Introduction
Hepatoblastoma is a common liver malignancy in
children, with an incidence ranking third among abdominal solid
tumors in children. This disease is often observed in boys aged
between 6 months and 3 years and is rarely diagnosed in adults.
Annually, ~0.5–1.5 children per million are diagnosed with
hepatoblastoma, accounting for 1% of malignancies in children and
50–79% of liver malignancies in children (1). Hepatoblastoma is a curable malignancy in
children and early diagnosis and therapy are effective in
increasing survival rates (2).
However, this type of cancer grows rapidly and has no specific
clinical manifestations. Children with hepatoblastoma often present
initially with a giant mass in the abdominal cavity, when the
hepatoblastoma is at stage II or higher, and with metastases to the
porta hepatis, portal vein or brain (3), leading to a poor prognosis. Color
Doppler ultrasound, CT and hepatic arteriography are routinely used
to diagnose hepatoblastoma, but these modalities are less effective
for early diagnosis (4). Thus, the
development of novel diagnostic methods is necessary for the early
diagnosis of hepatoblastoma.
Proteomics has been used previously in the field of
oncology to identify tumor-associated markers for the early
diagnosis of cancer, to investigate mechanisms of pathogenesis and
to identify therapeutic targets (5,6).
Biomarkers have been identified for a variety of tumor types
(7–9),
including nephroblastoma (10–12),
ovarian (13), prostate (14), pancreatic (15), colon (16) and breast cancer (17). The expression of certain serum
proteins may be altered in patients with cancer due to focal or
systemic inflammation; these proteins or inflammatory factors may
confound biomarker screening for malignancies in these patients
(18). Thus, these
inflammation-associated proteins must be excluded when screening
for biomarkers specific to malignancies.
The present study aimed to identify a pediatric
hepatoblastoma serum biomarker that is unaffected by inflammatory
factors. Serum was harvested from healthy children and those with
hepatoblastoma or systemic inflammatory response syndrome (SIRS). A
serum marker for hepatoblastoma was screened and identified
following the exclusion of interfering inflammatory factors.
Materials and methods
Clinical information
Participants were recruited from the First
Affiliated Hospital of Zhengzhou University of Henan Province
(Zhengzhou, China) between December 2011 and December 2013. There
were 30 children with pathologically proven hepatoblastoma,
including 19 males and 11 females, with a mean age of 39.80±16.50
months (range, 2–70 months). There were 20 children with SIRS,
including 12 male and 8 female, with a mean age of 47.26±19.76
months (range, 6–84 months). In addition, 20 healthy children were
recruited as controls, including 10 males and 10 females, with a
mean age of 38.53±18.46 months (range, 6–72 months). Fasting blood
was harvested from these subjects in the morning (05:00–06:00) and
placed at room temperature for 1 h, followed by centrifugation at
3,000 × g for 20 min. Supernatants were collected and stored at
−80°C. Prior to the study, informed consent was obtained from the
parents of these children. The study was approved by the ethics
committee of Zhengzhou University.
Screening of differentially expressed
proteins in the serum using SELDI-TOF-MS
Frozen sera were thawed in cold water, centrifuged
at 10,000 × g for 5 min at 4°C and the supernatants were collected.
MB-WCX binding buffer (10 µl), MB-WCX magnetic beads (10 µl)
(Profiling Kit 100 MB-WCX, Bruker Corporation, Ettlingen, Germany),
and serum (5 µl) were mixed in an eppendorf tube and incubated at
room temperature for 5 min. The tube was then placed in a bead
separator for 1 min. The solution (but not beads) was removed, and
the beads were washed twice with 100 µl MB-WCX wash buffer. The
solution was removed, and 5 µl MB-WCX elution buffer was added. The
tube was then placed into a bead separator for 2 min. The
supernatant was transferred to a 0.5-ml tube, to which 5 µl MB-WCX
stabilization buffer was added, followed by mass spectrometry (MS)
analysis.
The parameters for Ciphergen PBS II+ SELDI-TOF-MS
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) were set as
follows: Maximal molecular weight, 30,000 Da (optimal molecular
weight, 2,000–20,000 Da); optimal laser intensity, 190; and optimal
sensitivity, 7. The extracted proteins were added to a Ciphergen
WCX2 protein chip (Bio-Rad Laboratories, Inc.) that was placed in
the instrument for analysis. Following bioprocessor analysis,
different protein peaks and the corresponding mass-to-charge ratios
(m/z) were further analyzed; peaks with a difference in m/z of
<0.3% were regarded as representing the same protein. The data
were analyzed using the Wilcoxon rank sum test, and the proteins
differentially expressed between healthy children and those with
hepatoblastoma were identified based on the protein peaks and the
corresponding m/z. The proteins differentially expressed between
healthy children and those with SIRS (inflammatory proteins) were
identified based on protein peaks and the corresponding m/z. These
proteins were then excluded from those with potential as biomarkers
during comparison of the protein expression between healthy
children and those with hepatoblastoma.
Identification of target proteins by
MALDI-TOF-MS
Serum proteins (5 µl/lane) were separated by
SDS-PAGE on a separation gel with 12% acrylamide bis and a stacking
gel of 5% acrylamide bis (10x Tris/Glycine/SDS, pH 8.8, Bio-Rad
Laboratories, Inc.) at a voltage of 30 V through the separation gel
and at 90 V through the stacking gel. The gels were then stained
with Coomassie brilliant blue (Bio-Rad Laboratories, Inc.). The
target protein bands were cut from the gel and stored at −20°C for
further identification. Gel slices containing the target proteins
were placed in, 80 µl wash buffer (acetonitrile, Sangon Biotech Co.
