Introduction
Human papillomavirus (HPV) is a double-stranded DNA
virus, and is an established etiological agent that may lead to the
development of cervical cancer following infection. In total,
>100 different HPV genotypes have been identified. HPVL1 is the
main structural and major capsid protein of HPV, which contains
stable immunogenicity. Thus, HPVL1 detection can indicate HPV
infection. High-risk HPV16 and HPV18 are the most common oncogenic
types, leading to the development of cervical carcinomas and a
variety of different types of reproductive system malignant tumor
(1–4).
Lung cancer is one of the most common cancers and the leading cause
of cancer-associated mortality in the world. The pathogenesis of
lung cancer is complex, and is considered to be a result of the
interaction between environmental and genetic factors. Previous
studies have demonstrated that high-risk HPV16/18 may be closely
associated with lung cancer (5–9), but the
mechanisms are not yet fully elucidated.
The fragile histidine triad (FHIT) gene is a
candidate tumor suppressor gene located at chromosome 3p14.2 and
encompassing the FRA3B common fragile site (the most active
chromosome breakage site in the human genome). FHIT anomalies are
associated with the occurrence and development of a variety of
malignant tumors. When a tumor develops, tumor cell proliferation
gradually increases, while FHIT protein expression decreases. A
previous study of cervical squamous cell carcinomas (SCCs)
demonstrated that the HPV16 integration point was between FHIT exon
3 and 5. During the process of HPV16 integration, it may induce the
deletion of exons within FHIT leading to its inactivation (10–12).
Although data has indicated that there may be an association
between FHIT loss and HPV infection, the exact mechanism remains
poorly understood. FHIT loss is a frequent event in HPV-positive
lung cancer (13), indicating that
FHIT loss may be significant in the occurrence and development of
HPV-associated lung cancer. FHIT loss has been observed in the
early stages of tumorigenesis and may be a potential marker of
HPV-associated lung cancer.
Our previous study identified that mutations in p53
contributed to high-risk HPV16/18-infected lung cancer cases
(14). The role of p53 is to
safeguard the integrity of the genome by inducing cell cycle arrest
or apoptosis in response to DNA damage (15). In HPV16/18-infected lung cancer cases,
E6 is bound to the p53 tumor suppressor gene, and thereby induces
p53 degradation or mutation (14).
One hypothesis is that there is a correlation between high-risk
HPV16/18 infection and FHIT loss and p53 mutation. However, further
study has not been conducted to investigate this potential
association in HPV-associated lung cancer. The role of HPV16/18 in
the development of lung cancer has previously been investigated,
and FHIT loss and p53 mutation have been frequently observed in the
disease; however, the association between them is not well studied.
The present study further investigated the significance of HPV
infection, FHIT loss and p53 mutation in the development of lung
cancer and their possible associations.
Materials and methods
Ethical statement
The study was approved by the Ethics Committee of
Xi'an Jiaotong University (Xi'an, Shaanxi, China; no. 20120099).
Written informed consent was obtained from each patient prior to
participation in the study.
Patients and samples
A total of 290 samples, including 88 lung SCCs, 56
lung adenocarcinomas (AC), 36 lung small cell lung carcinomas
(SCLC) and 110 non-cancer controls were collected from patients
diagnosed in The First Affiliated Hospital of Medical School of
Xi'an Jiaotong University. All cases were selected from the period
between January 2012 and May 2014, and all specimens were
classified by three experienced pathologists according to the World
Health Organization classification.
The lung cancer samples included 85 undifferentiated
or poorly-differentiated carcinomas, 57 moderately-differentiated
carcinomas and 38 well-differentiated carcinomas. The samples were
obtained from 53 female (29.44%) and 127 male (70.56%) individuals,
with an age range between 30 and 80 years and a mean age of 51.56
years. A history of smoking was present in 124 cases (68.89%) and
absent in 56 cases (31.11%).
The 110 non-cancer control samples included 38
bronchiectasis samples, 21 inflammatory pseudotumors, 33 pulmonary
tuberculomas and 18 pulmonary cysts. The samples were from 18
female (16.36%) and 92 male (83.64%) individuals, with an age range
of between 25 and 75 years and a mean age of 51.48 years.
Reagents
The rabbit anti-FHIT polyclonal antibody (primary
antibody; dilution, 1:20; cat. no. PA5-32418; incubation, 4°C
overnight) was produced by Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). The Universal LSABTM+ immunohistochemical kit (cat. no.
