Introduction
Breast cancer is the most commonly diagnosed
neoplasm and the third leading cause of cancer-associated mortality
in the United States, with 22.2 mortalities per 100,000 women
associated with breast cancer each year. The five-year relative
survival rate for breast cancer has gradually increased since the
early 1990s and between 2007 and 2011 it was ~89.2%. However, the
prognosis of patients with breast cancer is dependent on the
disease stage at the time of diagnosis. In particular, the survival
rates of patients with localised disease and regional lymph node
metastasis at diagnosis are higher than those of patients
presenting with distant metastasis (1). A number of studies have established
molecular markers, which are associated with distinct
histopathological features, the response to adjuvant therapy and/or
the clinical outcome of breast cancer (2–7).
Furthermore, the following clinicopathological factors are
considered to be useful markers for predicting prognosis and
identifying therapeutic targets in patients with advanced breast
cancer: American Joint Committee on Cancer (AJCC) stage,
histological grade, oestrogen receptor (ER) and progesterone
receptor (PR) expression, human epithelial growth factor receptor 2
(HER2) amplification, p53 expression and Ki-67 labelling index
(2–6).
Based on data obtained from molecular or immunohistochemical (IHC)
analyses, breast cancer is classified into four major subtypes:
Luminal A, luminal B, basal-like and HER2-positive (7). More recently, the luminal B subtype has
been subdivided according to HER2 status and Ki-67 labelling index
(8).
Despite improvements in the treatment of breast
cancer, the high mortality rate of patients with direct invasion of
adjacent organs or distant metastases remains a problem (9). Therefore, improved understanding of the
molecular and cellular mechanisms of tumour invasion and metastasis
is required for the development of more effective treatment
strategies. The multi-step process of metastasis involves numerous
cellular events, including neovascularisation, stromal invasion,
lymphovascular invasion and growth at a secondary site (10,11).
Furthermore, increased tumour cell motility, combined with
extracellular matrix degradation at the invasive front of the
tumour, are critical early processes in metastasis. (12)
Fascin-1 is a 55-kDa cytoskeletal actin-binding
protein that packages actin filaments into tertiary structures,
including microspikes, stress fibres and membrane ruffles, within
dynamic cellular structures, resulting in the enhancement of cell
motility, migration and adhesion (13,14).
Fascin-1 (also known as fascin) is primarily expressed during
embryonic development, while its expression in adults is highly
restricted to neurons, glial cells, endothelial cells and
antigen-presenting dendritic cells (15). The additional forms of fascin,
fascin-2 and −3, are expressed in retinal photoreceptor cells and
the testes, respectively (16).
Published data has demonstrated that fascin is upregulated or
highly expressed in the human cancer of various organs, including
the oesophagus (17), breast
(18), colon (19), lung (20), stomach (21) and urinary bladder (22), as well as in individual tissues, and
that the expression of fascin is associated with aggressive
behaviour (23). Previous studies of
breast cancer have identified that fascin overexpression is
associated with factors representing aggressive tumor behaviour,
for example hormonal receptor negativity, a triple-negative subtype
and/or a basal-like phenotype (24–26).
However, to the best of our knowledge, no reports have thus far
identified a correlation between fascin expression and disease-free
or overall survival rates, according to the AJCC tumor node
metastasis (TNM) stage.
Therefore, the aim of the present study was to
investigate fascin expression in a large cohort of patients with
invasive ductal carcinoma (IDC) of the breast, and to assess any
statistical correlations between fascin expression, and
clinicopathologic parameters, molecular subtypes and patient
survival according to the AJCC stage of breast cancer.
Patients and methods
Patient selection
The present study included 194 Korean women
diagnosed with IDC at Kangbuk Samsung Hospital, Sungkyunkwan
University School of Medicine (Seoul, Republic of Korea) between
2000 and 2005. Various clinicopathological variables were
established by reviewing patient records and haematoxylin and
eosin-stained slides. For example, histological grade was
determined using the modified Bloom-Richardson-Elston grading
system (27). Additionally, tumours
were staged with reference to the size and/or extent of the tumour,
regional lymph node involvement and metastasis, using the seventh
edition of the AJCC TNM classification system (28).
Ethical Statement
The study was performed according to the Declaration
of Helsinki and approved by the local Ethics Committee of the
Kangbuk Samsung Hospital (KBSMC14011, Sungkyunkwan University
School of Medicine, Seoul, Republic of Korea).
Tissue microarray (TMA)
construction
A series of 194 tumour TMA specimens were assembled
using a tissue-array instrument (AccuMax™ Array; ISU ABXIS Co.
Ltd., Seoul, Korea). The TMAs consisted of 10×6 arrays of IDC
tissue cores measuring 2.0-mm in diameter, and the cores were
obtained from well-preserved, morphologically representative tumour
tissue samples in archived, formalin-fixed, paraffin-embedded
blocks. The assembled array was held in an X-Y position guide with
a 1-mm increment between the individual samples, a 2-mm depth
puncture prevention device and semi-automatic micrometers.
Considering the limitations associated with obtaining
representative areas of a tumour, the present study used duplicate
2.0-mm diameter tissue cores from each donor block. The percentage
of tumour in each tissue core was >70%.
Immunohistochemistry
For IHC staining, the TMA slides were deparaffinised
with heat at 55°C for 30 min, followed by three 5-min washes with
xylene (Duksan Pure Chemicals Co., Ltd., Ansan, Korea). The
sections were then rehydrated by a series of successive 5-min
washes in 100, 90 and 70% ethanol (Duksan Pure Chemicals Co.,
Ltd.). Antigens were retrieved by microwaving the samples for 4 min
20 sec in 250 ml of 10 mM sodium citrate (pH 6.0, Duksan Pure
Chemicals Co., Ltd.). Furthermore, endogenous peroxidase activity
was blocked by incubation with 0.3% hydrogen peroxidase (Duksan
Pure Chemicals Co., Ltd.) for 20 min. Immunostaining for PR, ER,
HER2, p53 and Ki-67 was performed using a DakoCytomation
Autostainer with a universal staining system and a ChemMate™ Dako
EnVision™ Detection kit (Dako North America, Inc., Carpinteria, CA,
USA). The primary antibodies used were as follows: Anti-PR (1:200
dilution; Dako, Glostrup, Denmark), anti-ER (1:200 dilution; Lab
Vision Corporation, Fremont, CA, USA), anti-p53 (1:5,000 dilution;
Sigma-Aldrich, St. Louis, MO, USA), anti-HER2 (1:200 dilution;
Dako) and anti-Ki-67 (1:200 dilution; Dako). For fascin,
affinity-purified rabbit anti-human-fascin polyclonal antibody
(1:500 dilution; Leica Microsystems Ltd., Milton Keynes, UK) was
used, and detection (4 min incubation at room temperature) was
performed with the UltraTech horseradish-peroxidase
streptavidin-biotin detection system (Beckman Coulter, Inc.,
Marseille, France) using an automatic staining machine from the
Bond™ Intense R Detection kit (Leica Microsystems Ltd., Milton
Keynes, UK).
