILD is the most severe
complication induced by radiotherapy, and may result in hepatic
failure and death. Furthermore, it has been demonstrated that HCC
may be more safely treated with spot-scanning proton therapy, in
order to reduce the risk of RILD, particularly if the nominal tumor
diameter is >6.3 cm (31).
Therefore, this alternating hyperfraction radiotherapy method may
offer more effective outcomes for the treatment of HCC in the
future.
In conclusion, the present study revealed that,
compared with intensity modulated radiotherapy, the use of
alternating hyperfraction radiotherapy may reduce the incidence
rate of radiation-induced liver injury, improve patient quality of
life and prolong the survival time, suggesting that it is an
effective radiation pattern for the treatment of massive HCC.
Acknowledgements
The present study was supported by the Wuhan Public
Health Bureau, China (grant no. WX13C36).
References
|
1
|
Center MM and Jemal A: International
trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers
Prev. 20:2362–2368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Willatt JM, Hussain HK, Adusumilli S and
Marrero JA: MR Imaging of hepatocellular carcinoma in the cirrhotic
liver: Challenges and controversies. Radiology. 247:311–330. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aitken KL and Hawkins MA: The role of
radiotherapy and chemoradiation in the management of primary liver
tumours. Clin Oncol (R Coll Radiol). 26:569–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vauthey JN, Lauwers GY, Esnaola NF, Do KA,
Belghiti J, Mirza N, et al: Simplified staging for hepatocellular
carcinoma. J Clin Oncol. 20:1527–1536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klein J and Dawson LA: Hepatocellular
carcinoma radiation therapy: Review of evidence and future
opportunities. Int J Radiat Oncol Biol Phys. 87:22–32. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lock MI, Hoyer M, Bydder SA, et al: An
international survey on liver metastases radiotherapy. Acta Oncol.
51:568–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Culleton S, Jiang H, Haddad CR, et al:
Outcomes following definitive stereotactic body radiotherapy for
patients with Child-Pugh B or C hepatocellular carcinoma. Radiother
Oncol. 111:412–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeng ZC, Tang ZY, Fan J, et al: A
comparison of chemoembolization combination with and without
radiotherapy for unresectable hepatocellular carcinoma. Cancer J.
10:307–316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park HC, Seong J, Tanaka M, et al:
Multidisciplinary management of nonresectable hepatocellular
carcinoma. Oncology. 81(Suppl 1): S134–S140. 2011.
|
|
12
|
Carr BI and Guerra V: Features of massive
hepatocellular carcinomas. Eur J Gastroenterol Hepatol. 26:101–108.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carr BI, Guerra V and Pancoska P:
Thrombocytopenia in relation to tumor size in patients with
hepatocellular carcinoma. Oncology. 83:339–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carr BI, Guerra V, de Giorgio M, Fagiuoli
S and Pancoska P: Small hepatocellular carcinomas and
thrombocytopenia. Oncology. 83:331–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Péus D, Newcomb N and Hofer S: Appraisal
of the Karnofsky Performance Status and proposal of a simple
algorithmic system for its evaluation. BMC Med Inform Decis Mak.
13:722013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwartz M, Roayaie S and Konstadoulakis
M: Strategies for the management of hepatocellular carcinoma. Nat
Clin Pract Oncol. 4:424–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B, Yu J, Wang L, et al: Study of local
three-dimensional conformal radiotherapy combined with
transcatheter arterial chemoembolization for patients with stage
III hepatocellular carcinoma. Am J Clin Oncol. 26:e92–e99. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the Radiation Therapy Oncology Group (RTOG) and the
European Organization for Research and Treatment of Cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamashita H, Onishi H, Matsumoto Y, et al:
Local effect of stereotactic body radiotherapy for primary and
metastatic liver tumors in 130 Japanese patients. Radiat Oncol.
9:1122014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Avila MA, Berasain C, Sangro B and Prieto
J: New therapies for hepatocellular carcinoma. Oncogene.
25:3866–3884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peck-Radosavljevic M: Hepatocellular
carcinoma: The place of new medical therapies. Therap Adv
Gastroenterol. 3:259–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lawrence TS, Robertson JM, Anscher MS,
Jirtle RL, Ensminger WD and Fajardo LF: Hepatic toxicity resulting
from cancer treatment. Int J Radiat Oncol Biol Phys. 31:1237–1248.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Purdy JA: 3D treatment planning and
intensity-modulated radiation therapy. Oncology (Williston Park).
13:155–168. 1999.PubMed/NCBI
|
|
25
|
Park HC, Seong J, Han KH, Chon CY, Moon YM
and Suh CO: Dose-response relationship in local radiotherapy for
hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 54:150–155.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada K, Izaki K, Sugimoto K, et al:
Prospective trial of combined transcatheter arterial
chemoembolization and three-dimensional conformal radiotherapy for
portal vein tumor thrombus in patients with unresectable
hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 57:113–119.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoon HI, Lee IJ, Han KH and Seong J:
Improved oncologic outcomes with image-guided intensity-modulated
radiation therapy using helical tomotherapy in locally advanced
hepatocellular carcinoma. J Cancer Res Clin Oncol. 140:1595–1605.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen D, Wang R, Meng X, et al: A
comparison of liver protection among 3-D conformal radiotherapy,
intensity-modulated radiotherapy and RapidArc for hepatocellular
carcinoma. Radiat Oncol. 9:482014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao Q, Zheng R, Xie G, et al:
Late-responding normal tissue cells benefit from high-precision
radiotherapy with prolonged fraction delivery times via enhanced
autophagy. Sci Rep. 5:91192015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mornex F, Girard N, Beziat C, et al:
Feasibility and efficacy of high-dose three-dimensional-conformal
radiotherapy in cirrhotic patients with small-size hepatocellular
carcinoma non-eligible for curative therapies - mature results of
the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys.
66:1152–1158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toramatsu C, Katoh N, Shimizu S, et al:
What is the appropriate size criterion for proton radiotherapy for
hepatocellular carcinoma? A dosimetric comparison of spot-scanning
proton therapy versus intensity-modulated radiation therapy. Radiat
Oncol. 8:482013. View Article : Google Scholar : PubMed/NCBI
|