Ltd., Shanghai, China), centrifuged at 10,000 × g at 37°C for 3–5
min 3 times, and dried at 90°C for 15 min. A total of 20 µl of
digest buffer [Dithiothreitol (10 mmol/l) /
NH4HCO3 (100 mmol/l), Sigma-Aldrich, St.
Louis, MO, USA] and 2 µl reducing agent [iodoacetamide (55 mmol/l)
/ NH4HCO3 (100mmol/l) (2 µl), Sigma-Aldrich]
were added to the dried proteins, followed by incubation at 37°C
for 10 min. After cooling to room temperature, 2 µl blocking agent
[NH4HCO3 (20mmol/l), Sangon Biotech Co. Ltd.]
and 0.5 µl diluted trypsin were added. The mixture was incubated at
37°C with constant vortexing, followed by centrifugation at 10,000
× g at 4°C for 10 min. The supernatants were transferred to fresh
tubes to which 0.1% formic acid (0.2 µl) and 50% acetonitrile (0.2
µl) were added. Following centrifugation at 10,000 × g for 10–15
min. Next, the supernatants were harvested for identification of
proteins.
Nano high-performance liquid chromatography was used
to purify supernatant proteins for application to a protein chip,
which was then loaded into the MALDI-TOF-MS instrument (Bruker
Corporation). Peptides were identified following protein digestion.
Mascot searching software (www.matrixscience.com/, Matrix Science Inc., Boston,
MA, USA) was used to search for corresponding proteins in the
SwissProt database (www.expasy.org/proteomics).
Confirmation of target protein
expression using quantitative polymerase chain reaction (qPCR)
The Whole Blood RNA Purification Mini kit (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used to extract
total RNA from the serum of children with hepatoblastoma and
healthy controls. To detect the mRNA expression of target proteins,
first-strand cDNA was synthesized from RNA using the Revert Aid
First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Inc.).
First-strand cDNA was amplified by qPCR using the Scientific Maxima
SYBR Green qPCR Master Mix (Thermo Fisher Scientific, Inc.). The
PCR primers for the amplification of the target protein
apolipoprotein A-1 (Apo A-I) were produced by Sangon Biotech Co.
Ltd. and the sequences were as follows: Forward, 5′-ACA GCG TGA CCT
CCA CCTT-3′ and reverse, 5′-CTT GCT CAT CTC CTG CCT CA-3′. PCR was
carried out as follows: Pre-treatment with uracil-DNA glycosylase
(Sangon Biotech Co. Ltd.) at 50°C for 2 min, pre-denaturation at
95°C for 10 min, 40 cycles of denaturation at 95°C for 15 sec,
annealing at 60°C for 30 sec, and extension at 60°C for 30 sec.
Molecular Analyst ABI 7500 Software, version 2.0.1 (Bio-Rad
Laboratories, Inc.) was used to analyze the PCR products, and the
target protein mRNA expression levels were normalized against
β-actin.
Enzyme linked-immunosorbent assay
(ELISA)
ELISA was used to detect the expression of Apo A-I
protein in the serum of children with hepatoblastoma and healthy
controls, using a Human apoprotein A1, apo-A1 ELISA kit (Sangon
Biotech Co. Ltd.). Standard Apo A-I protein solutions (0, 62.5,
125, 250, 500, 1,000, 2,000 and 4,000 pg/ml) were prepared, and
triplicate 100-µl samples at each concentration were added to a
96-well plate coated with monoclonal rabbit anti-human Apo A-I
antibody (1:100 dilution). Serum collected from children with
hepatoblastoma (n=10) and healthy controls (n=10) was diluted 1:100
with the ABC working solution from the apo-A1 ELISA Kit, and
triplicate 100-µl serum samples were added to the same plate.
Following incubation at 37°C for 90 min, the supernatant in each
well was removed, and biotin-conjugated anti-human Apo A-I antibody
(100 µl) was added, followed by incubation at 37°C for 60 min.
After washing, ABC working solution was added for visualization and
the samples were incubated at 37°C for 30 min. The samples were
washed, 3,3′,5,5′-tetramethylbenzidine (90 µl) was added to each
well, and stop solution (100 µl) was added. The optical density was
measured at 450 nm, and a standard curve was generated. The
concentration of each sample was calculated according to the
standard curve.
Statistical analysis
To distinguish between the differentially expressed
proteins in the serum, as screened by MS, a nonlinear support
vector machine (SVM) classifier was used as previously described
(19). It was originally developed by
Vladimir Vapnik, with a radialbased function kernel, a parameter γ
of 0.6, and a cost of the constrain violation of 19. The
leave-one-out crossing validation approach was applied to estimate
the accuracy of this classifier (19). The capability of each peak in
distinguishing data of different groups was estimated by the
P-value of the Wilcoxon t-test. P≤0.01 was considered to indicate a
statistically significant difference.
In confirming the Apo A-I expression by qPCR and
ELISA, statistical analyses were performed using SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). The independent
samples t-test was used to compare mean values between two groups,
and P<0.05 was considered to indicate a statistically
significant difference for all analyses.