Interpretation of IHC staining
The sections were observed using an Olympus BX51
light microscope (Olympus Corporation, Tokyo, Japan). The IHC
results were used to classify the tumours into the following five
molecular subtypes: Luminal A (ER- and/or PR-positive,
HER2-negative, Ki-67 low), luminal B HER2-positive (ER- and/or
PR-positive, HER2-positive), luminal B HER2-negative (ER- and/or
PR-positive, HER2-negative, Ki-67 high), HER2-positive (ER- and
PR-negative, HER2-positive) and triple-negative (ER-, PR- and
HER2-negative) (9,29–31).
Positive fascin immunostaining was defined as
exclusive cytoplasmic staining with no nuclear staining. Normal
tissue composed of endothelial cells was used as the positive
control tissue. Staining intensity of tumour cells was graded on a
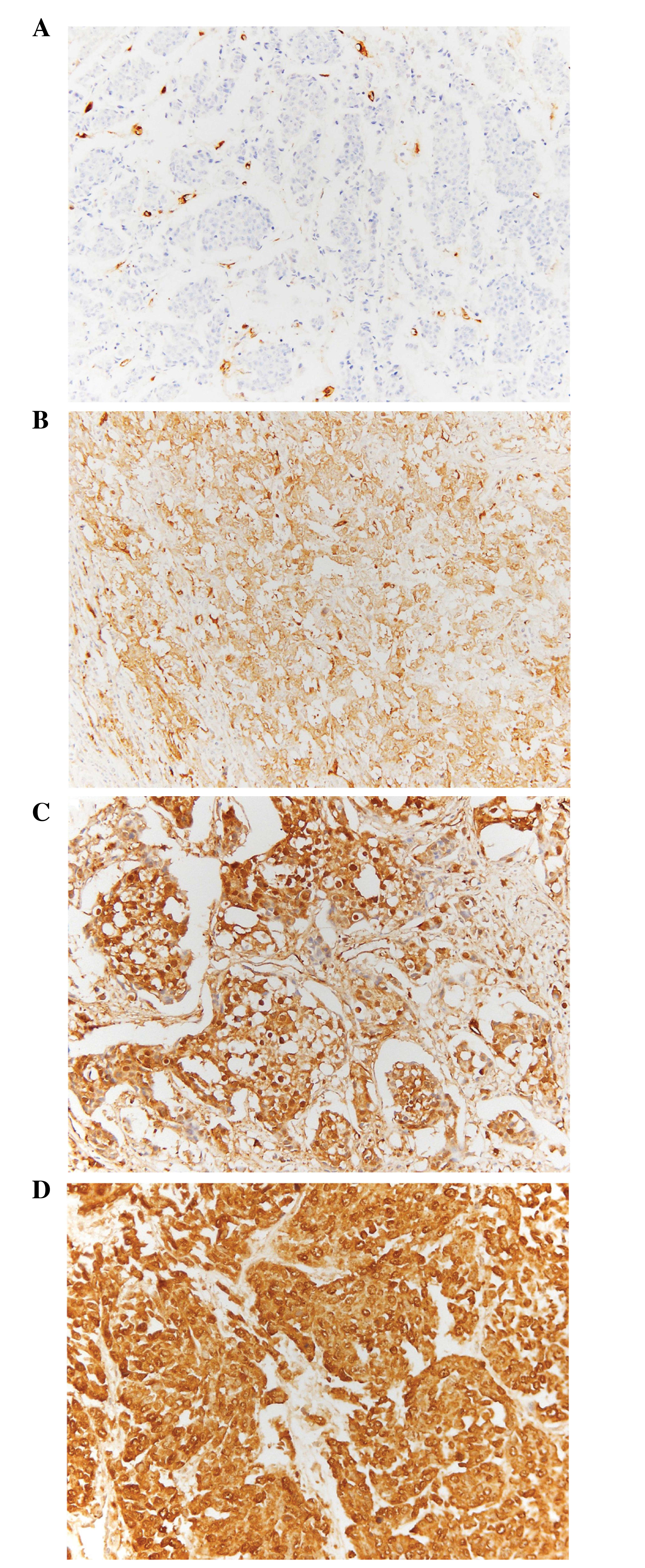
scale of 0–3, as follows: No staining, 0; weak 1; moderate, 2; or
strong, 3 (Fig. 1). In addition, the
extent of tumour staining was scored based on the percentage of
tumour cells exhibiting staining, using the following scoring
system: 1–25% staining, 1; 26–50% staining, 2; 51–75% staining, 3;
or 76–100% staining, 4. In cases with a discrepancy between the
duplicated cores, the highest score of the two tissue cores was
used as the final score. To calculate the combined immunoreactive
score, the staining intensity and extent of tumour staining scores
were multiplied (9,32).
