Introduction
Perivascular epithelioid cell neoplasm (PEComa) is
rare type of tumor that originates in mesenchymal tissues, and was
first reported in 1996 (1). PEComa is
defined by the World Health Organization Classification of Tumors
of Soft Tissue and Bone as a mesenchymal tumor composed of
perivascular epithelioid cells with unique histological properties
and immunophenotypes (2). The PEComa
family includes angiomyolipoma (AML), clear cell ‘sugar’ tumor
(CCST), lymphangioleiomyomatosis (LAM), clear cell myomelanocytic
tumor of the ligamentum teres/falciform ligament (CCMMT) and PEComa
not otherwise specified (PEComa NOS) (3). PEComas rarely originate in the
retroperitoneum and, even when this does occur, the majority of
tumors are benign (4).
Retroperitoneal PEComas generally occur in women of ~50 years of
age (4). Patients with
retroperitoneal PEComa generally do not present with any
discomfort, but occasionally exhibit non-specific symptoms, for
example back pain, abdominal pain or a sense of oppression
(4,5).
The current study reports a rare case of primary
retroperitoneal malignant PEComa involving the nearby
retroperitoneal organs, which demonstrated similar imaging
manifestations to those of a stromal tumor.
Case report
A 51-year-old female was admitted to hospital after
experiencing stiffness and discomfort in the upper right abdomen
for longer than one month, and subsequently, an opacity in the
retroperitoneum was revealed by ultrasonography. The patient's
symptoms of stiffness and discomfort were accompanied by a
limitation in deep breathing and nighttime dyscoimesis of unknown
cause. The stiffness recovered spontaneously after several hours.
The patient did not present with any additional symptoms, for
example nausea, vomiting, diarrhoea, melena, coughing or
expectoration. During laboratory tests, routine urine analysis
revealed the following: Erythrocytes, 27.2/µl (normal,
0.0–22.7/µl); urobilinogen, 50 µmol/l (normal, 1.7–30.0 µmol/l) and
bacteria, 425.6/µl (normal, 0.0–130.7/µl). Blood routine was