Results
Screening of proteins differentially
expressed between children with hepatoblastoma and healthy
controls
The data collected following MS from children with
hepatoblastoma and healthy controls were standardized and the
protein peaks and corresponding m/z values were compared using the
Wilcoxon rank sum test. A total of 10 peaks with differences were
identified (P<0.01). The results demonstrated that 4 highly
expressed proteins and 6 proteins with low expression were
identified in the serum of children with hepatoblastoma. Following
screening with the SVM, a combined model with the highest Youden
index was screened, and a protein with an m/z of 9,348 Da was
identified as a marker. The Youden index is a method which
evaluates the reliability of the screening test. Assuming that the
false negative (the missed diagnosis) and the false positives (the
misdiagnosis) result in the same outcome, the Youden index may be
used. The Youden index is the sum of the sensitivity and
specificity minus 1, and in this contexts refers to the total
capacity of the screening method in identifying the difference
between the real patients and the false negative and positives. The
greater the Youdenindex value, the larger and more significant the
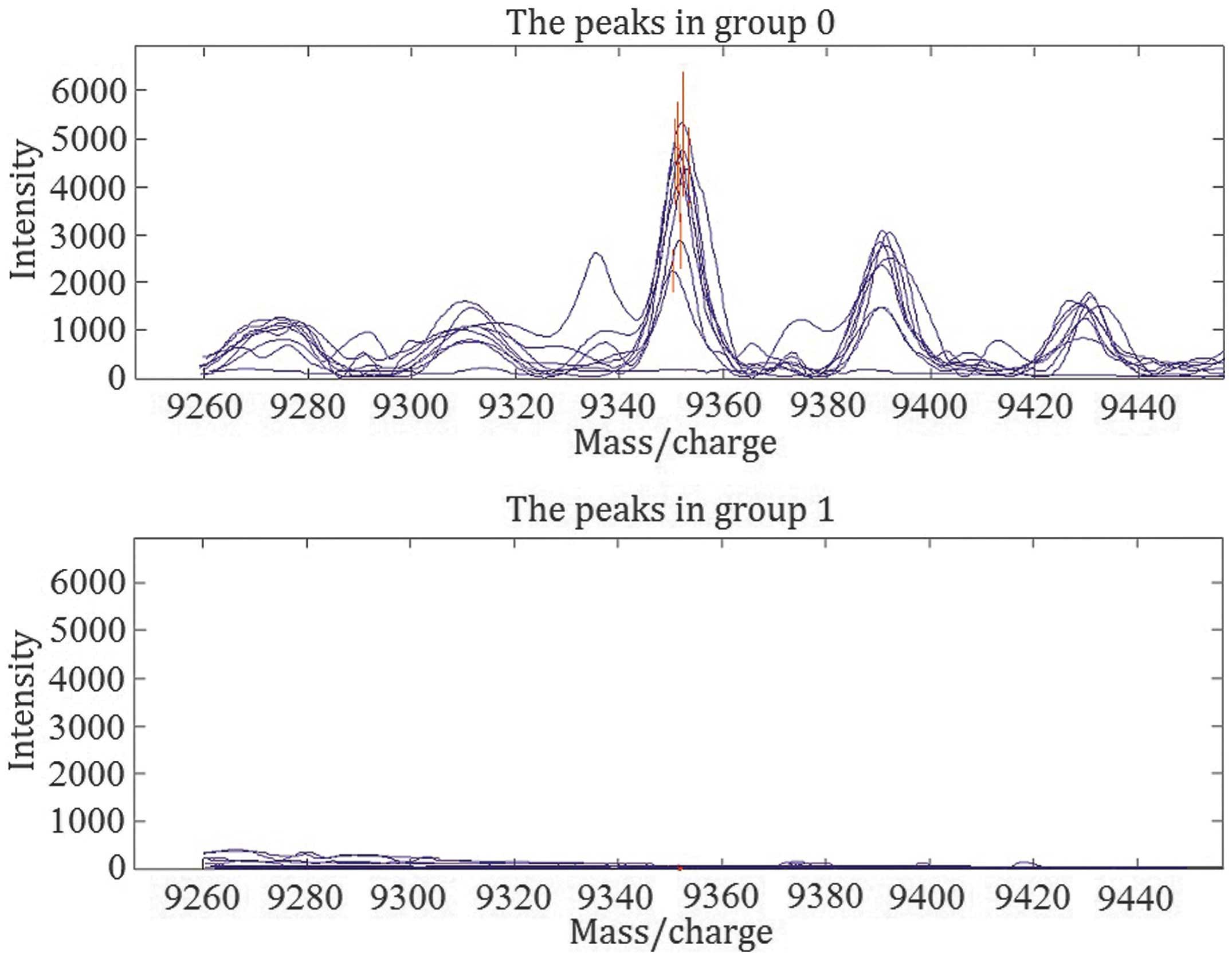
effect and the accuracy of the screening experiment (20). As presented in Fig. 1, the expression of this protein was
significantly reduced in children with hepatoblastoma compared with
healthy controls (29.0±20.9 vs. 2,036.7±881.5, respectively;
P<0.01).