K0679), including the biotinylated goat anti-rabbit antibody
(secondary antibody; dilution, 1:50; incubation, 37°C for 15 min)
and enzyme-chain avidin complexes (incubation, 37°C for 15 min),
was produced by Dako (Glostrup, Denmark).
DNA extraction
The 4% paraformaldehyde-fixed and paraffin-embedded
tissue samples were cut into 10-µm slices and placed into 1.5-ml
Eppendorf tubes as described previously (16). The specimens were then treated with 1
ml of xylene, followed by 1 ml of ethanol. Following centrifugation
at 14,020 × g at 4°C for 5 min, the pellet, which contained the
precipitated DNA, was resuspended in digestion buffer (50 mM
Tris-Cl, pH 8.0; 1 mM EDTA, pH 8.0; 0.5% Tween-20) containing 200
µg of proteinase K (Invitrogen Life Technologies, Carlsbad, CA,
USA) and incubated at 56°C overnight. After incubation, the
solution was heated at 100°C for 10 min and centrifuged at 14,020 ×
g at 4°C for 10 min. An aliquot of the supernatant, containing the
precipitated DNA, was stored at −20°C prior to use.
β-globin polymerase chain reaction
(PCR)
The method for β-globin detection and evaluation was
performed as previously described (6). PCO3/PCO4 (110 bp)
primers (PCO3 primer, 5′-ACACAACTGTGTTCACTGC-3′; and
PCO4 primer, 5′-CAACTTCATCCACGTTCACC-3′) were used as
the internal positive control. The PCR conditions were as follows:
Initial denaturation at 95°C for 4 min, 40 cycles with a cycling
profile of 95°C for 1 min, 52°C for 1 min and 72°C for 2 min, and a
final extension for 5 min at 72°C.
Quantitative PCR
The thermal conditions of amplification were as
follows: Initial denaturation at 94°C for 5 min, followed by 30
cycles of 94°C for 30 sec, 45–55°C for 30 sec and 72°C for 1 min,
and a final extension at 72°C for 5 min. The primer sets were
synthesized by Shaanxi Jintai Biological Engineering Co., Ltd.,
(Xi'an, China) and are presented in Table
I.
 | Table I.HPV primer sets and length. |
Table I.
HPV primer sets and length.
| Primer | Sequence | Length, bp |
|---|
| HPVL1 |
5′-CGTCCCAGGGGATACTGATC-3′ | 450 |
|
|
5′-GCCCAGGGTCATAATAATGG-3′ |
|
| HPV16 |
5′-GAACAGCAATACAACAAACCCG-3′ | 315 |
|
|
5′-CCATGCATGATTACAGCTGG-3′ |
|
| HPV18 |
5′-TGCCAGAAACCGTTGAATCC-3′ | 152 |
Negative and positive controls were designed for