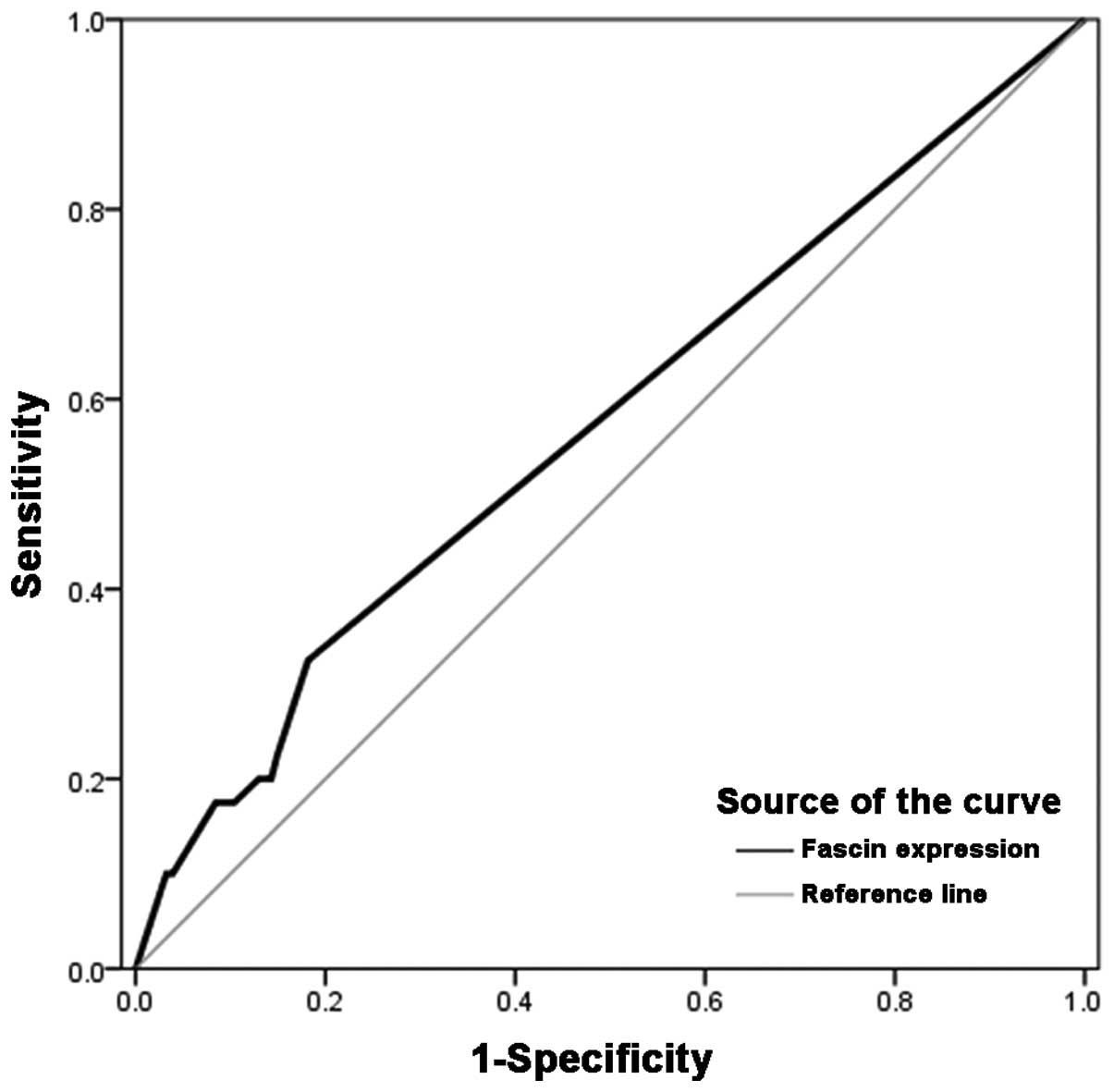
The optimal cut-off values for fascin expression
were calculated by plotting receiver operating characteristic (ROC)
curves of sensitivity versus 1-specificity. The cut-off value
calculated from the ROC curve was then used to evaluate the
association between patient mortality and fascin expression. The
ROC curves revealed effective discriminatory power for the
correlation between overall survival and fascin expression in the
tumour samples (area under the ROC curve, 0.572; Fig. 2). Using the ROC curve, fascin
expression was classified as negative (intensity score, <1) or
positive (intensity score, ≥1).
Statistical analysis
Correlations between specific clinicopathological
parameters and fascin expression were analysed by performing the
χ2 test, the linear by linear association test and
Fisher's exact test. Furthermore, a Student's t-test was used to
examine the association between fascin protein expression and
continuous variables, including p53 expression and Ki-67 labelling
index. Disease-free survival was defined as the time from the date
of diagnosis to the date of recurrence or development of novel
distant metastasis. Similarly, overall survival was defined as the
time from the date of treatment to the final follow-up visit or
cancer-associated mortality. Survival curves were generated using
the Kaplan-Meier method and were compared by performing the
Tarone-Ware test. Additionally, multivariate analysis was performed
to identify independent prognostic markers for disease-free and
overall survival using a Cox multistep regression model. P<0.05
was considered to indicate a statistically significant difference,
and all statistical analyses were performed using SPSS statistical
software for Windows (version 18.0; SPSS Inc., Chicago, IL,
USA).
Results
Patient characteristics
The mean and median ages of the patients were 48.2
and 47.0 years, respectively (range, 26–79 years). Treatment
strategies for these cases of breast cancer included modified
radical mastectomy with axillary lymph node dissection (151
patients; 77.8%), modified radical mastectomy (25 patients; 12.9%),
breast-conserving surgery with axillary lymph node dissection (8
patients; 4.1%) and breast-conserving surgery without axillary
lymph node dissection (10 patients; 5.2%). At the time of surgery,
the T and N classifications of the current cohort were as follows:
T1, 66 patients (34%); T2, 114 patients (58.8%); T3, 14 patients
(7.2%); and N0, 86 patients (44.3%); N1, 63 patients (32.5%); N2,
22 patients (11.3%); N3, 23 patients (11.9%). Furthermore, the
distribution of AJCC staging was as follows: Stage I, 38 patients
(19.6%); stage II, 109 patients (56.2%); and stage III, 47 patients
(24.2%). Overall, 149 patients received combination chemotherapy
with trastuzumab and tamoxifen, 36 received trastuzumab
chemotherapy alone and 7 received tamoxifen chemotherapy alone. The
remaining 2 patients, who did not receive further treatment, were
diagnosed with tubular carcinoma and a 2-mm IDC, respectively.
Following surgery, 49 (25.3%) patients developed local recurrence
or novel distant metastases, and 40 (20.6%) patients succumbed
during the mean follow-up period of 74.2 months.
Fascin expression is correlated with
certain clinicopathological parameters and molecular subtype
In the present study, fascin expression was positive
in 41/194 (21.1%) and negative in 153/194 (78.9%) cases of IDC.
Fascin expression was significantly associated with local
recurrence, a high histological grade, tumour necrosis, ER- and
PR-negativity, and high expression of p53 and Ki-67 (all
P<0.05), but was not significantly associated with HER2
positivity (Table I). Notably, fascin
expression was significantly correlated with resistance to adjuvant
therapy (P<0.001).
 | Table I.Correlation between
clinicopathological parameters and fascin expression in invasive
ductal carcinoma. |
Table I.
Correlation between
clinicopathological parameters and fascin expression in invasive
ductal carcinoma.