Screening of proteins differentially
expressed between children with SIRS and healthy controls
Following processing and statistical analysis of
data collected following MS, 10 protein peaks were identified that
differed significantly (P<0.01) between children with SIRS and
healthy controls. The results indicated 6 proteins with increased
expression and 4 proteins with reduced expression compared with
controls in the serum of children with SIRS. Following SVM
screening, a combined model with the highest Louden index was
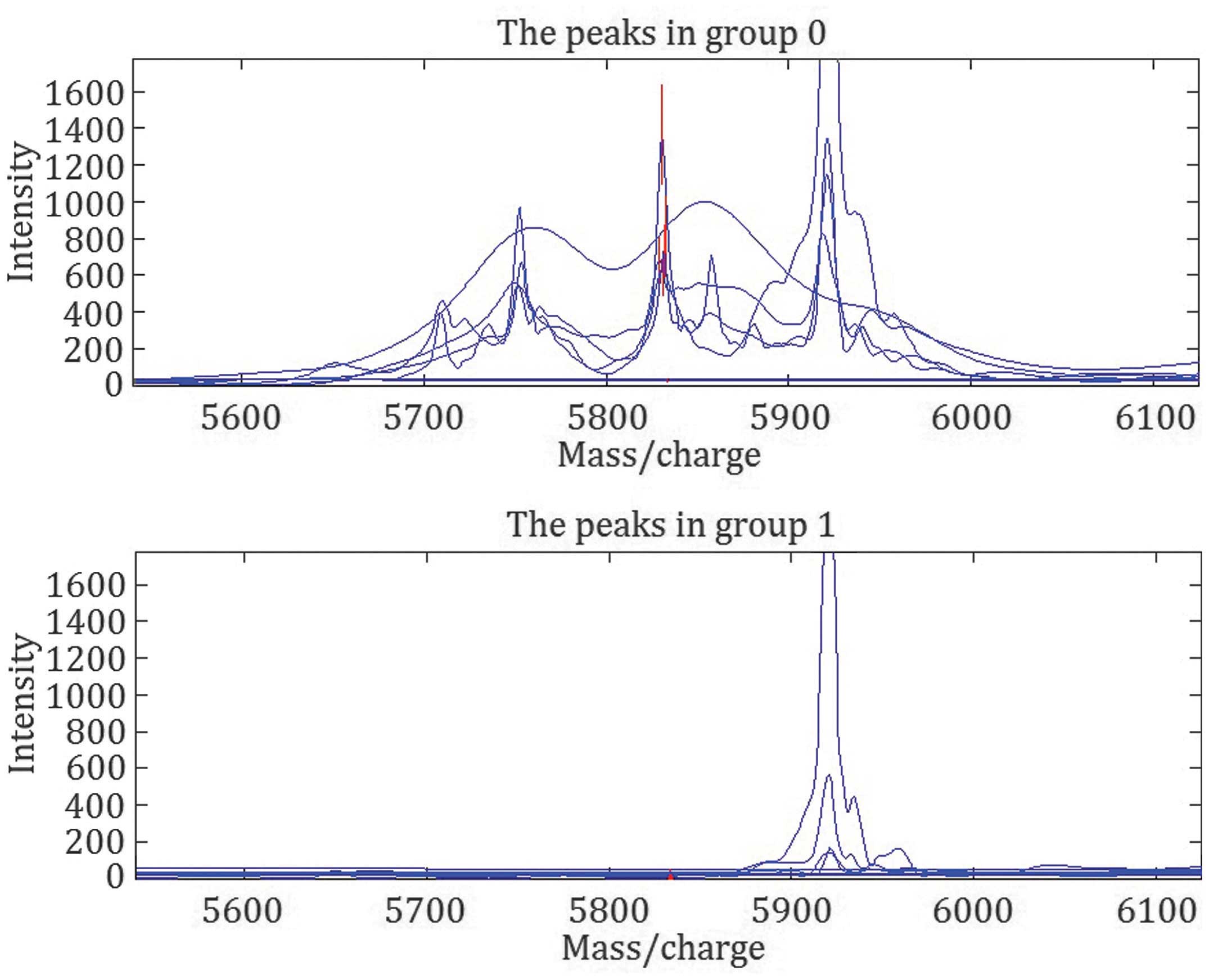
screened, and a protein with m/z of 5,833 Da was identified. As
presented in Fig. 2, the expression
of this protein was significantly increased in children with SIRS
compared with healthy controls (1,283.0±943.3 vs. 75.7±75.1,
respectively; P<0.01).
Exclusion of interfering inflammatory
factors
SELDI-TOF-MS was performed to identify protein peaks
that differed between children with hepatoblastoma and healthy
controls, and between children with SIRS and healthy controls.
Paired comparison indicated that one protein with an m/z of 9,348
was not observed in the serum of children with SIRS (range, ±0.3%),
indicating that this protein was a serum biomarker for
hepatoblastoma.