each experiment. Sterilized water was used as the negative control
and a HPV-infected condyloma acuminatum specimen was used as the
HPV general positive control. The HPV16- and HPV18-positive control
was DNA purified from infected SiHa cells and HeLa cells,
respectively.
Immunohistochemisty (IHC)
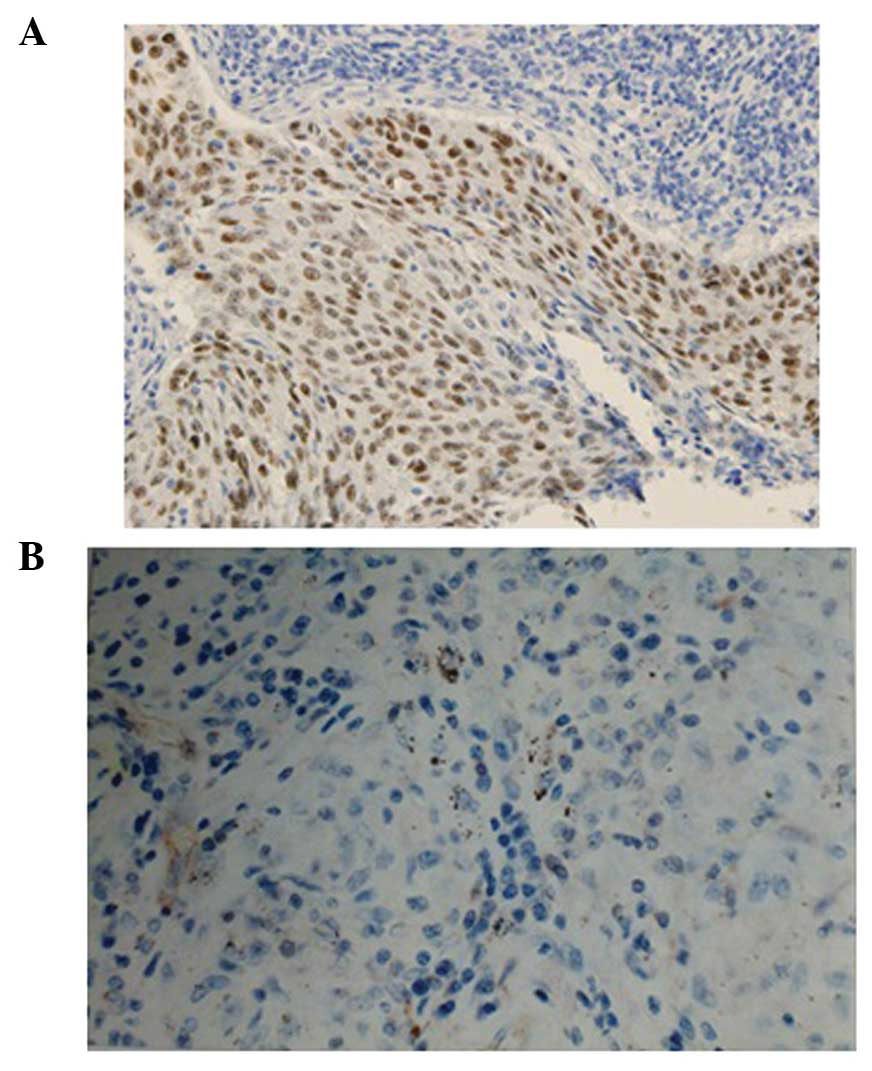
IHC was used to detect the FHIT protein expression
and p53 mutation. The rabbit anti-human FHIT polyclonal antibody
and the SP kit were produced by Thermo Fisher Scientific, Inc.
Phosphate-buffered saline was used as a negative control instead of
the primary antibody. The IHC was undertaken according to the
manufacturer's instructions. The results were evaluated
independently by three observers. FHIT protein was expressed in the
cytoplasm. Staining of FHIT loss was characterized by no
cytoplasmic staining in the tumor cells. Each slide was evaluated
in 10 visual fields under a microscope (BH-2 Optical Microscope,
Olympus Corporation, Tokyo, Japan) at x400 magnification. In each
visual field, 100 cells were evaluated, resulting in a total of
1,000 cells per section; the percentage of positive expression of
each section was then calculated from these observations. The
methods for detecting and evaluating mutations in p53 have been
previously described (15).
Statistical analysis
The SPSS software program, version 17.0 (SPSS, Inc.,
Chicago, IL, USA) was used to apply the χ2 test,
Fisher's exact test and Pearson correlation test for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference. The demographic characteristics review and
statistical analysis were approved by the Department of Public
Health, Xi'an Jiaotong University School of Medicine.
Results
Detection of HPVL1/16/18
infection
In the lung cancer cases and non-cancer controls,
HPVL1 was detected in 100/180 (55.56%) and 8/110 (7.27%) cases,
HPV16 was detected in 67/180 (37.22%) and 7/110 (6.36%) cases and
HPV18 was detected in 56/180 (31.11%) and 6/110 (5.45%) cases,
respectively. The HPVL1, HPV16 and HPV18 detection rate was
increased in the lung cancer group compared with the non-cancer
control group (P<0.001, P<0.001 and P<0.001, respectively)
(Table II).
 | Table II.Detection of HPVL1/16/18
infection. |
Table II.
Detection of HPVL1/16/18
infection.