|
|
| Fascin expression,
n (%) |
|
|---|
|
|
|
|
|
|---|
| Parameter | Patients, n
(n=194) | Negative
(n=153) | Positive
(n=41) | P-value |
|---|
| Age, years |
|
|
|
|
|
≤47 | 104 | 82 (53.6) | 22 (53.7) | 0.994a |
|
>47 | 90 | 71 (46.4) | 19 (46.3) |
|
| AJCC stage |
|
|
|
|
| I | 38 | 29 (19.0) | 9 (22.0) | 0.441b |
| II | 109 | 85 (55.6) | 24 (58.5) |
|
|
III | 47 | 39 (25.5) | 8 (19.5) |
|
| T category |
|
|
|
|
| T1 | 66 | 55 (35.9) | 11 (26.8) | 0.369b |
| T2 | 114 | 87 (56.9) | 27 (65.9) |
|
| T3 | 14 | 11 (7.2) | 3 (7.3) |
|
| N category |
|
|
|
|
| N0 | 86 | 61 (39.9) | 25 (61.0) | 0.155b |
| N1 | 63 | 54 (35.3) | 9 (22.0) |
|
| N2 | 22 | 21 (13.7) | 1 (2.4) |
|
| N3 | 23 | 17 (11.1) | 6 (14.6) |
|
| Local
recurrence |
|
|
|
|
|
Absence | 188 | 151 (98.7) | 37 (90.2) | 0.019c,d |
|
Presence | 6 | 2 (1.3) | 4 (9.8) |
|
| Distant
metastasis |
|
|
|
|
|
Absence | 147 | 118 (77.1) | 29 (70.7) | 0.396a |
|
Presence | 47 | 35 (22.9) | 12 (29.3) |
|
| Tumour size,
cm |
|
|
|
|
| ≤2 | 69 | 58 (37.9) | 11 (26.8) | 0.188a |
|
>2 | 125 | 95 (62.1) | 30 (73.2) |
|
| Tumour border |
|
|
|
|
|
Well-defined | 40 | 30 (19.6) | 10 (24.4) | 0.501a |
|
Ill-defined | 154 | 123 (80.4) | 31 (75.6) |
|
| Number of
tumours |
|
|
|
|
|
Single | 181 | 142 (92.8) | 39 (95.1) | 0.599a |
|
Multiple | 13 | 11 (7.2) | 2 (4.9) |
|
| Paget's
disease |
|
|
|
|
|
Absence | 187 | 146 (95.4) | 41 (100) | 0.349a |
|
Presence | 7 | 7 (4.6) | 0 (0.0) |
|
| Histological
grade |
|
|
|
|
| 1 | 27 | 26 (17.0) | 1 (2.4) |
<0.001b,d |
| 2 | 89 | 76 (49.7) | 13 (31.7) |
|
| 3 | 78 | 51 (33.3) | 27 (65.9) |
|
| Lymphatic
invasion |
|
|
|
|
|
Negative | 91 | 72 (47.1) | 19 (46.3) | 0.935a |
|
Positive | 103 | 81 (52.9) | 22 (53.7) |
|
| Vascular
invasion |
|
|
|
|
|
Negative | 179 | 144 (94.1) | 35 (85.4) | 0.093c |
|
Positive | 15 | 9 (5.9) | 6 (14.6) |
|
| Perineural
invasion |
|
|
|
|
|
Negative | 163 | 127 (83.0) | 36 (87.8) | 0.456a |
|
Positive | 31 | 26 (17.0) | 5 (12.2) |
|
| Tumour
necrosis |
|
|
|
|
|
Absence | 109 | 95 (62.1) | 14 (34.1) | 0.001d |
|
Presence | 85 | 58 (37.9) | 27 (65.9) |
|
| Central tumour
fibrosis |
|
|
|
|
|
Absence | 158 | 122 (79.7) | 36 (87.8) | 0.238a |
|
Presence | 36 | 31 (20.3) | 5 (12.2) |
|
| EIC |
|
|
|
|
|
Absence | 163 | 125 (81.7) | 38 (92.7) | 0.088a |
|
Presence | 31 | 28 (18.3) | 3 (7.3) |
|
| Oestrogen
receptor |
|
|
|
|
|
Negative | 65 | 33 (21.6) | 32 (78) |
<0.001f |
|
Positive | 129 | 120 (78.4) | 9 (22) |
