Introduction
Osteosarcoma is one of the most common childhood
malignancies, with ~400 novel cases of osteosarcoma diagnosed in
the USA each year (1).
Pathologically, osteosarcoma arises from mesenchymal cells, which
are characterized by spindle cells and aberrant osteoid formation.
The primary sites of osteosarcoma are typically the distal femur,
the proximal tibia and the distal humerus, with 64% of lesions
arising from the knee (2). The
administration of neoadjuvant chemotherapy and surgical
intervention has increased the five-year survival rate from ~10% to
70% in the last 30 years (3). Despite
the additional improvements in the treatment of osteosarcoma over
the previous 20 years, the overall survival rate of patients has
reached a plateau (4). In
osteosarcoma, a poor response to chemotherapy is associated with a
poorer outcome and a lower survival rate (5). However, it has been demonstrated that an
increase in drug efflux, a decrease drug influx, inactivation of
the agent, alterations in the drug targets, increased DNA damage
repair and evasion of apoptosis all contribute to intrinsic or
acquired tumor chemoresistance (6).
In modern treatment regimens, various chemotherapeutic agents are
administered in combination, either intravenously or orally, pre-
and post-operatively (7–9). However, the majority of chemotherapeutic
agents also carry the risk of short- and long-term toxic and
adverse effects (10). Therefore,
identification of the effector molecules and signal transduction
pathways that are responsible for the regulation of carcinogenesis
and malignant development is vital to understand and isolate
potential molecular targets that may be used to disrupt the tumor
machinery, whilst protecting the integrity and function of normal
tissue.
The acridine family is extensive (11) and includes a wide variety of nitro,
halo, hydroxy, cyano, alkyl and aryl derivatives. The
chemotherapeutic action of acridines is their most notable
property. In particular, the 3,6-diaminoacridine dye proflavin is
also an acridine derivative. Acridine derivatives were initially
developed as dyes, and during the early 20th century, the
pharmacological properties of the derivatives were evaluated.
During this time, proflavin was used as a topical antibacterial and
antifungal agent. This acridine derivative is well known for its
disinfectant bacteriostatic properties against numerous
gram-positive bacteria. Proflavin is used as a topical antiseptic
in the form of dihydrochloride and hemisuphate salts, and was
formerly used as a urinary antiseptic. The roles of proflavin as a
mutagen and as an enzyme inhibitor have been extensively studied
(12,13). Proflavin has been used as a mitotic
inhibitor that interferes with nucleic acid synthesis in
vivo (14,15) and in vitro, and it also acts as
a potent mutagen (16) that
intercalates itself between adjacent base pairs of the DNA
molecule, causing deletion errors during replication (17). These effects are of interest for the
study of genic action. Proflavin was included in a systematic
investigation into the action of various types of antimetabolites
on nucleolar fine structure. The lesions produced by proflavin in
the nucleus and the nucleolus were identified to be so specific
that this compound received particular attention (18). However, to the best of our knowledge,
no studies have been performed on the action of proflavin against
osteosarcoma at present. Therefore, the present study aimed to
determine the effects of proflavin on osteosarcoma cells and to
predict the underlying mechanism with emphasis on anticancer
activity. The present study hypothesized that proflavin exerts
anticancer activity in osteosarcoma with involvement of apoptosis
and autophagy. The human osteosarcoma MG-63 cell line was used to
explore the anticancer property of proflavin and changes in cell
viability with focuses on apoptosis and autophagy was also assessed
by real-time PCR and immunoblotting methods.
Materials and methods
Materials
The human osteosarcoma MG63 cell line (catalog no.,
CRL-1427) was obtained from the American Type Culture Collection
(Manassas, VA, USA) and maintained in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and
100 µg/ml penicillin/streptomycin at 37°C in a humidified
atmosphere containing 5% CO2. DMEM, FBS and trypsin-EDTA
were purchased from Gibco Life Technologies (Gaithersburg, MD,
USA). Proflavin, penicillin, streptomycin and dimethyl sulfoxide
(DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Antibodies against caspase-3 (polyclonal rabbit anti-human; catalog
no. 9662), caspase-9 (polyclonal rabbit anti-human; catalog no.