| Type | Lung cancer
cases | Non-cancer
controls | P-value |
|---|
|
HPVL1+ | 100 (55.56) | 8 (7.27) | <0.001 |
|
HPVL1− | 80 (44.44) | 102 (92.73) |
|
|
HPV16+ | 67 (37.22) | 7 (6.36) | <0.001 |
|
HPV16− | 113 (62.78) | 103 (93.64) |
|
|
HPV18+ | 56 (31.11) | 6 (5.45) | <0.001 |
|
HPV18− | 124 (68.89) | 104 (94.55) |
|
Association between HPV16 infection,
loss of FHIT and pathological characteristics in lung cancer
The HPV16 detection rate was increased in the lung
cancer group compared with the non-cancer control group
(P<0.001; Table III). HPV16
detection was not correlated with the degree of cell
differentiation, tumor-node-metastasis (TNM) stage, lymph node
metastasis, gender or age (P>0.05); however, HPV infection was
significantly associated with the histological type (P=0.006) and
smoking history (P=0.033). The HPV16 detection rate was increased
in the cases with lung SCC (51.14%) and those with a smoking
history (44.35%) compared with the cases of AC (26.79%), SCLC
(19.44%) and those with no smoking history (21.43%). The rate of
FHIT loss was increased in the lung cancer group (44.44%) compared
with the non-cancer control group (7.27%) (P<0.001). In the lung
cancer group, FHIT loss was not correlated with TNM stage, lymph
node metastasis, gender or age (P>0.05); however FHIT loss was
significantly associated with the histological type (P=0.003), the
degree of cell differentiation (P=0.007) and a smoking history
(P<0.001). The rate of FHIT loss was increased in the cases of
lung SCC (60.23%), and in those with a low degree of cell
differentiation (57.65%) or a smoking history (55.65%) compared
with the cases of SCLC (33.33%), AC (26.79%) or those with a
moderate degree of cell differentiation (38.60%), high degree of
cell differentiation (23.68%) or no smoking history (19.64%)
(Table III). In addition, the
coexistence rate of FHIT loss and a smoking history in the 180 lung
cancer cases was 38.33% (69/180) and the Pearson contingency
coefficient was r=0.318, (P<0.001) (Table IV).
 | Table III.Associations between HPV16 infection,
FHIT loss and clinicopathological characteristics in lung
cancer. |
Table III.
Associations between HPV16 infection,
FHIT loss and clinicopathological characteristics in lung
cancer.
| Characteristic |
HPV16+ |
HPV16− | P-value | FHIT
loss+ | FHIT
loss− | P-value |
|---|
| Non-cancer
controls | 7 (6.36) | 103 (93.64) | <0.001 | 8 (7.27) | 102 (92.73) | <0.001 |
| Lung cancer
cases | 67 (37.22) | 113 (62.78) |
| 80 (44.44) | 100 (55.56) |
|
| SCC | 45 (51.14) | 43 (48.86) | 0.006 | 53 (60.23) | 35 (39.77) | 0.003 |
| AC | 15 (26.79) | 41 (73.21) |
| 15 (26.79) | 41 (73.21) |
|
| SCLC | 7 (19.44) | 29 (80.56) |
| 12 (33.33) | 24 (66.67) |
|
| Cell
differentiation |
|
| 0.052 |
|
| 0.007 |
| Poor | 39 (45.88) | 46 (54.12) |
| 49 (57.65) | 36 (42.35) |
|
|
Moderate | 21 (36.84) | 36 (63.16) |
| 22 (38.60) | 35 (61.40) |
|
| Well | 7 (18.42) | 31 (81.58) |
| 9 (23.68) | 29 (76.32) |
|
| TNM stage of
tumors |
|
| 0.723 |
|
| 0.654 |
|
I+II | 43 (38.39) | 69 (61.61) |
| 45 (40.18) | 67 (59.82) |
|
|
III+IV | 24 (35.29) | 44 (64.71) |
| 35 (51.47) | 33 (48.53) |
|
| Lymph node
metastasis |
|
| 0.997 |
|
| 0.965 |
| N0 | 36 (37.50) | 60 (62.50) |
| 42 (43.75) | 54 (56.25) |
|
|
N1–3 | 31 (36.91) | 53 (63.09) |
| 38 (45.24) | 46 (54.76) |
|
| Smoking
history |
|
| 0.033 |
|
| <0.001 |
|
Yes | 55 (44.35) | 69 (55.65) |
| 69 (55.65) | 55 (44.35) |
|
| No | 12 (21.43) | 44 (78.57) |
| 11 (19.64) | 45 (80.36) |
|
| Gender |
|
| 0.706 |
|
| 0.162 |
|
Male | 47 (37.01) | 80 (62.99) |
| 65 (51.18) | 62 (48.82) |
|
|
Female | 20 (37.74) | 33 (62.26) |
| 15 (28.30) | 38 (71.70) |
|
| Age, years |
|
| 0.340 |
|
| 0.866 |
|
<55 | 32 (38.10) | 52 (61.90) |
| 39 (46.43) | 45 (53.57) |
|
|
≥55 | 35 (36.46) | 61 (63.54) |
| 41 (42.71) | 55 (57.29) |
|
 | Table IV.Correlation between FHIT loss and
smoking history in lung cancer cases. |
Table IV.