normal, while levels of tumor markers, including α-fetoprotein,
carcinoembryonic antigen, carbohydrate antigens (19-9, 125 and
15-3) and ferritin, were also normal. Whole abdomen enhanced
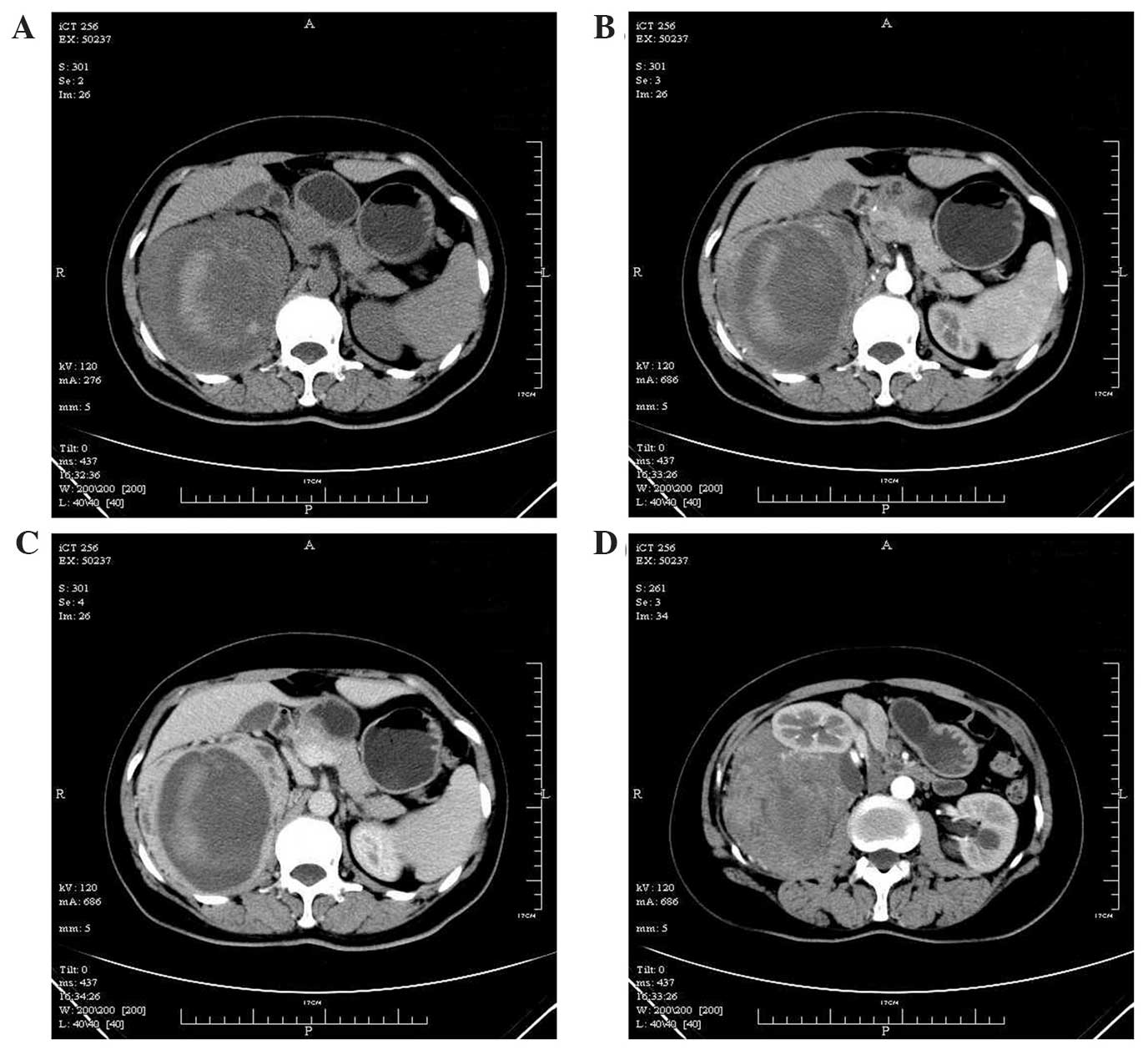
computed tomography (CT) identified a massive soft-tissue density
shadow of 11.1×10.7×20.0 cm to the rear of the right kidney.
Multi-nodular and massive low-density shadows were observed in the
mass, with striped high-density shadows within the low-density area
(Fig. 1A). A plain scan revealed a CT
value of 29.2 Hounsfield units (HU) in the solid section, while an
enhanced scan demonstrated an unevenly enhanced solid section with
a CT value of 44.6 HU at arterial phase and 77.1 HU at venous phase
(Fig. 1B and C). The cystic section
of the tumor was not enhanced. The tumor was enclosed and fed with
an artery branching from the abdominal aorta and was clearly
demarcated; however, the tumor borderline with the right kidney was
unclear, and the right adrenal gland and kidney were displaced
forward (Fig. 1D). The patient was
preoperatively diagnosed as most likely having a retroperitoneal
stromal tumor. Following complete preoperative preparation, the
patient received resection of the retroperitoneal tumor and the
involved right kidney. The findings observed during the surgery
were as follows: A large mass (12×20 cm) occupied the right side of
the retroperitoneum with a clear margin and capsule. The upper pole
of the tumor reached the diaphragmatic dome and was partially
connected with the liver and the diaphragm muscle, while the lower
pole remained dissociated, and its interior margin abutted the
right kidney, which was also found to be partially involved during
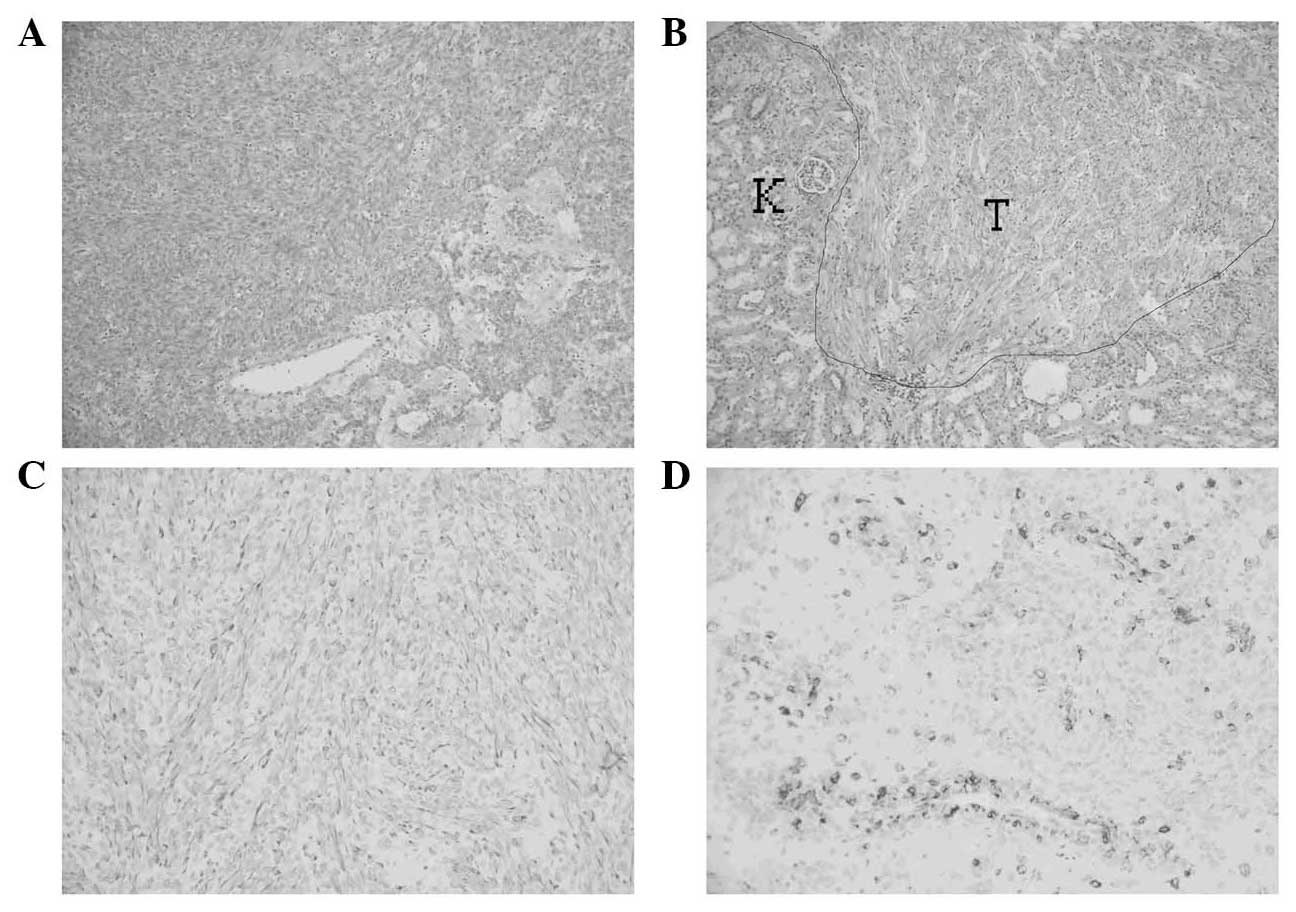
the separation. Pathological examination with hematoxylin and eosin
staining revealed that the tumor cells were fusiform and arranged
in bundles and microcapsules (Fig.
2A). The uniformly-sized cells occasionally demonstrated
mitotic figures, and the focus invaded the kidney (Fig. 2B), but not the adrenal gland.