9502), B-cell lymphoma 2 (Bcl-2; polyclonal rabbit anti-human;
catalog no. 2872), Bcl-2-associated X protein (Bax; polyclonal
rabbit anti-human; catalog no. 2774), light chain 3 (LC3)-I
(monoclonal rabbit anti-human; catalog no. 4599), LC3-II
(monoclonal rabbit anti-human; catalog no. 4599), poly(adenosine
diphosphate-ribose) polymerase (PARP; monoclonal rabbit anti-human;
catalog no. 9532) and β-actin (polyclonal rabbit anti-human;
catalog no. 4967) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Horseradish peroxidase-conjugated
secondary antibodies against mouse immunoglobulin G (IgG)
(polyclonal rabbit; catalog no. ab6728) and rabbit IgG (polyclonal
goat; catalog no. ab6721) were purchased from Abcam (Cambridge, MA,
USA).
Cell culture and MTT assay
The cells were seeded in six-well culture plates at
an initial density of 1×105 cells/ml and grown to ~80%
confluence. For experimental treatments, the cells were
administered with proflavin at concentrations of 0, 0.1, 1, 5 and
10 µM in serum-free DMEM for 24 h. Following treatment, the cells
were washed with phosphate-buffered saline (PBS) that contained 25
mM sodium phosphate and 150 mM NaCl (pH 7.2), and were harvested
for the subsequent analyses.
An MTT assay was performed to determine cell
viability. The cells were seeded overnight in 96-well plates at a
density of 1×104 cells/well prior to treatment.
Following treatment, the medium was replaced with 0.5 mg/ml MTT
solution. The supernatant was removed after 3 h of incubation at
37°C and DMSO was subsequently added. Absorbance was measured at
540 nm, with background subtraction at 650 nm, on EMax Endpoint
ELISA Microplate Reader (Molecular Devices LLC, Sunnyvale, CA,
USA). Percentage survival was defined as: Survival (%) = 100 × (T -
T0) / (C - T0), when (T - T0) = 0.
When T<T0, cell death had occurred and the cytotoxic
activity was determined as follows: Cytotoxic activity (%) = (T -
T0) / T0, where T is the optical density
(OD)540 value at the time-point in question, and
T0 is the OD540 value at the moment of drug
administration. C indicates the OD540 value of the
untreated control group at the time-point in question.
IC50 values were calculated from three independent
experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following treatment in the present study, total RNA
was extracted using TRIzol reagent (Ambion Life Technologies,
Carlsbad, CA, USA). The quantity of RNA was determined using the
Qubit RNA Assay kit (Invitrogen, Carlsbad, CA, USA). Reverse
transcription was performed in a 20-µl reaction with 200 ng total
RNA using high-capacity cDNA reverse transcription kits (Applied
Biosystems Life Technologies, Foster City, CA, USA). Relative
quantification of apoptosis and autophagy markers were assessed
using the ABI 7900 HT system (Applied Biosystems Life
Technologies). The housekeeping gene GAPDH was used as an internal
control. The primer sequences used are reported in Table I.
 | Table I.Primers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| Bax |
TGGAGCTGCAGAGGATGATTG |
GGCCTTGAGCACCAGTTTG |
| Bcl-2 |
GCTATAACTGGAGAGTGCTGAAGATTG |
TGATGTTGTATTTTTTAAGTACAGCATGAT |
| Atg5 |
ATGGACAGTTGCACACACTAGGA |
ATCTTCAGGATCAATAGCAGAAGGA |
| Beclin1 |
CTCACAGCTCCATTACTTACCACAG |
TCAATAAATGGCTCCTCTCCTGA |
| GAPDH |
AATGGAAATCCCATCACCATCT |
CAGCATCGCCCCACTTG |
Immunoblotting
Subsequent to treatment with proflavin, the cells
were washed with PBS and incubated with RIPA buffer (Sigma-Aldrich)
on ice for 5 min. The resulting samples were then centrifuged at
23,800 × g for 15 min at 4°C. The supernatants were collected for
the subsequent western blotting. The protein concentration was
measured using Qubit Protein Assay kit. Equal amounts of cellular
protein (20 µg/sample) were electrophoresed using 12% SDS-PAGE and
the gels were then transferred to polyvinylidene difluoride
membranes (Amersham Pharmacia Biotech, Zurich, Switzerland) at 100
V for 1 h at 4°C. The blots were incubated with 5% bovine serum
albulmin in Tris-buffered saline in Tween-20 (TBST; Sigma-Aldrich).