|
| Progesterone
receptor |
|
|
|
|
|
Negative | 92 | 56 (36.6) | 36 (87.8) |
<0.001f |
|
Positive | 102 | 97 (63.4) | 5 (12.2) |
|
| HER2 |
|
|
|
|
|
Negative | 143 | 113 (73.9) | 30 (73.2) | 0.929a |
|
Positive | 51 | 40 (26.1) | 11 (26.8) |
|
| Adjuvant
therapyf |
|
|
|
|
|
Responder | 175 | 145 (96) | 30 (73.2) |
<0.001d |
|
Non-responder | 17 | 6 (4.0) | 11 (26.8) |
|
| p53 expression |
| 20.84 | 63.33 |
<0.001d,e |
| Ki-67 labelling
index |
| 2.56 | 7.37 |
<0.001d,e |
Compared with the other subtypes investigated,
triple-negative breast cancer was associated with old age and
vascular invasion. HER2 and triple-negative breast cancer were
frequently observed in patients with a high histological grade and
tumour necrosis (all P<0.05; data not shown). Furthermore,
luminal B HER2-negative and HER2 breast cancer were correlated with
lymphatic invasion, and patients with luminal B HER2-negative
breast cancer exhibited a good response to adjuvant therapy
compared with that of other subtypes (all P<0.05; data not
shown).
Fascin expression is associated with
triple-negative breast cancer and correlated with patient
survival
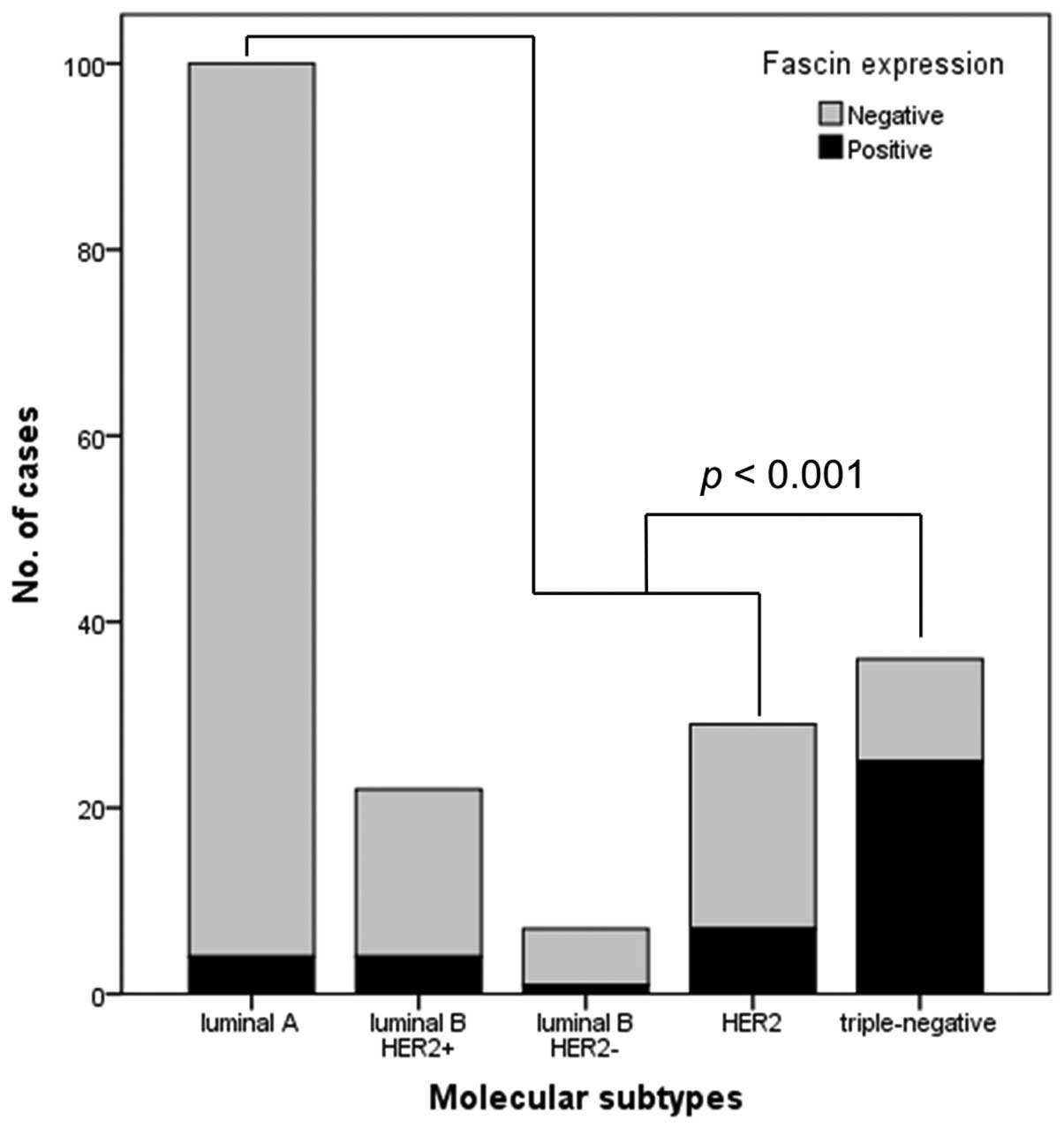
With respect to molecular subtype, the distribution
of fascin expression was as follows: Four cases of luminal A (4%),
1 case of luminal B HER2-negative (14.3%), 4 cases of luminal B
HER2-positive (18.2%), 25 cases of triple-negative (69.4%) and 7
cases of HER2 (24.1%) breast cancer. A comparison of the various
molecular subtypes revealed significantly greater occurrence of
fascin expression in the triple-negative subtype than in the
luminal A, luminal B or HER2 subtypes (P<0.001; Fig. 3).
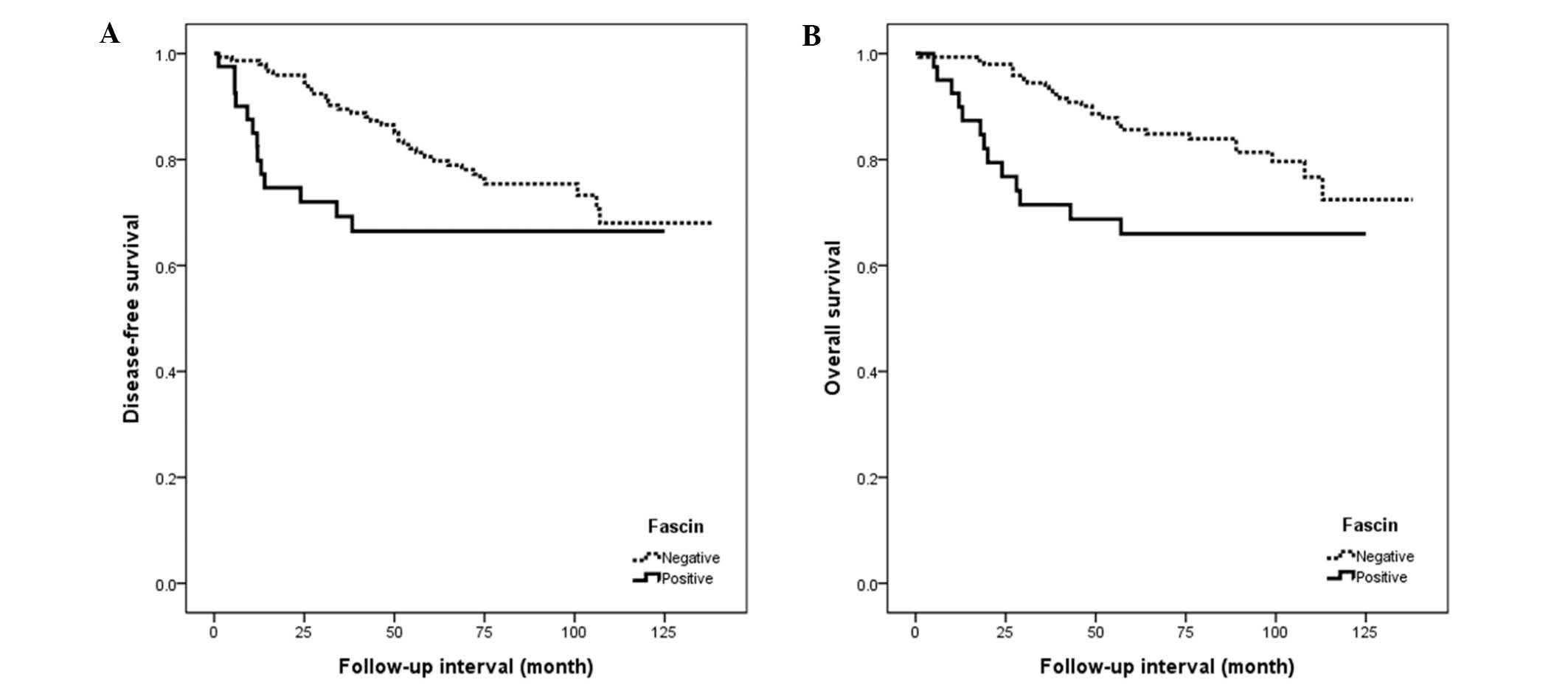
Analysis of patient survival using the Kaplan-Meier
method identified that fascin expression was significantly
correlated with poor disease-free and overall survival rates
(P<0.05; Fig. 4). Other prognostic
factors, including advanced tumour stage, high histological grade
and lymphatic and perineural invasions, were also correlated with
worse disease-free and overall survival in univariate analysis (all
P<0.05; Table II). In
multivariate analyses, the significant correlation between fascin
and overall survival persisted (P=0.013), whereas fascin expression
only exhibited a marginal association with decreased disease-free
survival (P<0.069).
 | Table II.Disease-free and overall survival
analyses (n=194). |
Table II.