Immunohistochemical (IHC) staining results were:
Pan-cytokeratin(–), Melan-A(–), HMB-45(+), smooth muscle actin
(SMA)(+), Desmin(+), CD34(–), P53(+) and Ki-67(+, 2%) (Fig. 2C and D). The final diagnosis was
retroperitoneal PEComa involving the kidney. The patient recovered
well following surgery, without further treatment. No recurrence
was identified during the follow up at 7 months post surgery.
Written informed consent for the use of medical data
for teaching and research was obtained from the patient upon
admission.
Discussion
PEComa is a rare type of tumor originating in
mesenchymal tissues. The characteristics of PEComas-NOS are that,
microscopically, the majority of perivascular epithelioid cells
surround the blood vessels and are arranged radially around the
lumen, forming bunch- and web-shaped structures (1–3). A PEComa
is composed of two sections, the epithelioid cells surrounding the
vessels and the spindle cells distal to the vessels, and the
proportions of the two parts may vary significantly (3). Certain cases demonstrate clear nuclear
atypia and mitosis (3). Sclerosing
PEComa occurs when the tumor cells are embedded in the collagenized
or hyalinized tumor stroma (4). A
group of sclerosing PEComas were previously reported, and the
majority occurred in the retroperitoneum (4). The case in the present study presented
with typical manifestations of PEComa, but did not demonstrate
obvious interstitial sclerosis. The typical IHC characteristics of
PEComa are tumor cell expression of melanocyte and muscle cell
markers; however, the expression levels of these markers differ
between patients (4). The most
sensitive markers are Desmin and HMB-45, followed by SMA,
Caldesmon, and MiTF, while Melan-A and S-100 may be positively
expressed in a lower proportion of cases (4). In the present case, HMB-45 was expressed
positively and diffusely in the epithelioid cells, while SMA and
Desmin were expressed diffusely in the spindle cells. Though most
PEComas are benign, approximately half of PEComas-NOS are malignant
(5,6).