Correlation between FHIT loss and
smoking history in lung cancer cases.
| Parameter |
Smoking+ |
Smoking− | Total | χ2 | r |
|---|
| FHIT
loss+ | 69 | 11 | 80 |
|
|
| FHIT
loss− | 55 | 45 | 100 |
|
|
| Total | 124 | 56 | 180 | 20.251 | 0.318 |
FHIT loss and p53 mutation in lung
cancer cases and non-cancer controls
For the lung cancer cases and non-cancer controls,
the rate of FHIT loss in the HPVL1-positive group (69.00% and
62.50%, respectively) was significantly increased compared with
that in the HPV-negative group (42.50 and 8.82%; P=0.002 and
P<0.001, respectively; Table V).
In the lung cancer cases, the rate of FHIT loss in the HPV16- and
18-positive groups (77.61 and 71.43%, respectively) was
significantly increased compared with the rate in the HPV16- and
18-negative groups (47.79 and 49.19%; P=0.003 and P=0.029,
respectively).
 | Table V.FHIT loss and p53 mutation in lung
cancer cases and non-cancer controls. |
Table V.
FHIT loss and p53 mutation in lung
cancer cases and non-cancer controls.
| HPV | FHIT
loss+ | FHIT
loss− | P-value |
p53+ |
p53− | P-value |
|---|
| Non-cancer
controls |
|
|
|
|
|
|
|
HPVL1+ | 5
(62.50) | 3
(37.50) | <0.001 | 1 (12.50) | 7 (87.50) | 0.987 |
|
HPVL1− | 9 (8.82) | 93 (91.18) |
| 11 (10.78) | 91 (89.22) |
|
| Lung cancer
cases |
|
|
|
|
|
|
|
HPVL1+ | 69 (69.00) | 31 (31.00) | 0.002 | 71 (71.00) | 29 (29.00) | 0.016 |
|
HPVL1− | 34 (42.50) | 46 (57.50) |
| 39 (48.75) | 41 (51.25) |
|
|
HPV16+ | 52 (77.61) | 15 (22.39) | 0.003 | 51 (76.12) | 16 (23.88) | 0.014 |
|
HPV16− | 54 (47.79) | 59 (52.21) |
| 59 (52.21) | 54 (47.79) |
|
|
HPV18+ | 40 (71.43) | 16 (28.57) | 0.029 | 35 (62.50) | 21 (37.50) | 0.072 |
|
HPV18− | 61 (49.19) | 63 (50.81) |
| 54 (43.55) | 70 (56.45) |
|
IHC was used to investigate p53 mutation in the lung
tissues. In the non-cancer controls, no significant difference was
observed in the p53 mutation rate between the HPVL1-positive group
and the HPVL1-negative group (P=0.987). By contrast, in the lung
cancer cases, the p53 mutation rate in the HPVL1- and
HPV16-positive groups (71.00 and 76.12%, respectively) was
significantly increased compared with the HPVL1- and HPV16-negative
groups (48.75 and 52.21%; P=0.016 and P=0.014, respectively).
However, no significant difference in p53 mutation was observed
between the HPV18-positive group and the HPV18-negative group
(P=0.072) (Table V).
Correlation between FHIT loss and p53
mutation in HPV-positive lung cancer cases
After obtaining the results showing that HPV
infection correlated with FHIT loss and p53 mutation in the lung
tissues, it was also observed that FHIT loss coexisted with p53
mutation in 57.00% (57/100) of the HPV-positive lung cancer cases.
Due to the high coexistence of FHIT loss and p53 mutation in the
HPV-positive lung cancer cases, a correlative study of them was
conducted. The analysis demonstrated that FHIT loss and p53
mutation possessed synergistic effects in the lung cancer cases
with HPV-infection (Pearson contingency coefficient, r=0.357;
P<0.001) (Table VI; Fig. 1).
 | Table VI.Correlation between FHIT loss and p53
mutation in HPV-positive lung cancer cases. |
Table VI.
Correlation between FHIT loss and p53
mutation in HPV-positive lung cancer cases.
| Parameter | p53
mutation+ | p53
mutation− | Total | χ2 | r |
|---|
| FHIT
loss+ | 57 | 12 | 69 |
|
|
| FHIT
loss− | 14 | 17 | 31 |
|
|
| Total | 71 | 29 | 100 | 14.568 | 0.357 |
Discussion
HPV infection, particularly high-risk HPV16/18
infection, has been identified as a risk factor for the development
of cervical cancer, lung cancer and other malignant tumors
(1,2,17,18). Previous studies have reported that the
involvement of HPV16/18 infection in lung tumorigenesis may be
mediated through FHIT and p53 (7,13,14,19,20).