Disease-free and overall survival
analyses (n=194).
| Survival | Univariate
significancea | Multivariate
significanceb | Hazard ratio | 95% CI |
|---|
| Disease-free |
|
|
|
|
| Fascin
expression (negative vs. positive) | 0.045 | 0.069 | 1.878 | 0.952–3.705 |
| AJCC
stage (I or II vs. III) |
<0.001c | 0.047c | 1.898 | 1.01–3.568 |
|
Histological grade (1 or 2 vs.
3) | 0.003c | 0.182 | 1.555 | 0.813–2.975 |
|
Lymphatic invasion (absence
vs. presence) | 0.002c | 0.206 | 1.600 | 0.772–3.316 |
|
Perineural invasion (absence
vs. presence) |
<0.001c |
<0.001c | 3.594 | 1.92–6.726 |
| Overall |
|
|
|
|
| Fascin
expression (negative vs. positive) | 0.004c | 0.013 | 2.475 | 1.214–5.042 |
| AJCC
stage (I or II vs. III) | <0.001 | 0.059 | 1.954 | 0.976–3.912 |
|
Histological grade (1 or 2 vs.
3) |
<0.001c | 0.205 | 1.616 | 0.769–3.393 |
|
Lymphatic invasion (absence
vs. presence) | 0.001c | 0.127 | 1.936 | 0.829–4.519 |
|
Perineural invasion (absence
vs. presence) |
<0.001c | 0.001c | 3.148 | 1.577–6.283 |
Fascin expression is correlated with
patient survival, according to AJCC TNM staging and response to
adjuvant therapy
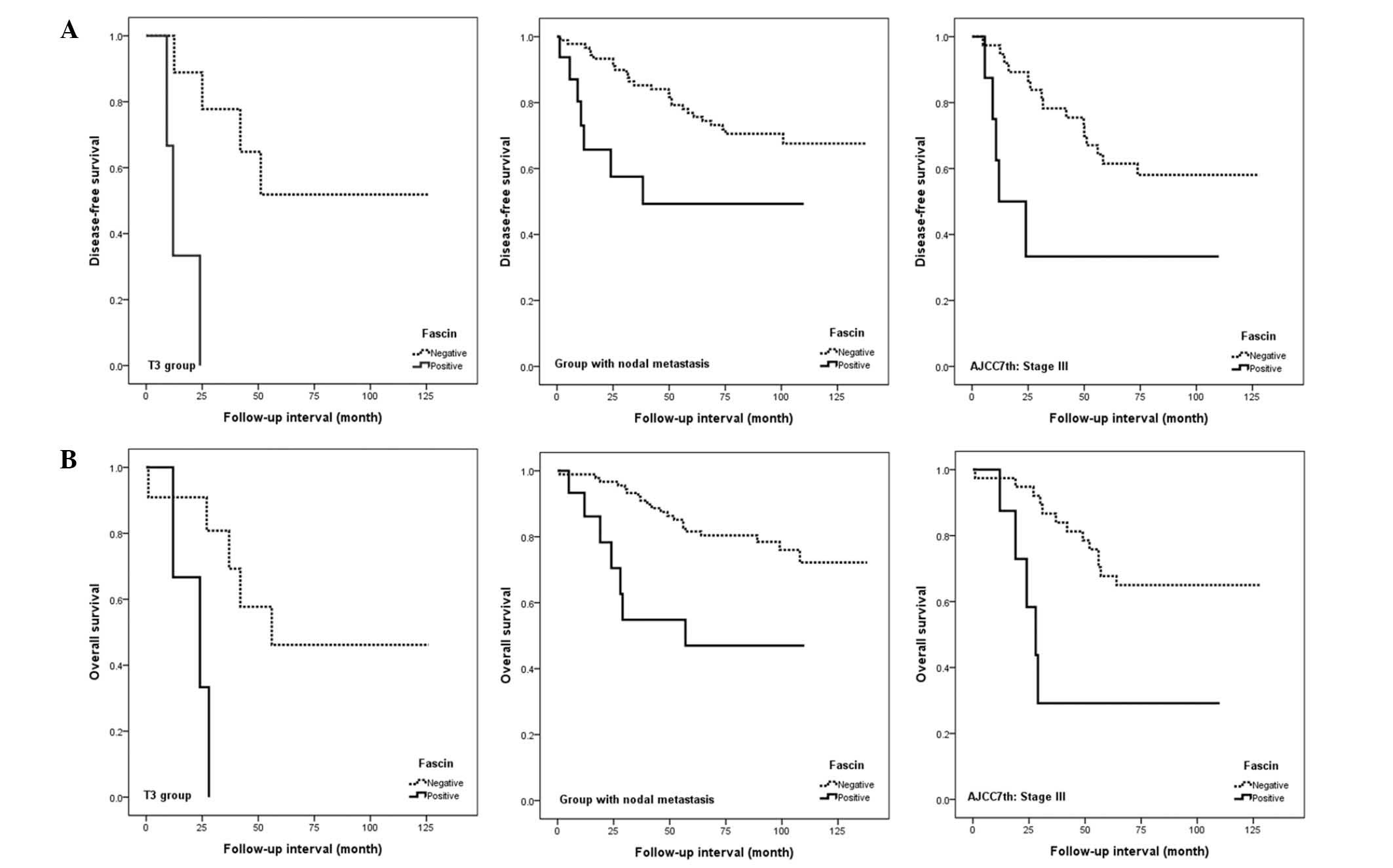
Fascin expression was associated with poor
disease-free and overall survival in patients with an advanced
tumour stage, nodal metastasis or advanced AJCC stage (all
P<0.05; Fig. 5). However, no
significant correlation was observed between fascin and survival
rate for patients with early stage disease. In multivariate
analyses, the significance of the association between fascin
expression and disease-free or overall survival was retained for
patients with an advanced AJCC stage, and the association between
fascin expression and disease-free survival was retained for
patients with an advanced T classification (all P<0.05; Table III).
 | Table III.Correlations between disease-free and
overall survival, and fascin expression according to the size
and/or extent of the tumour, nodal metastasis and AJCC stage
group. |
Table III.