As the number of reported cases is small, there are no consistent
criteria for the diagnosis of benign or malignant PEComas. However,
empirically-based criteria are defined as follows: Benign tumor,
diameter <5 cm, no necrosis or vascular invasion, no
infiltrative growth, with occasional nuclear atypia, and nuclear
division ≤1/50 HPF, without definite malignant potential, with only
nuclear pleomorphism/polykaryocytes or only tumor diameter >5
cm; malignant tumor, diameter >5 cm, necrosis, infiltrative
growth or vascular invasion, clear nuclear atypia and nuclear
division ≥1/50 HPF (7). The present
case exhibited occasional nuclear division, but the diameter of the
lesion was >5 cm, and presented with bleeding, necrosis and
invasion into the right kidney, all of which were consistent with
the definition of malignant PEComa.
PEComas are associated with specific imaging
manifestations according to their location. AMLs commonly occur in
the liver or kidney, and may be diagnosed by the detection of fat
components on CT or magnetic resonance (MR) imaging, with obvious
enhancement on the enhanced scan. The key in diagnostic imaging is
still the detection of fat components in low-fat AML or epithelioid
AML (8–10). CCSTs are mainly benign and manifest as
peripheral and smooth-edged quasi-circular nodules, without
cavities or calcification, which may be markedly enhanced (11,12).
Malignant CCSTs are manifested as multi-nodules or masses, with
occasional calcification and metastasis to the liver, adrenal gland
or brain (13). LAM manifest on
high-resolution CT as quasi-circular and almost wall-less cysts in
diffuse distribution, likely accompanied by pneumothorax, pleural
effusion or chylothorax, and occasionally with small AML and
retroperitoneal lymphangioma in the kidneys (14). There has been little research
regarding the imaging characteristics of CCMMT (15,16). The
imaging features of a group of malignant PEComas include signs of
occurrence in the retroperitoneum, smooth-edged masses and uneven
T2WI signals, likely with additional symptoms, including
calcification, necrosis, bleeding, invasion into nearby veins and
marked enhancement, however these imaging features are mostly
non-specific (17). The major target
organs of metastasis are the lungs and liver (17). The majority of reported PEComas occur
in retroperitoneal organs. However, the case described in the
present study originated in the retroperitoneum. Furthermore, it
was initially reported that the PEComa invaded the nearby
retroperitoneal organs (the right kidney), which may explain the
increased erythrocyte content detected in the urine. In another 33
cases of PEComa occurring at various sites, two retroperitoneal
cases displayed large lesions and enhancement, of which one was
detected by fat components (16). Two
recently reported primary retroperitoneal PEComas manifested as
fat-rich masses with enhanced nodules or vascular structure
(5,18). Therefore, the detection of fat
components and the display of blood-supply-rich components may be
two valuable clues for imaging-based diagnosis of retroperitoneal
PEComas. In the present case, the enhanced CT only clarified the
rich-blood-supply soft tissues, and thus other rich-blood-supply
lesions, for example sarcoma or stromal tumor, should be
distinguished. No fat component was detected by plain CT scan or
histopathological examination, however, marked intratumoral
bleeding was detected, all of which differed from the results of
previous imaging reports. It was hypothesized that the immaturity
of tumor blood vessels is likely to induce ischemic necrosis and
cystic degeneration in the focus, while the incomplete vascular
walls may result in the rupture and bleeding of microvessels.
At present, primary and isolated metastatic PEComas
are mainly treated by surgery, with good prognosis (3). Malignant PEComas may be treated with
chemotherapy or immunotherapy, but with poor prognosis (19,20). In
the present case, the mass possessed clear edges, but had locally
invaded the right kidney, and thus the excision of the tumor and
associated right kidney was performed. Since the tumor was
completely excised, no further treatment was applied. No recurrence
was identified during the follow-up at 7 months post surgery.
In conclusion, retroperitoneal PEComa is a rare type
of tumor, and its diagnosis remains difficult at preoperative
imaging. However, diagnostic imaging will facilitate the selection
of an appropriate treatment strategy. Locally invasive PEComa may
be treated with surgery. The imaging characteristics and
therapeutic methods for the diagnosis and treatment of
retroperitoneal PEComa should be further evaluated.