However, the interaction between HPV and FHIT is not fully
understood, and the association between p53 and FHIT in
HPV-infected lung cancer has not been well studied. Therefore the
present study aimed to investigate this area of research.
The present study demonstrated that HPV infection
was more common in lung cancer cases compared with non-cancer
controls (P<0.001). The predominant genotypes were the high-risk
HPV16 and/or HPV18 genotypes. HPV16 detection was not correlated
with the degree of cell differentiation, TNM stage, lymph node
metastasis, gender or age (P>0.05), however, HPV16 infection was
significantly associated with the histological type (P=0.006) and
smoking history (P=0.033). The HPV16 detection rate was increased
in the cases of lung SCC (51.14%) and in those with a smoking
history (44.35%) compared with the cases of AC (26.79%), SCLC
(19.44%) and those with no smoking history (21.43%). Similar to
previous findings (6), the present
study indicated that HPV, particularly high-risk HPV16/18, may be
responsible for lung cancer development.
The integration of high-risk HPV16/18 DNA genotypes
into the host chromosome from the fragile site, FRA3B, which is
adjacent to FHIT, is crucial in HPV-induced cervical carcinogenesis
(21). As an important tumor
suppressor gene, FHIT protein inhibits tumor progression with a
wide range of tumor suppressive functions. FHIT loss consequently
leads to the development of tumors (10,12,22).
Therefore, the present study further investigated FHIT loss in
association with HPV infection and the tumor behavior of lung
cancer.
The results of the present study demonstrated that
the rate of FHIT loss was increased in the lung cancer group
compared with the non-cancer control group (P<0.001), indicating
that FHIT loss was associated with lung cancer. Pathological
analysis demonstrated that FHIT loss was not correlated with the
TNM stage of tumors, lymph node metastasis, gender or age
(P>0.05); however, FHIT loss was significantly associated with
the histological type (P=0.003), degree of cell differentiation
(P=0.007) and smoking history (P<0.001). FHIT loss in cases of
lung SCC, a low degree of cell differentiation or a smoking history
was significantly increased compared with FHIT loss in cases of AC,
a high degree of cell differentiation or no smoking history. This
finding indicated that FHIT loss may be important in the occurrence
of lung cancer, particularly in lung SCC.
Previous studies identified that the FRA3B fragile
site, which is covered by FHIT, is targeted by tobacco (23). Tobacco exposure results in an
increased level of chromosome fragility at the FRA3B fragile site.
The fragility of the chromosome may be related to abnormalities in
replication, resulting in single-strand DNA gaps that, if
unrepaired, may lead to chromosomal damage, including the deletion
of distal genes, deletions within the fragile site, and
translocations or other rearrangements with a break at the FRA3B
fragile site. FHIT gene loss or a reduction in its expression may
be associated with the instability of FRA3B (24). Therefore, this may explain the
observation that the rate of FHIT loss was increased in the cases
with a history of smoking compared with no smoking history in the
present study.
A previous study of cervical cancer demonstrated
that FHIT anomalies were closely associated with high-risk HPV16/18
infection (23,24). The results of the present study
demonstrated that FHIT loss was also associated with HPV infection
in lung cancer. FHIT loss in the HPV-positive group was
significantly increased compared with the HPV-negative group. It
was similar in the HPV16/18-positive group and the
HPV16/18-negative group. These results demonstrated that FHIT loss
is associated with HPV infection, particularly high-risk HPV16/18
infection. The present study further indicated the role of FHIT in
the tumorigenesis process and may also assist in the understanding
of the role of FHIT in lung cancer. In vitro studies have
demonstrated that HPV is able to insert its genes into the fragile
site FRA3B adjacent to FHIT resulting in allele loss of the gene
(12). Therefore, it may reasonably
be considered that HPV infection, particularly high-risk HPV16/18
infection, may induce a fissure or breaking point in the FRA3B
fragile site, into which HPV-DNA may integrate into the host cell
(6), resulting in the loss of FHIT
protein expression. These observations indicate that FHIT loss may
provide the integration site for HPV, while at the same time, the
integration of HPV results in the inactivation of FHIT. Therefore,
the involvement of HPV infection in lung tumorigenesis may, at
least in part, be mediated through FHIT loss.
In the present study, in the non-cancer controls and
lung cancer cases, the rate of FHIT loss in the HPV-positive group
was significantly increased compared with the HPV-negative group
(P<0.001 and P=0.002, respectively). For the lung cancer cases,
the rate of FHIT loss in the HPV16- and 18-positive groups was
significantly increased compared with the HPV16/18-negative group
(P=0.003 and P=0.029, respectively). The coexistence of FHIT loss
and a smoking history in the 180 lung cancer cases was 38.33%
(69/180) and the Pearson contingency coefficient was r=0.318
(P<0.001). These results may further explain the contributions
to the lung tumorigenesis of FHIT loss and indicate that FHIT loss
may be an early indicator for lung cancer, particularly for
patients with a history of smoking.