The membranes were incubated with a 1:1,000 dilution of antibodies
against caspase-9, caspase-3, PARP and β-actin for 1 h at room
temperature. The blots were washed with TBST and incubated with
secondary antibody (dilution, 1:2,000) for 30 min at room
temperature. For signal detection, the blots were then incubated
with peroxidase-coupled goat anti-rabbit immunoglobulin (Amersham
Pharmacia Biotech) and the enhanced chemiluminescence (Amersham
Pharmacia Biotech) reagents were used to detect the signals,
according to the manufacturer's instructions.
Statistical analysis
The data were expressed as the mean ± standard error
of the mean of the three independent experiments. The statistical
significance of differences was determined using one-way analysis
of variance followed by Dunnett's test for multiple comparisons
with the control. P<0.05 was considered to indicate a
statistically significant difference.
Results
Proflavin inhibition of osteosarcoma
MG-63 cells
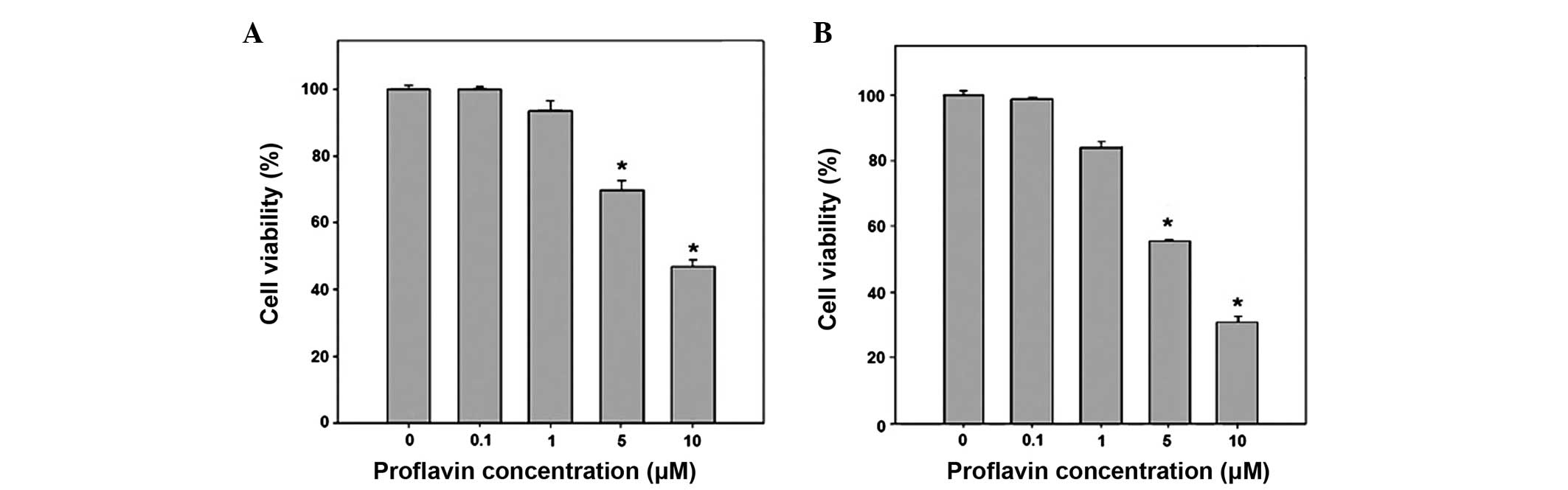
An MTT assay determined the inhibitory effect of
proflavin on the growth of MG63 osteosarcoma cells. In the MTT
assay, the MG-63 osteosarcoma cells were treated with proflavin at
concentrations of 0, 0.1, 1, 5 and 10 µM for 24 or 48 h, which was
followed by viability analysis. As shown in Fig. 1, the treatment of the MG-63 cells with
0–10 µM proflavin resulted in the suppression of cell growth in a
dose-dependent manner. The viability was significantly decreased to
68.7±3.0 and 47.7±1.5% of the viability of the control cells in
response to treatment with 5 and 10 µM of proflavin for 24 h,
respectively (P<0.05 vs. control). The prolonged treatment with
proflavin led to a greater inhibition of cell viability, which was
54.8±0.4 and 32.1±1.5% of control with 5 and 10 µM of proflavin,
respectively (P<0.01 vs. control).