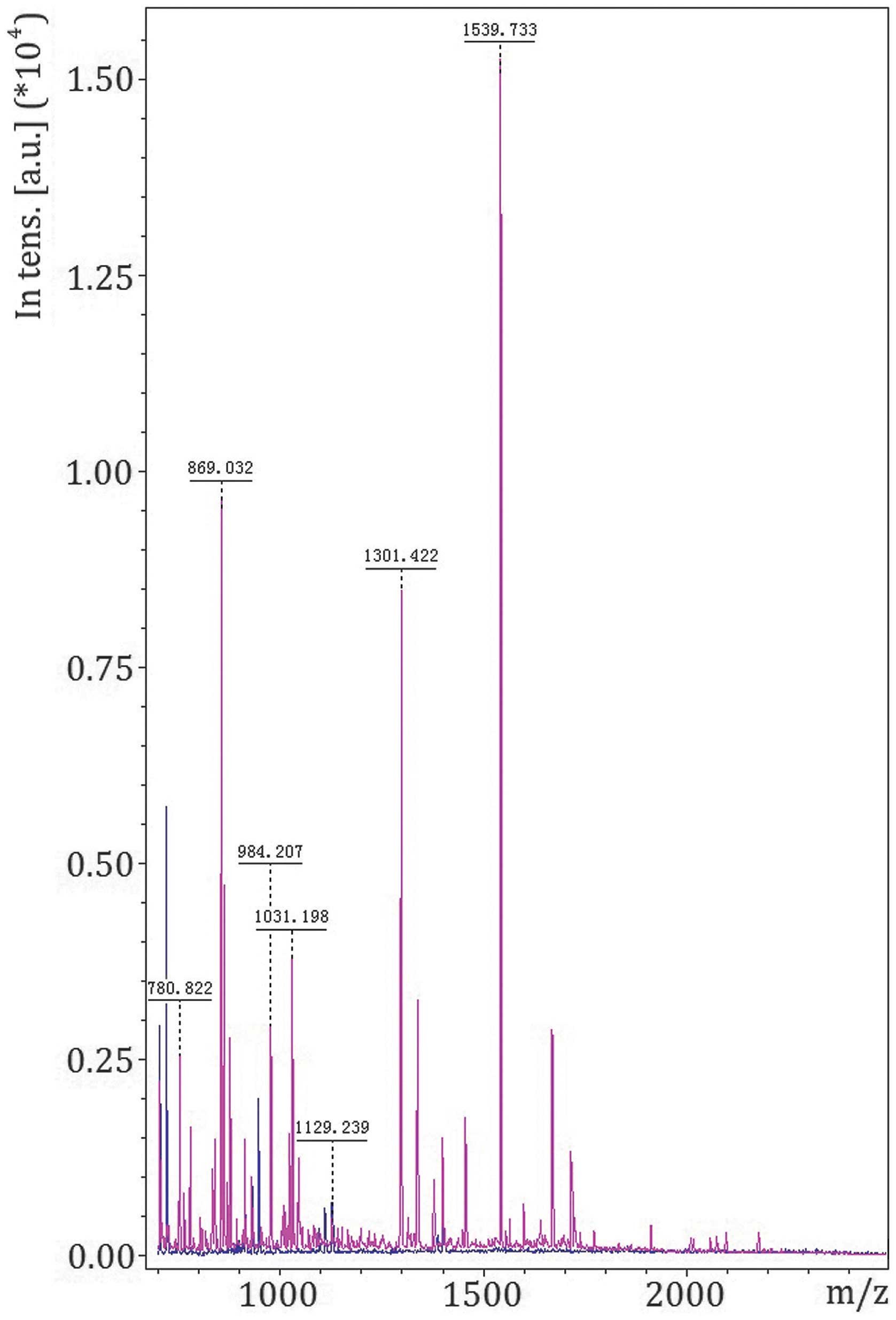
Target protein identification
Following gel electrophoresis, the target protein
with an m/z of 9,348 Da was harvested. The protein band
corresponding to the protein peak was collected and treated using
in-gel digestion. MALDI-TOF-MS was used for MS of peptides
following digestion (Fig. 3), and the
amino acid sequences were determined (Table I). The peptide sequences were analyzed
for matching and recombination to generate the amino acid sequence
of the complete protein (Table II).
Mascot searching software was used to search for a corresponding
protein in the SwissProt database, revealing a protein with an m/z
of 9,348 Da and a coverage rate of 45.0%; the match score with Apo
A-I was 88.
 | Table I.Amino acid sequence of each peptide
yielded by protein digestion as determined by matrix-assisted laser
desorption/ionization-time of flight-mass spectrometry. |
Table I.
Amino acid sequence of each peptide
yielded by protein digestion as determined by matrix-assisted laser
desorption/ionization-time of flight-mass spectrometry.
| M/Z | Protein | Peptides
identified | Sequence |
|---|
| 9,348 | Apolipoprotein
A-I | K.WQEEMELYRQK.V |
KWQEEMELYRQKVEPLRAE |
|
|
| R.QKVEPLR.A |
LQEGARQKLHELQEKLSPLGE |
|
|
| R.AELQEGARQK.L |
EMRDRARAHVDALRTHLAPY |
|
|
| K.LSPLGEEMR.D |
SDELRQRLAARLEALKENG |
|
|
| R.AHVDALR.T |
|
|
|
| R.THLAPYSDELR.Q |
|
|
|
| R.LAARLEALK.E |
|
 | Table II.Amino acid sequence of the full-length
protein obtained by matching and recombination of peptides. |
Table II.