Correlations between disease-free and
overall survival, and fascin expression according to the size
and/or extent of the tumour, nodal metastasis and AJCC stage
group.
| Survival |
Recurrence/total | Univariate
significancea | Multivariate
significanceb | Hazard ratio | 95% CI |
|---|
| T stage |
|
|
|
|
|
| T1 | 14/66 | 0.969 | 0.502 | 1.757 | 0.338–9.126 |
| T2 | 28/114 | 0.177 | 0.491 | 1.363 | 0.565–3.291 |
| T3 | 7/14 | 0.001c | 0.044c | 15.512 | 1.08–222.759 |
| Nodal
metastasis |
|
|
|
|
|
| No | 16/86 | 0.217 | 0.456 | 1.511 | 0.51–4.479 |
|
Yes | 33/108 | 0.009c | 0.186 | 1.842 | 0.745–4.556 |
| AJCC stage |
|
|
|
|
|
| I | 7/38 | 0.605 | 0.491 | 1.920 | 0.301–12.26 |
| II | 22/109 | 0.203 | 0.661 | 1.251 | 0.459–3.41 |
|
III | 20/47 | 0.007c | 0.029c | 3.800 | 1.148–12.58 |
|
| B, Overall
survival |
|
|
|
|
|
|
| Survival |
Mortality/total | Univariate
significancea | Multivariate
significanceb | Hazard ratio | 95% CI |
| T stage |
|
|
|
|
|
| T1 | 12/66 | 0.701 | 0.283 | 2.562 | 0.461–14.245 |
| T2 | 20/114 | 0.013c | 0.065 | 2.551 | 0.942–6.904 |
| T3 | 8/14 | 0.013c | 0.067 | 9.952 | 0.848–116.797 |
| Nodal
metastasis |
|
|
|
|
|
| No | 13/86 | 0.660 | 0.204 | 2.143 | 0.66–6.958 |
|
Yes | 27/108 | 0.001c | 0.101 | 2.185 | 0.858–5.566 |
| AJCC stage |
|
|
|
|
|
| I | 5/38 | 0.361 | 0.507 | 1.880 | 0.292–12.125 |
| II | 17/109 | 0.024 | 0.098 | 2.554 | 0.84–7.767 |
|
III | 18/47 | 0.004c | 0.048c | 3.239 | 1.012–10.366 |
Analysis according to the adjuvant treatment
response, identified that fascin expression was significantly
associated with disease-free and overall survival in patients with
resistance to adjuvant therapy, however, no significance was
observed following adjustment for the aforementioned potential
confounders (data not shown).
Discussion
During cancer progression, an increase in cell
motility is essential for tumour invasion and subsequent
dissemination or metastasis. This increase in motility occurs via
the modulation of actin filaments to form finger-like plasma
membrane protrusions termed invadopodia, (33,34). Such
dynamic rearrangement of the actin cytoskeleton is regulated by
numerous actin-binding proteins (35), including fascin. Fascin is an actin
cross-linking protein, which localises to filopodia at the leading
edge of migratory cells. Enhanced fascin expression is associated
with increased cell migration and invasion (36). Studies have demonstrated that fascin
expression is significantly associated with triple-negativity, as
well as poor clinical outcome, in hormone receptor-negative or
triple-negative breast cancer (25,26).
However, to the best of our knowledge, no previous studies have,
thus far, identified an association between fascin expression and
survival in patients with late-stage disease or resistance to
adjuvant therapy.
Previous studies have demonstrated that fascin
expression is associated with reduced survival in various types of
cancer (20–23,26,37). The
expression of fascin in tumours was initially considered to be
important in promoting tumour progression, and numerous studies of
colon cancer have proposed that fascin is more important in
advanced stage disease compared with early stage disease (20,37,38).
However, a study based on lung cancer reported that fascin
expression may be associated with shorter survival in patients with
early stage disease (22), although
an alternative study of lung cancer did not observe a significant
association between fascin expression and survival in early stage
disease (39). Thus, there is
controversy regarding the association between fascin expression and
the clinical outcomes of patients with cancer. In the present
study, fascin expression was significantly associated with poor
disease-free and overall survival in patients with advanced stage
breast cancer. Additionally, fascin expression was significantly
associated with worse disease-free and overall survival in patients
who were resistant to adjuvant therapy in univariate analyses,
although this significance did not remain following multivariate
analyses.
Fascin may enhance tumour cell motility and invasion
in addition to accelerating tumour cell proliferation, thereby
enhancing cancer cell survival and contributing to the development
of additional distant metastases (40,41). In
the present study, fascin expression was significantly associated
with local recurrence and the survival of patients with late stage
disease. This may be due to the role of fascin in upregulating
other proteins known to be critical for the execution of
metastasis, for example urokinase-type plasminogen activator, and
matrix metalloproteinases-2 and −9 (19). However, the precise mechanisms by
which fascin promotes cancer development and progression are not
fully understood, and the potential prognostic value of fascin may
vary according to biological factors, the degree of cancer
progression and therapeutic tolerance.
There were a number of limitations to the present
study. Firstly, it shares similarities with a previous
retrospective study that did not identify continuous associations
over time (42). Therefore, the
current results did not clarify whether associations with fascin
are maintained throughout invasive tumour growth or whether they
contribute only to the initiation of invasive growth. This is a
significant consideration when determining therapeutic strategies
based on the control of fascin activity. In addition, the number of
patients with late-stage disease or resistance to adjuvant therapy
was relatively small, potentially limiting the statistical power of
the study in future analyses.
In conclusion, the results of the present study
demonstrated that fascin expression was significantly correlated
with breast cancer patient survival, particularly for those with
late stage disease. In addition, fascin expression was
significantly correlated with known predictors of poor survival,
including high histological grade, tumour necrosis, ER- and
PR-negativity, resistance to adjuvant therapy and elevated
expression of p53 and Ki-67. Thus, fascin may be considered as a
key protein in the promotion of tumour progression, and may
facilitate the prediction of outcomes and improvement of prognostic
models in breast cancer. Furthermore, fascin may present a
promising therapeutic target for the inhibition of metastasis,
particularly in patients with advanced breast cancer.
References
|
1
|
National Cancer Institute, . Cancer
statistics: SEER stat fact sheets, breast cancer. http://seer.cancer.gov/statfacts/html/breast.html
|
|
2
|
Bouchalova P, Nenutil R, Muller P, et al:
Mutant p53 accumulation in human breast cancer is not an intrinsic
property or dependent on structural or functional disruption but is
regulated by exogenous stress and receptor status. J Pathol.
233:238–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harris L, Fritsche H, Mennel R, et al:
American Society of Clinical Oncology 2007 update of
recommendations for the use of tumor markers in breast cancer. J
Clin Oncol. 25:5287–5312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin Q, Liu Y, Chen H, et al: Survivin,
Ki-67 and tumor grade as predictors of response to docetaxel-based
neoadjuvant chemotherapy in locally advanced breast cancer. Mol
Clin Oncol. 1:839–844. 2013.PubMed/NCBI
|
|
5
|
Mason BH, Holdaway IM, Mullins PR, Yee LH
and Kay RG: Progesterone and estrogen receptors as prognostic
variables in breast cancer. Cancer Res. 43:2985–2990.