Meanwhile, FHIT loss and p53 mutation have
previously been observed to frequently occur in lung cancer and may
therefore contribute to the development of lung cancer. The present
study further investigated p53 mutations using IHC. In the lung
cancer cases, the p53 mutation rate in the HPVL1- and
HPV16-positive groups was demonstrated to be significantly
increased compared with the HPVL1- and HPV16-negative groups
(P=0.016 and P=0.014, respectively). These findings indicated that
p53 mutation is associated with HPV infection, particularly HPV16
infection. The co-expression studies demonstrated that FHIT loss
and p53 mutation had synergistic effects in the HPV-infected lung
cancer cases, with a Pearson contingency coefficient of r=0.357
(P<0.001), indicating that FHIT loss and p53 mutation may
coordinate in the development of HPV-infected lung cancer.
In light of the findings of the present study and
our previous studies (6,14), one hypothesis is that following
infection of the lung tissue with HPV, HPV-DNA integrates into the
host-cell DNA at the FRA3B fragile site and results in FHIT loss.
This integration may result in the upregulation of the
transcription of the E6 and E7 oncogenes. E6 expression accelerates
p53 mutation (25), which increases
the possibility of a HPV-infected cells becoming malignant.
Abnormal loss of FHIT occurs early and frequently in the
progression from normal to malignant lesions. The results of the
present study provide early epidemiological data that require
further study in high-risk HPV16/18-infected lung SCC patients.
In summary, the results of the present study
indicated that the involvement of HPV infection in lung
tumorigenesis may, at least in part, be mediated through FHIT loss.
FHIT loss and p53 mutation may be coordinated in the development of
HPV-associated lung cancer, and may accelerate the occurrence and
development of the disease.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81273148) and the
International Cooperation Foundation of Shaanxi Province (grant no.
2013KW32-05). The authors would like to acknowledge the
contributions of Professor Suminori Akiba (Kagoshima University,
Japan), who provided assistance in analyzing the data. The authors
would also like to thank Dr Guanjun Zhang (Director of the
Department of Pathology, the First Affiliated Hospital of Medical
School of Xi'an Jiaotong University) for contributing to the
pathological diagnosis and for assistance in the preparation of the
paraffin-embedded slices.
References
|
1
|
Al-Badawi IA, Al-Suwaine A, Al-Aker M,
Asaad L, Alaidan A, Tulbah A, Fe Bohol M and Munkarah AR: Detection
and genotyping of human papilloma virus in cervical cancer
specimens from Saudi patients. Int J Gynecol Cancer. 21:907–910.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Shabanah OA, Hafez MM, Hassan ZK,
Sayed-Ahmed MM, Abozeed WN, Al-Rejaie SS and Alsheikh AA: Human
papillomavirus genotyping and integration in ovarian cancer Saudi
patients. Virol J. 10:343–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang X and Lu L: Expression of HPV-16 E6
protein and p53 inactivation increases the uterine cervical cancer
invasion. Drug Res (Stuttg). 65:70–73. 2015.PubMed/NCBI
|
|
4
|
Aumayr K, Susani M, Horvat R, Wrba F,
Mazal P, Klatte T, Koller A, Neudert B and Haitel A: P16INK4A
immunohistochemistry for detection of human papilloma
virus-associated penile squamous cell carcinoma is superior to
in-situ hybridization. Int J Immunopathol Pharmacol. 26:611–620.
2013.PubMed/NCBI
|
|
5
|
Sarchianaki E, Derdas SP, Ntaoukakis M, et
al: Detection and genotype analysis of human papillomavirus in
non-small cell lung cancer patients. Tumour Biol. 35:3203–3209.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu Y, Yang A, Hu S and Yan H: Correlation
of HPV-16/18 infection of human papillomavirus with lung squamous
cell carcinomas in Western China. Oncol Rep. 21:1627–1632.
2009.PubMed/NCBI
|
|
7
|
Jafari H, Gharemohammadlou R, Fakhrjou A,
et al: Genotyping of human papillomavirus and TP53 mutations at
exons 5 to 7 in lung cancer patients from Iran. Bioimpacts.