Amino acid sequence of the full-length
protein obtained by matching and recombination of peptides.
| M/Z | Protein | Confirmed
peptide | Coverage rate
(%) | Score |
|---|
| 9,348 | Apolipoprotein
A-I |
KWQEEMELYRQKVEPLRA | 45.0 | 88 |
|
|
|
ELQEGARQKLHELQEKLS |
|
|
|
|
|
PLGEEMRDRARAHVDAL |
|
|
|
|
|
RTHLAPYSDELRQRLAAR |
|
|
|
|
| LEALKENG |
|
|
Confirmation of Apo A-I expression by
qPCR and ELISA
To confirm that the protein identified by
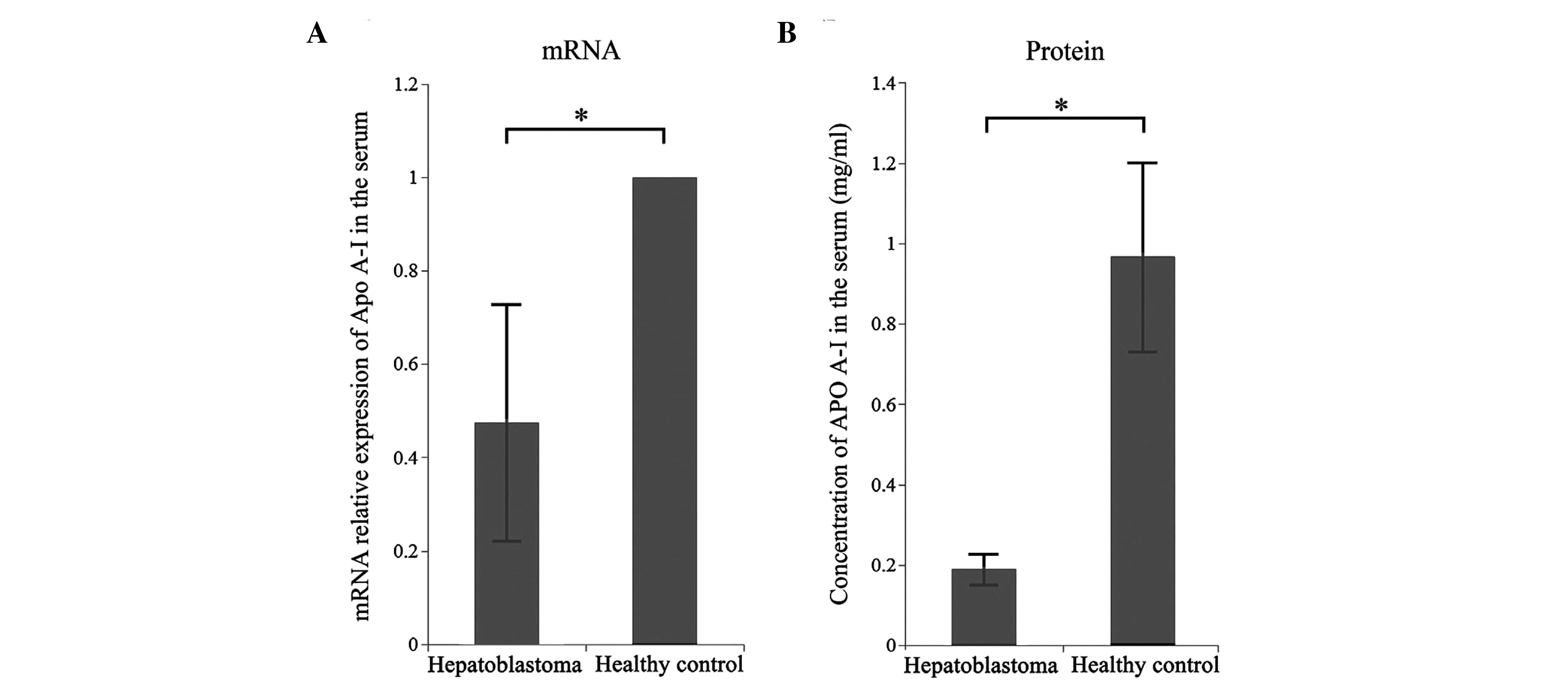
MALDI-TOF-MS was Apo A-I, fluorescence qPCR was performed to detect
Apo A-I mRNA expression in serum. The results demonstrated that Apo
A-I mRNA expression levels were significantly reduced in children
with hepatoblastoma compared with healthy children (P<0.05;
Fig. 4A).
In addition, ELISA was employed to detect the levels
of Apo A-I protein in the serum of children with hepatoblastoma and
healthy children. A standard curve was constructed using standard
samples at different concentrations, and the concentration of Apo
A-I in the serum of children with hepatoblastoma and healthy
children was calculated according to the standard curve. The Apo
A-I concentration was significantly reduced in children with
hepatoblastoma compared with healthy controls (0.1898±0.0387 vs.
0.9707±0.2372 mg/ml, respectively; P<0.05; Fig. 4B).
Discussion
In the present study, SELDI-TOF-MS revealed a
protein with an m/z of 9,348 Da that was expressed at significantly
reduced levels in the serum of children with hepatoblastoma
compared with healthy controls (P<0.01); the interference of
inflammatory proteins in this result was excluded. Sequence
analysis identified this protein as Apo A-I. qPCR and ELISA
demonstrated that the expression levels of Apo A-I mRNA and protein
were significantly reduced in children with hepatoblastoma compared
with healthy controls (P<0.05). Collectively, these findings
indicate that Apo A-I is a potential biomarker for
hepatoblastoma.