Apoptosis studies
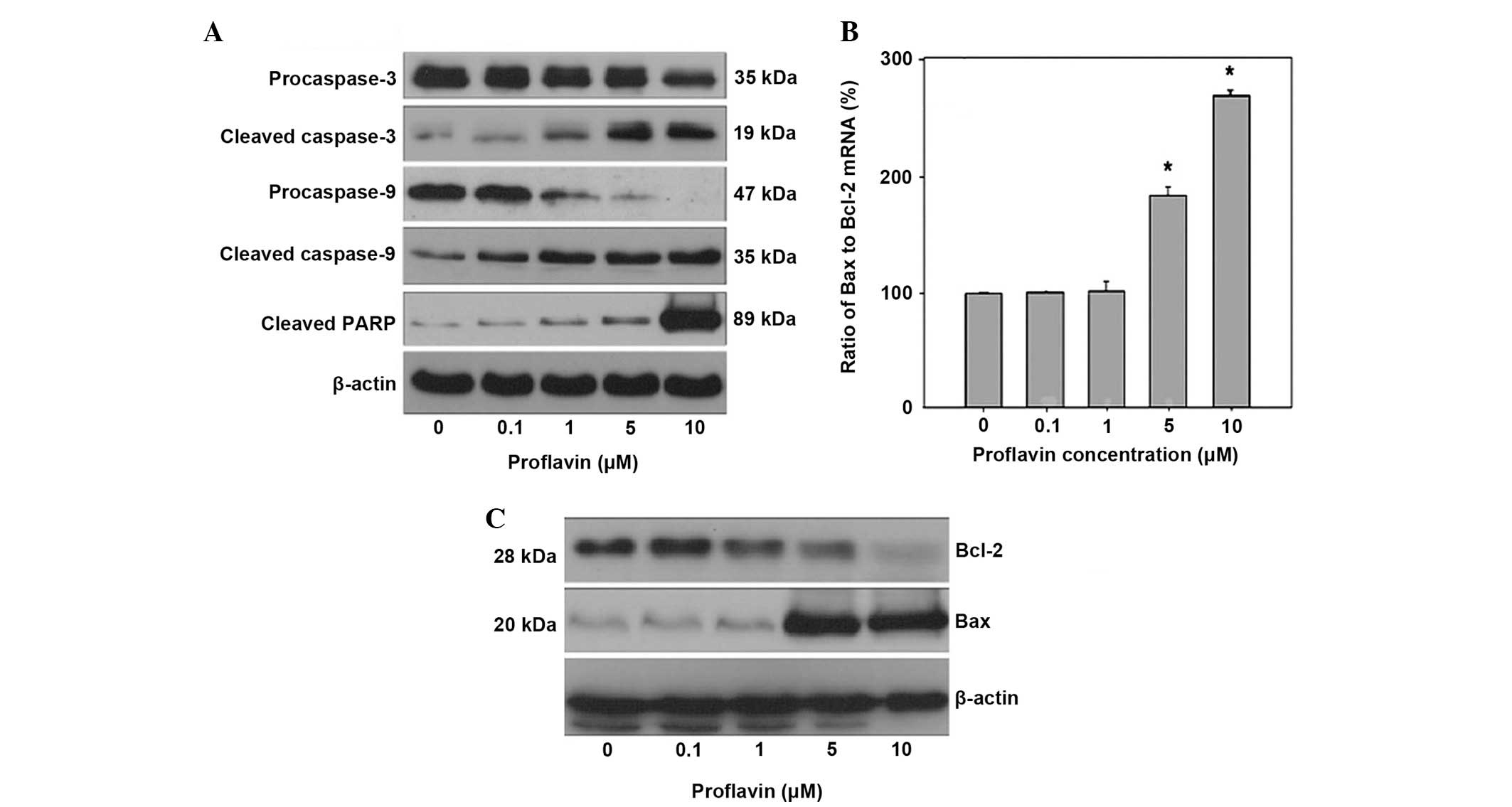
Apoptosis assays were performed to elucidate the
mechanisms underlying proflavin-induced growth inhibition on MG-63
cells treated with proflavin at various concentrations. The present
study identified that the induction of apoptosis was based on the
alteration of apoptotic biomarkers in proflavin-treated MG-63
cells. From the results shown in Fig.