References
|
1
|
Zamboni C, Pea M, Martignoni G, et al:
Clear cell ‘sugar’ tumor of the pancreas. A novel member of the
family of lesions characterized by the presence of perivascular
epithelioid cells. Am J Surg Pathol. 20:722–730. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folpe AL: Neoplasms with perivascular
epithelioid cell differentiation (PEComas)World Health Organization
Classification of Tumors: Pathology and Genetics of Tumors of Soft
Tissue and Bone. Fletcher CDM, Unni KK and Mertens F: IARC Press;
Lyon: pp. 221–222. 2002
|
|
3
|
Armah HB and Parwani AV: Perivascular
epithelioid cell tumor. Arch Pathol Lab Med. 133:648–654.
2009.PubMed/NCBI
|
|
4
|
Hornick JL and Fletcher CD: Sclerosing
PEComa: Clinicopathologic analysis of a distinctive variant with a
predilection for the retroperitoneum. Am J Surg Pathol. 32:493–501.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pata G, Tironi A, Solaini L, et al:
Perivascular epithelioid cell tumor located retroperitoneally with
pulmonary lymphangioleiomyomatosis: Report of a case. Surg Today.
44:572–576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alaggio R, Cecchetto G, Martignoni G, et
al: Malignant perivascular epithelioid cell tumor in children:
Description of a case and review of the literature. J Pediatr Surg.
47:e31–e40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folpe AL, Mentzel T, Lehr HA, et al:
Perivascular epithelioid cell neoplasms of soft tissue and
gynecologic origin. Am J Surg Pathol. 29:1558–1575. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Y, Ouyang H, Wang X, et al: MRI
manifestations of liver epithelioid and nonepithelioid
angiomyolipoma. J Magn Reson Imaging. 39:1502–1508. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JY, Kim JK, Kim N and Cho KS: CT
histogram analysis: Differentiation of angiomyolipoma without
visible fat from renal cell carcinoma at CT imaging. Radiology.
246:472–479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Froemming AT, Boland J, Cheville J, et al:
Renal epithelioid angiomyolipoma: Imaging characteristics in nine
cases with radiologic-pathologic correlation and review of the
literature. AJR Am J Roentgenol. 200:178–186. 2013. View Article : Google Scholar
|
|
11
|
Seo JB, Im JG, Seo JW and Yeon KM: Clear
cell tumor of the lung. AJR Am J Roentgenol. 166:730–731. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santana AN, Nunes FS, Ho N and Takagaki
TY: A rare cause of hemoptysis: Benign sugar (clear) cell tumor of
the lung. Eur J Cardiothorac Surg. 25:652–654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lim HJ, Lee HY, Han J, et al: Uncommon of
the uncommon: Malignant perivascular epithelioid cell tumor of the
lung. Korean J Radiol. 14:692–696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pallisa E, Sanz P, Roman A, et al:
Lymphangioleiomyomatosis: Pulmonary and abdominal findings with
pathologic correlation. Radiographics. 22:185–198. 2002. View Article : Google Scholar
|
|
15
|
Folpe AL, Goodman ZD, Ishak KG, et al:
Clear cell myomelanocytic tumor of the falciform
ligament/ligamentum teres: A novel member of the
perivascularepithelioid clear cell family of tumors with a
predilection for children and young adults. Am J Surg Pathol.
24:1239–1246. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan Y, Zhang H and Xiao EH: Perivascular
epithelioid cell tumour: Dynamic CT, MRI and clinicopathological
characteristics - analysis of 32 cases and review of the
literature. Clin Radiol. 68:555–561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tirumani SH, Shinagare AB, Hargreaves J,
et al: Imaging features of primary and metastatic malignant
perivascular epithelioid cell tumors. AJR Am J Roentgenol.
202:252–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wildgruber M, Becker K, Feith M and Gaa J:
Perivascular epitheloid cell tumor (PEComa) mimicking
retroperitoneal liposarcoma. World J Surg Oncol. 12:32014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeon IS and Lee SM: Multimodal treatment
using surgery, radiotherapy and chemotherapy in a patient with a
perivascular epithelioid cell tumor of the uterus. J Pediatr
Hematol Oncol. 27:681–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parfitt JR, Bella AJ, Wehrli BM and Izawa
JI: Primary PEComa of the bladder treated with primary excision and
adjuvant interferon-α immunotherapy: A case report. BMC Urol.
6:202006. View Article : Google Scholar : PubMed/NCBI
|