A variety of inflammatory factors and proteins are
important in the occurrence and development of malignancies, and
their expression may further augment the inflammatory response. The
association between inflammation and malignancies is therefore
complex (21,22). Inflammatory proteins may confound the
identification of markers that are solely specific to tumors
(18). Previous studies have
demonstrated that high serum levels of IL-10, IL-1, IL-6, and TNF-α
are closely associated with renal, colon and oral cancer (23–25). To
overcome the influence of inflammatory proteins in the search for
biomarkers specific to hepatoblastoma, the present study identified
a differentially expressed protein with m/z of 5,833 Da that was
associated with inflammation by comparative screening of healthy
controls and children with SIRS. This protein was different to the
protein with an m/z of 9,348 Da that was identified to be
differentially expressed between hepatoblastoma and healthy
controls. The protein with an m/z of 5,833 Da identified in this
screening was then eliminated as an interfering factor, and the aim
was to identify a biomarker of hepatoblastoma that is not
associated with inflammation.
SELDI-TOF-MS analysis demonstrated that the Apo A-I
protein was expressed at significantly reduced levels in the serum
of children with hepatoblastoma compared with healthy controls.
Since this protein was not eliminated in the SIRS inflammatory
protein screening, this protein may be considered to be a specific
marker for hepatoblastoma. Apo A-I is an important structural
protein in the high-density lipoprotein family, and the most
abundant member of the Apo A family. This 28 kDa, 243-amino-acid
protein is predominantly synthesized in the liver but is expressed
in the majority of tissues (26). Apo
A-I is involved in several steps of lipid metabolism, stabilization
of lipoprotein structure and the regulation of lipoprotein
metabolism (27). Abnormalities in
the structure or concentration of Apo A-I can lead to lipid
metabolism disorders and induce pathological changes, including
hypertension and insulin resistance (28,29).
An association between Apo A-I and malignancies has
been reported: A previous study demonstrated that Apo A-I is a
serum biomarker specific for early-stage ovarian cancer that can be
used for diagnosis (32). In order to
study Apo A-I function further, 18 amino-acid peptides (Apo A-I
mimetic peptides) have been designed (31). These peptides are of different
subtypes (2F, 3F, 4F, 5F, 6F and 7F); all have helical structures
similar to that of Apo A-I and anti-oxidative and hydrophilic
properties. Gao et al (32)
demonstrated that Apo A-I mimetic peptide L-4F regulates hypoxia
inducible factor-1α expression to inhibit the expression of
vascular endothelial cell growth factor and angiogenesis in a
number of different types of cancer. L-4F also induces the
apoptosis of ovarian cancer cells, exerting antitumor effects on
ovarian cancer (32). The expression
of Apo A-I in early stage ovarian cancer does not preclude the use
of Apo A-I as a specific biomarker for the early diagnosis of
hepatoblastoma in children as ovarian cancer does not occur in
preschool children.
In conclusion, the present study demonstrates that
Apo A-I expression is reduced in children with hepatoblastoma
compared with healthy controls. These findings indicate that Apo
A-I may be used as a serum biomarker for hepatoblastoma in
children. Additional studies are required to determine the actual
efficacy of Apo A-I as a biomarker in clinical practice. Studies
with a large patient cohort are required to determine the
specificity and sensitivity of Apo A-I in diagnosing hepatoblastoma
in children, and to identify any advantages of Apo A-I over other
diagnostic tools. In addition, the association between Apo A-I and
the occurrence and development of hepatoblastoma should be
investigated to determine the role of Apo A-I in the pathogenesis
of hepatoblastoma.
Acknowledgements
The present study was conducted within the
Department of Pediatric Surgery, supported by the First Affiliated
Hospital of Zhengzhou University. The authors thank the personnel
of the Institute of Cancer, The Second Affiliated Hospital, College
of Medicine of Zhejiang University (Hangzhou, China) for their
excellent assistance. The study was financially supported by the
National Natural Science Foundation of China (grant no. 81172085).
The authors would also like to thank all the patients who
participated.
References
|
1
|
Zynger DL, Gupta A, Luan C, Chou PM, Yang
GY and Yang XJ: Expression of glypican 3 in hepatoblastoma: an
immunohistochemical study of 65 cases. Hum Pathol. 39:224–230.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ismail H, Broniszczak D, Kaliciński P,
Dembowska-Bagińska B, Perek D, Teisseyre J, Kluge P, Kościesza A,
Lembas A and Markiewicz M: Changing treatment and outcome of
children with hepatoblastoma: analysis of a single center
experience over the last 20 years. J Pediatr Surg. 47:1331–1339.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baertschiger RM, Ozsahin H, Rougemont AL,
et al: Cure of multifocal panhepatic hepatoblastoma: is liver
transplantation always necessary? J Pediatr Surg. 45:1030–1036.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan FS, Xu M, Wang W, Zhou LY and Xie XY:
Infantile hepatic hemangioendothelioma in comparison with
hepatoblastoma in children: clinical and ultrasound features. Hepat
Mon. 13:e111032013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alvarez-Chaver P, Otero-Estévez O, Páez de
la Cadena M, Rodríguez-Berrocal FJ and Martínez-Zorzano VS:
Proteomics for discovery of candidate colorectal cancer biomarkers.
World J Gastroenterol. 20:3804–3824. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Silberring J and Ciborowski P: Biomarker
discovery and clinical proteomics. Trends Analyt Chem. 29:128–140.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maurya P, Meleady P, Dowling P and Clynes
M: Proteomic approaches for serum biomarker discovery in cancer.
Anticancer Res. 27((3A)): 1247–1255. 2007.PubMed/NCBI
|
|
8
|
de Wit M, Fijneman RJ, Verheul HM, Meijer
GA and Jimenez CR: Proteomics in colorectal cancer translational
research: biomarker discovery for clinical applications. Clin
Biochem. 46:466–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Humphries JM, Penno MA, Weiland F, et al:
Identification and validation of novel candidate protein biomarkers
for the detection of human gastric cancer. Biochim Biophys Acta.