3:135–140. 2013.PubMed/NCBI
|
|
8
|
Ragin C, Obikoya-Malomo M, Kim S, et al:
HPV-associated lung cancers: An international pooled analysis.
Carcinogenesis. 35:1267–1275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hasegawa Y, Ando M, Kubo A, Isa S,
Yamamoto S, Tsujino K, Kurata T, Ou SH, Takada M and Kawaguchi T:
Human papilloma virus in non-small cell lung cancer in never
smokers: A systematic review of the literature. Lung Cancer.
83:8–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohta M, Inoue H, Cotticelli MG, et al: The
FHIT gene, spanning the chromosome 3p14.2 fragile site and renal
carcinoma-associated t(3;8) breakpoint, is abnormal in digestive
tract cancers. Cell. 84:587–597. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wistuba II, Montellano FD, Milchgrub S, et
al: Deletions of chromosome 3p are frequent and early events in the
pathogenesis of uterine cervical carcinoma. Cancer Res.
57:3154–3158. 1997.PubMed/NCBI
|
|
12
|
Wilke CM, Hall BK, Hoge A, Paradee W,
Smith DI and Glover TW: FRA3B extends over a broad region and
contains a spontaneous HPV16 integration site: Direct evidence for
the coincidence of viral integration sites and fragile sites. Hum
Mol Genet. 5:187–195. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Cheng YW, Wu DW, Chen JT, Chen CY,
Chou MC and Lee H: Frequent FHIT gene loss of heterozygosity in
human papillomavirus-infected non-smoking female lung cancer in
Taiwan. Cancer Lett. 235:18–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Y, Yang A, Hu S, Zhang J and Yan H:
Significance of human papillomavirus 16/18 infection in association
with p53 mutation in lung carcinomas. Clin Respir J. 7:27–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Münger K, Baldwin A, Edwards KM, Hayakawa
H, Nguyen CL, Owens M, Grace M and Huh K: Mechanisms of human
papillomavirus-induced oncogenesis. J Virol. 78:11451–11460. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greer CE, Wheeler CM and Manos MM: PCR
amplification from paraffin-embedded tissues: sample preparation
and the effects of fixationPCR Primer: a laboratory manual. Carl WD
and Gabriela SD: Cold Spring Harbor Laboratory Press; New York: pp.
99–112. 1995
|
|
17
|
Wang JL, Fang CL, Wang M, Yu MC, Bai KJ,
Lu PC and Liu HE: Human papillomavirus infections as a marker to
predict overall survival in lung adenocarcinoma. Int J Cancer.
134:65–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Badillo-Almaraz I, Zapata-Benavides P,
Saavedra-Alonso S, Zamora-Avila D, Reséndez-Pérez D, Tamez-Guerra
R, Herrera-Esparza R and Rodríguez-Padilla C: Human papillomavirus
16/18 infections in lung cancer patients in Mexico. Intervirology.
56:310–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwakawa R, Kohno T, Enari M, Kiyono T and
Yokota J: Prevalence of human papillomavirus 16/18/33 infection and
p53 mutation in lung adenocarcinoma. Cancer Sci. 101:1891–1896.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bahnassy AA, Zekri AR, Madbouly MS,
El-Naggar M, El-Khelany ZF and El-Merzebany MM: The correlation
between FHIT, P53 and MMR genes in human papillomavirus-associated
cervical carcinoma. J Egypt Natl Canc Inst. 18:191–202.
2006.PubMed/NCBI
|
|
21
|
Giarnieri E, Zanesi N, Bottoni A,
Alderisio M, Lukic A, Vecchione A, Ziparo V, Croce CM and Mancini
R: Oncosuppressor proteins of fragile sites are reduced in cervical
cancer. Cancer Lett. 289:40–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sozzi G, Veronese ML, Neghni M, et al: The
FHIT gene 3p14.2 is abnormal in lung cancer. Cell. 85:17–26. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kapitanović S, Čačev T, Lončar B, Catela
Ivković T, Križanac Š and Pavelić K: Reduced FHIT expression is
associated with tumor progression in sporadic colon adenocarcinoma.
Exp Mol Pathol. 96:92–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stein CK, Glover TW, Palmer JL and Glisson
BS: Direct correlation between FRA3B expression and cigarette
smoking. Genes Chromosomes Cancer. 34:333–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neyaz MK, Hussain S, Hassan MI, et al:
Novel missense mutation in FHIT gene: Interpreting the effect in
HPV-mediated cervical cancer in Indian women. Mol Cell Biochem.
335:53–58. 2010. View Article : Google Scholar : PubMed/NCBI
|