1983.PubMed/NCBI
|
|
6
|
Ogston KN, Miller ID, Payne S, et al: A
new histological grading system to assess response of breast
cancers to primary chemotherapy: Prognostic significance and
survival. Breast. 12:320–327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peppercorn J, Perou CM and Carey LA:
Molecular subtypes in breast cancer evaluation and management:
Divide and conquer. Cancer Invest. 26:1–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feeley LP, Mulligan AM, Pinnaduwage D,
Bull SB and Andrulis IL: Distinguishing luminal breast cancer
subtypes by Ki67, progesterone receptor or TP53 status provides
prognostic information. Mod Pathol. 27:554–561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chambers AF, Naumov GN, Varghese HJ, et
al: Critical steps in hematogenous metastasis: An overview. Surg
Oncol Clin N Am. 10:243–255. 2001.PubMed/NCBI
|
|
10
|
Saaristo A, Karpanen T and Alitalo K:
Mechanisms of angiogenesis and their use in the inhibition of tumor
growth and metastasis. Oncogene. 19:6122–6129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woodhouse EC, Chuaqui RF and Liotta LA:
General mechanisms of metastasis. Cancer. 80(Suppl): S1529–S1537.
1997.
|
|
12
|
Min KW, Kim DH, Do SI, et al: Diagnostic
and prognostic relevance of MMP-11 expression in the stromal
fibroblast-like cells adjacent to invasive ductal carcinoma of the
breast. Ann Surg Oncol. 20(Suppl 3): S433–S442. 2013.PubMed/NCBI
|
|
13
|
Jiang P, Enomoto A and Takahashi M: Cell
biology of the movement of breast cancer cells: Intracellular
signalling and the actin cytoskeleton. Cancer Lett. 284:122–130.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edwards RA and Bryan J: Fascins, a family
of actin bundling proteins. Cell Motil Cytoskeleton. 32:1–9. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mosialos G, Yamashiro S, Baughman RW, et
al: Epstein-Barr virus infection induces expression in B
lymphocytes of a novel gene encoding an evolutionarily conserved
55-kilodalton actin-bundling protein. J Virol. 68:7320–7328.
1994.PubMed/NCBI
|
|
16
|
Hashimoto Y, Skacel M and Adams JC: Roles
of fascin in human carcinoma motility and signaling: Prospects for
a novel biomarker? Int J Biochem Cell Biol. 37:1787–1804. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu KF, Lin CK, Yu CP, et al: Cortactin,
fascin, and survivin expression associated with clinicopathological
parameters in esophageal squamous cell carcinoma. Dis Esophagus.
22:402–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Alwan M, Olabi S, Ghebeh H, et al:
Fascin is a key regulator of breast cancer invasion that acts via
the modification of metastasis-associated molecules. PLoS One.
6:e273392011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hashimoto Y, Skacel M, Lavery IC, et al:
Prognostic significance of fascin expression in advanced colorectal
cancer: An immunohistochemical study of colorectal adenomas and
adenocarcinomas. BMC Cancer. 6:2412006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pelosi G, Pastorino U, Pasini F, et al:
Independent prognostic value of fascin immunoreactivity in stage I
nonsmall cell lung cancer. Br J Cancer. 88:537–547. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hashimoto Y, Shimada Y, Kawamura J, et al:
The prognostic relevance of fascin expression in human gastric
carcinoma. Oncology. 67:262–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tong GX, Yee H, Chiriboga L, Hernandez O
and Waisman J: Fascin-1 expression in papillary and invasive
urothelial carcinomas of the urinary bladder. Hum Pathol.
36:741–746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tan VY, Lewis SJ, Adams JC and Martin RM:
Association of fascin-1 with mortality, disease progression and
metastasis in carcinomas: A systematic review and meta-analysis.
BMC Med. 11:522013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grothey A, Hashizume R, Sahin AA and
McCrea PD: Fascin, an actin-bundling protein associated with cell
motility, is upregulated in hormone receptor negative breast
cancer. Br J Cancer. 83:870–873. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoder BJ, Tso E, Skacel M, et al: The
expression of fascin, an actin-bundling motility protein,
correlates with hormone receptor-negative breast cancer and a more
aggressive clinical course. Clin Cancer Res. 11:186–192.
2005.PubMed/NCBI
|
|
26
|
Esnakula AK, Ricks-Santi L, Kwagyan J, et
al: Strong association of fascin expression with triple negative
breast cancer and basal-like phenotype in African-American women. J
Clin Pathol. 67:153–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robbins P, Pinder S, de Klerk N, et al:
Histological grading of breast carcinomas: A study of interobserver
agreement. Hum Pathol. 26:873–879. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edge SB, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th. Springer; New York, NY, USA: 2010
|
|
29
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
30
|
Carey LA, Perou CM, Livasy CA, et al:
Race, breast cancer subtypes, and survival in the Carolina Breast
Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wolff AC, Hammond ME, Hicks DG, et al:
American Society of Clinical Oncology; College of American
Pathologists: Recommendations for human epidermal growth factor
receptor 2 testing in breast cancer: American Society of Clinical
Oncology/College of American Pathologists clinical practice
guideline update. J Clin Oncol. 31:3997–4013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.PubMed/NCBI
|
|
33
|
Jayo A and Parsons M: Fascin: A key
regulator of cytoskeletal dynamics. Int J Biochem Cell Biol.
42:1614–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li A, Dawson JC, Forero-Vargas M, et al:
The actin-bundling protein fascin stabilizes actin in invadopodia
and potentiates protrusive invasion. Curr Biol. 20:339–345. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsudaira P: Actin crosslinking proteins
at the leading edge. Semin Cell Biol. 5:165–174. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vignjevic D, Schoumacher M, Gavert N, et
al: Fascin, a novel target of beta-catenin-TCF signaling, is
expressed at the invasive front of human colon cancer. Cancer Res.
67:6844–6853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oh SY, Kim YB, Suh KW, et al: Prognostic
impact of fascin-1 expression is more significant in advanced
colorectal cancer. J Surg Res. 172:102–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Puppa G, Maisonneuve P, Sonzogni A, et al:
Independent prognostic value of fascin immunoreactivity in stage
III-IV colonic adenocarcinoma. Br J Cancer. 96:1118–1126. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roh MS, Um SJ, Choi Y, et al: Prognostic
significance of fascin expression in stage I non-small cell lung
cancer. Tuberc Respir Dis (Seoul). 65:105–109. 2008. View Article : Google Scholar
|
|
40
|
Xing P, Li JG, Jin F, et al: Fascin, an
actin-bundling protein, promotes breast cancer progression in
vitro. Cell Biochem Funct. 29:303–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie JJ, Xu LY, Wu JY, et al: Involvement
of CYR61 and CTGF in the fascin-mediated proliferation and
invasiveness of esophageal squamous cell carcinomas cells. Am J
Pathol. 176:939–951. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gu MJ, Kim JY and Park JB: Fascin
expression predicts lymph node metastasis and worse survival in
small intestinal carcinoma. Pathology. 46:21–24. 2014. View Article : Google Scholar : PubMed/NCBI
|