1844:1051–1058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Q, Wang J, Dong R, Yang S and Zheng
S: Identification of novel serum biomarkers in child nephroblastoma
using proteomics technology. Mol Biol Rep. 38:631–638. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Q, Shan Y, Wang JX, Dong R, Yang SB
and Zheng S: Detection of biomarkers in children with Wilms' tumor
using proteinchip technology. Chin Med J (Engl). 123:1939–1941.
2010.PubMed/NCBI
|
|
12
|
Wang J, Wang L, Zhang D, Fan Y, Jia Z, Qin
P, Yu J, Zheng S and Yang F: Identification of potential serum
biomarkers for Wilms tumor after excluding confounding effects of
common systemic inflammatory factors. Mol Biol Rep. 39:5095–5104.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Zhang X, Ge X, Guo H, Xiong G and
Zhu Y: Proteomic studies of early-stage and advanced ovarian cancer
patients. Gynecol Oncol. 111:111–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Skytt A, Thysell E, Stattin P, Stenman UH,
Antti H and Wikström P: SELDI-TOF MS versus prostate specific
antigen analysis of prospective plasma samples in a nested
case-control study of prostate cancer. Int J Cancer. 121:615–620.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu D, Cao L, Yu J, Que R, Jiang W, Zhou Y
and Zhu L: Diagnosis of pancreatic adenocarcinoma using protein
chip technology. Pancreatology. 9:127–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hundt S, Haug U and Brenner H: Blood
markers for early detection of colorectal cancer: a systematic
review. Cancer Epidemiol Biomarkers Prev. 16:1935–1953. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gonçalves A, Bertucci F, Birnbaum D and
Borg JP: Protein profiling SELDI-TOF and breast cancer: clinical
potential applications. Med Sci (Paris). 23:23–26. 2007.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kelly-Spratt KS, Pitteri SJ, Gurley KE, et
al: Plasma proteome profiles associated with inflammation,
angiogenesis, and cancer. PLoS One. 6:e197212011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan Y, Shi L, Liu Q, et al: Discovery and
identification of potential biomarkers of papillary thyroid
carcinoma. Mol Cancer. 8:792009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu T, Wang J and Fang Y: A model-free
estimation for the covariate-adjusted Youden index and its
associated cut-point. Stat Med. 33:4963–4974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chechlinska M, Kowalewska M and Nowak R:
Systemic inflammation as a confounding factor in cancer biomarker
discovery and validation. Nat Rev Cancer. 10:2–3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou JM, Zhao X, Tian L, et al:
Immunotherapy of tumors with recombinant adenovirus encoding
macrophage inflammatory protein 3beta induces tumor-specific immune
response in immunocompetent tumor-bearing mice. Acta Pharmacol Sin.
30:355–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kondo T, Ito F, Nakazawa H, Horita S,
Osaka Y and Toma H: High expression of chemokine gene as a
favorable prognostic factor in renal cell carcinoma. J Urol.
171:2171–2175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Grevenstein WM, Hofland LJ, van Rossen
ME, van Koetsveld PM, Jeekel J and van Eijck CH: Inflammatory
cytokines stimulate the adhesion of colon carcinoma cells to
mesothelial monolayers. Dig Dis Sci. 52:2775–2783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jablonska E, Piotrowski L and Grabowska Z:
Serum Levels of IL-1β, IL-6, TNF-α, sTNF-RI and CRP in Patients
with Oral Cavity Cancer. Pathol Oncol Res. 3:126–129. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Law SW, Lackner KJ, Fojo SS, Hospattankar
A, Monge JC and Brewer HB Jr: The molecular biology of human
apoA-I, apoA-II, apoC-II and apoB. Adv Exp Med Biol. 201:151–162.
1986.PubMed/NCBI
|
|
27
|
Frank PG and Marcel YL: Apolipoprotein
A-I: Structure-function relationships. J Lipid Res. 41:853–872.
2000.PubMed/NCBI
|
|
28
|
Ma YQ, Thomas GN and Tomlinson B:
Association of two apolipoprotein A-I gene MspI polymorphisms with
lipid and blood pressure levels. Int J Cardiol. 102:309–314. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du T, Yuan G, Zhang M, Zhou X, Sun X and
Yu X: Clinical usefulness of lipid ratios, visceral adiposity
indicators, and the triglycerides and glucose index as risk markers
of insulin resistance. Cardiovasc Diabetol. 13:1462014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su F, Lang J, Kumar A, et al: Validation
of candidate serum ovarian cancer biomarkers for early detection.
Biomark Insights. 2:369–375. 2007.PubMed/NCBI
|
|
31
|
Getz GS, Wool GD and Reardon CA:
Apoprotein A-I mimetic peptides and their potential
anti-atherogenic mechanisms of action. Curr Opin Lipidol.
20:171–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao F, Chattopadhyay A, Navab M, et al:
Apolipoprotein A-I mimetic peptides inhibit expression and activity
of hypoxia-inducible factor-1α in human ovarian cancer cell lines
and a mouse ovarian cancer model. J Pharmacol Exp Ther.
342:255–262. 2012. View Article : Google Scholar : PubMed/NCBI
|