2A, a significant decrease in the level of procaspase-3 was
observed to be in association with increased cleaved caspase-3
levels at concentrations of 5 and 10 µM compared with the controls.
The results also revealed that proflavin induced an increase in
cleaved caspase-9 levels in a dose-dependent manner and proflavin
treatment at 10 µM led to significant cleavages of PARP in MG63
cells. In addition, the effect of proflavin treatment on the
expression of apoptotic genes was investigated using RT-qPCR and
western blotting. As shown in Fig.
2B, the ratio of Bax to Bcl-2 mRNA was significantly elevated
in the cells treated with proflavin at higher concentrations
compared with the controls. In addition, the response to the
alteration in protein levels of Bax and Bcl-2 in MG-63 cells
treated with proflavin was identified and is shown in Fig. 2C.
Proflavin induced autophagy
Autophagy plays an important role in protecting
against cancer, and potentially in contributing to the growth of
cancer (19). Autophagy may protect
against cancer by isolating damaged organelles, allowing cell
differentiation, increasing protein catabolism and even promoting
cell death in cancerous cells (20).
However, autophagy also contributes to cancer by promoting the
survival of tumor cells that have been starved. The role of
autophagy in cancer is one that has been highly investigated and
reviewed. There is evidence that emphasizes the role of autophagy
as a tumor suppressor and a factor in tumor cell survival. However,
previous studies have been able to demonstrate that autophagy is
more likely to be used as a tumor suppressor, according to several
models (19,20).
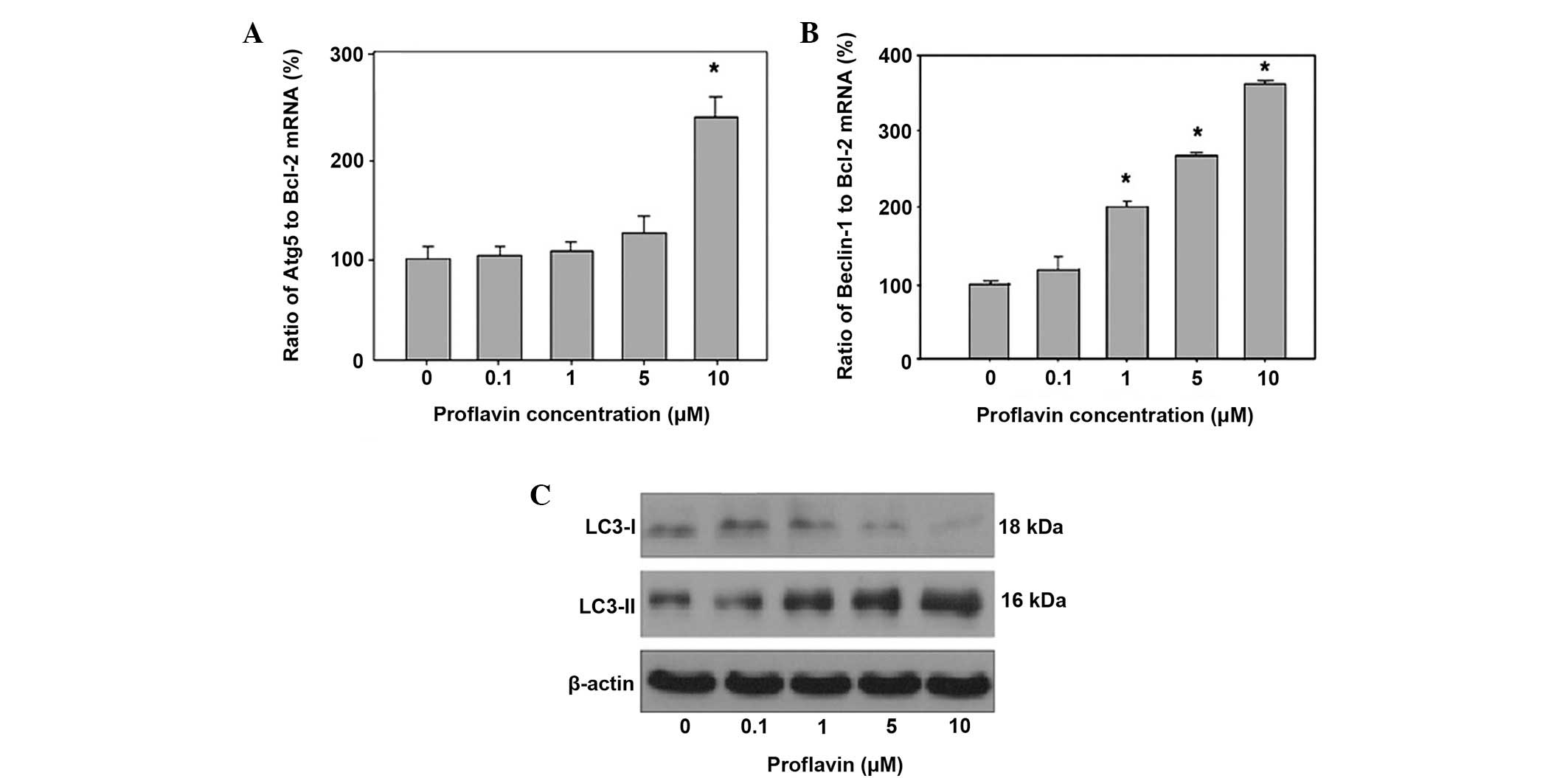
The role of autophagy in proflavin-induced cell
death was then investigated in osteosarcoma cells. The results
revealed that autophagy contributed to cell death in the presence
of drug treatment against osteosarcoma cells. The experimental data
revealed that exposure to 5 and 10 µM proflavin led to an elevated
autophagy related 5 (Atg5) to Bcl-2 mRNA ratio, up to a 1.2- and
2.4-fold increase compared with the ratio in the control and MG-63
cells, respectively. The ratio of the autophagy-associated genes
Beclin 1 and Bcl-2 was also assessed in proflavin-treated MG-63
cells. The resulting data indicated that proflavin treatment
increased the ratio of Beclin1 to Bcl-2 at concentrations of 5 and
10 µM up to 2.5- and 3.5-fold compared to the ratio in control
cells. Also, the influence of proflavin treatment on LC3-I and
LC3-II levels was examined by western blot analysis, and the
results demonstrated that the levels of LC3-II were significantly
increased, whereas the LC3-I levels was were decreased, with a
concomitant increase in the concentration of proflavin administered
to MG-63 cells (Fig. 3).
Discussion
Acridines are generally known for their
antibacterial and antiseptic activity. Several studies have
indicated that acridines possess properties that inhibit tumor
growth and cancer cell proliferation (21–24).
Acriflavine, an acridine derivative has previously been used as an
imaging contrast agent due to the ability to pass through the cell
membrane and strongly label acidic constituents (25,26). In
addition, the molecular mechanism of the anticancer activity of
acriflavine appears to vary depending on the type and origin of
cancer. However, the mechanism underlying the inhibitory effect of
acriflavine in osteogenic sarcoma cells remains to be elucidated.
Acriflavine has been reported to initiate the apoptosis pathways in
eukaryotic cells (27), and the
underlying mechanism remains unclear. In yeast cells, acriflavine
induces apoptosis through the alteration of the mitochondrial
membrane, which subsequently results in apoptosis (12). In mammalian cells, acriflavine has
been demonstrated to induce apoptosis in association with
radiotherapy through p53-dependent mitochondrial pathways and ER
stress signals (24).
Proflavin induces a series of ultrastructural
alterations in cultured rat embryonic cells. Non-specific
cytoplasmic lesions include disorganization of the endoplasmic
reticulum and large cytoplasmic inclusions with osmiophilic
material and myelin figures (28),
which is similar to the abnormalities described in HeLa cells
treated with acridine orange and in liver and pancreatic acinar
cells of rats fed with ethionine and azaserine (18). However, characteristic nuclear and
nucleolar lesions, including clumping of the chromatin with
unsticking from the nuclear membrane, disappearance of the
nucleoplasmic matrix and segregation of nucleolar components, occur
with increased duration and concentration of treatment (18). Two other antimetabolites, daunomycin
and ethidium bromide, which are chemically dissimilar to proflavin,
produce identical nuclear and nucleolar lesions to proflavin at
adequate concentrations. Since proflavin, daunomycin and ethidium
bromide form complexes with DNA by intercalation between base
pairs, the nuclear and nucleolar lesions may represent the
morphological expression for a specific molecular action (18,28).
In the present study, novel evidence that proflavin
directly inhibits growth and promotes apoptosis in osteosarcoma
cells was provided. Proflavin was found to significantly induce
apoptosis in MG-63 cells at concentrations >5 µM. The present
results demonstrated that exposure of osteosarcoma cells to
proflavin leads to dose-dependent PARP cleavage and the catalytic
activation of caspases-3 and -9. It was suggested in the current
study that proflavin induced apoptosis of MG-63 cells through the
activation of the intrinsic caspase pathway. Since the activation
of caspase-9 and PARP cleavage implicated mitochondrial apoptosis,
the ratio of Bcl-2 and Bax mRNA expression was examined in MG-63
cells treated with proflavin. Treatment of MG-63 with proflavin
increased the Bax/Bcl-2 ratio, indicating that proflavin causes
mitochondrial membrane disruption that leads to the initiation of
mitochondrial apoptosis. Since proflavin passes through the cell
membrane, proflavin-induced cell death is likely to involve the
activation of intrinsic apoptosis through caspases-3 and -9 and
mitochondrial dysfunction. The present study observed that
proflavin significantly induced autophagy in a dose-dependent
manner in MG-63 cells. The expression of Beclin 1 and Atg5 was
significantly upregulated in response to proflavin treatment,
suggesting that proflavin induced autophagy in MG-63 cells by
activating the Beclin 1/Atg5 pathway, which may be considered as a
marker reflecting autophagic activity. The LC3 protein is
distributed ubiquitously in mammalian tissues, as well as in
cultured cells. It is known that the level of LC3-I in cells is
relatively stable and that LC3-II is more sensitive to
immunoblotting compared to LC3-I (29). As autophagy occurs, LC3-I is
conjugated to phosphatidyl ethanolamine to form LC3-II, while
autophagosomes take up cytoplasmic components. In the present
study, autophagy was also characterized by an accumulation of
cleaved LC3-II and a decrease in the level of LC3-I. The
accumulation of LC3-II indicated the formation of autophagosomes in
the presence of proflavin. Similar to the trend of apoptosis, the
significant autophagic effect demonstrated by proflavin was
observed in the cells treated with relatively high (10 µM)
concentrations of proflavin. As Beclin 1, Atg5 and LC3-II were
upregulated, it is implied that proflavin induces autophagy the
HIF-1α-dependent and HIF-1α-independent pathways.
In conclusion, the present study demonstrated that
proflavin arrested the growth of MG63 osteosarcoma cells through
the activation of apoptotic cascades. Additionally, autophagy was
revealed to synergistically contribute to the proflavin-inhibited
growth of osteosarcoma cells. Additional studies are required to
elucidate and explore the mechanism by which proflavin suppresses
osteosarcoma cells in vivo.
Acknowledgements
The authors thank Natural Science Foundation of
Shandong Province (grant no. ZR2009CL027), Fund of Science Star
program of Technology bureau of Jinan city (grant no., 20100118)
and Medical Found of Academy of Medical Sciences of Shandong
Province (grant no., 201023) for the financial assistance.
References
|
1
|
Kaatsch P: Epidemiology of childhood
cancer. Cancer Treat Rev. 36:277–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JA, Kim MS, Kim DH, Lim JS, Yoo JY,
Koh JS, Lee SY, Jeon DG and Park KD: Relative tumor burden
predticts metastasis-free survival in pediatric osteosarcoma.
Pediatr Blood Cancer. 50:195–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bacci G, Longhi A, Versari M, Mercuri M,
Briccoli A and Picci A: Prognostic factors for osteosarcoma of the
extremity treated with neoadjuvant chemotherapy: 15-year experience
in 789 patients treated at a single institution. Cancer.
106:1154–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Driscoll L: Mechanisms of drug
sensitivity and resistance in cancer. Curr Cancer Drug Targets.
9:250–251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AH, Hogendoorn PC and Egeler AM: Chemotherapeutic
adjuvant treatment for osteosarcoma: Where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment - where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McTiernan A, Jinks RC, Sydes MR, Uscinska
B, Hook JM, van Glabbeke M, Bramwell V, Lewis IJ, Taminiau AH,
Nooij MA, et al: Presence of chemotherapy-induced toxicity predicts
improved survival in patients with localised extremity osteosarcoma
treated with doxorubicin and cisplatin: A report from the European
osteosarcoma intergroup. Eur J Cancer. 48:703–712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Janeway KA and Grier HE: Sequelae of
osteosarcoma medical therapy: A review of rare acute toxicities and
late effects. Lancet Oncol. 11:670–678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Acheson RM: Acridines. Interscience
Publishers; New York, NY: pp. 10–90. 1973
|
|
12
|
Li HJ and Crothers DM: Relaxation studies
of the proflavine-DNA complex: The kinetics of an intercalation
reaction. J Mol Biol. 39:461–477. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Faller LD and LaFond RE: Binding
properties of oligomeric alpha-chymotrypsin. Biochemistry.
10:1033–1041. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bubel HC and Wolff DA: Proflavine
inhibition of vaccinia virus synthesis. J Bacteriol. 89:977–983.
1965.PubMed/NCBI
|
|
15
|
Scholtissek C and Rott R: Binding of
proflavine to deoxyribonucleic acid and ribonucleic acid and its
biological significance. Nature. 204:39–43. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orgel A and Brenner S: Mutagenesis of
bacteriophage T4 by acridines. J Mol Biol. 3:762–768. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lerman LS: Structural consideration in the
interaction of DNA and acridines. J Mol Biol. 3:18–30. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simard R: Specific nuclear and nucleolar
ultrastructural lesions induced by proflavin and similarly acting
antimetabolites in tissue culture. Cancer Res. 26:2316–2328.
1966.PubMed/NCBI
|
|
19
|
Furuya N, Liang XH and Levin B: Autophagy
and cancerAutophagy. Klionsky DJ: Landes Bioscience; Georgetown,
TX: pp. 244–253. 2004
|
|
20
|
Mathew R, Karp CM, Beaudoin B, Vuong N,
Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hassan S, Laryea D, Mahteme H, Felth J,
Fryknäs M, Fayad W, Linder S, Rickardson L, Gullbo J, Graf W, et
al: Novel activity of acriflavine against colorectal cancer tumor
cells. Cancer Sci. 102:2206–2213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Strese S, Fryknäs M, Larsson R and Gullbo
J: Effects of hypoxia on human cancer cell line chemosensitivity.
BMC Cancer. 13:3312013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong CC, Zhang H, Gilkes DM, Chen J, Wei
H, Chaturvedi P, Hubbi ME and Semenza GL: Inhibitors of
hypoxia-inducible factor 1 block breast cancer metastatic niche
formation and lung metastasis. J Mol Med (Berl). 90:803–815. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim MJ, Ahn JY, Han Y, Yu CH, Kim MH, Lee
SL, Lim DS and Song JY: Acriflavine enhances radiosensitivity of
colon cancer cells through endoplasmic reticulum stress-mediated
apoptosis. Int J Biochem Cell Biol. 44:1214–1222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Polglase AL, McLaren WJ, Skinner SA,
Kiesslich R, Neurath MF and Delaney PM: A fluorescence confocal
endomicroscope for in vivo microscopy of the upper- and the
lower-GI tract. Gastrointest Endosc. 62:686–695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kiesslich R, Goetz M, Vieth M, Galle PR
and Neurath MF: Confocal laser endomicroscopy. Gastrointest Endosc
Clin N Am. 15:715–731. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keyhani E, Khavari-Nejad S, Keyhani J and
Attar F: Acriflavine-mediated apoptosis and necrosis in yeast
Candida utilis. Ann NY Acad Sci. 1171:284–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simard R, Langelier, Mandeville NM and
Royal A: Inhibitors as tools in elucidating the structure and
function of the nucleusThe Cell Nucleus. Busch H: 3. Academic Press
Inc.; London: pp. 447–487. 1974
|
|
29
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|