Introduction
Malignancies of the paranasal cavity are rare,
representing ~3% of all head and neck cancers and ≤1% of total
malignancies; the combination of surgery, chemotherapy and
radiation therapy (RT) has been widely used in patients with the
disease (1–3). Among paranasal cavity malignancies,
cancer of the ethmoid sinus complex is even rarer, and surgical
approaches are usually complicated by a lack of satisfactory
surgical clearance and the risk of serious dysfunction of the
surrounding normal tissues, including the brain stem and optic
nerve (4). Consequently, definitive
RT for patients with unresectable squamous cell carcinoma of the
ethmoid sinus (SCC-ES) has been performed, but it can be difficult
to deliver a curative irradiation dose to the tumor without severe
complications, such as visual loss and brain necrosis, among others
(5). Thus, there is little
information on the efficacy of RT for SCC-ES at present.
Proton beam therapy (PBT) offers advantageous
physical properties to RT for a variety of cancers. Since proton
beams exhibit a spread-out Bragg peak and achieve an improved dose
distribution of the target volume using specified beam modulations
compared with photon beams (6–10), PBT
delivers a large irradiation dose to the tumor using limited
numbers of portals while sparing the surrounding normal tissues.
Hence, the technique may yield improved disease control with
minimum morbidity compared with previous conventional RT (11). A number of previous studies have
reported favorable outcomes for patients with head and neck cancers
treated with PBT (8–10), but SCC-ES has not been an area of
focus, as there is a limited number of patients with this disease.
Therefore, the efficacy of PBT in addition to RT using photon beams
in the management of SCC-ES remains unknown.
The present study reports the clinical outcomes of
patients with SCC-ES who were treated with PBT for the first time,
and reviews the literature regarding the role of RT in the
management of SCC-ES. Furthermore, three-dimensional conformal RT
(3D-CRT) and intensity-modulated RT (IMRT) treatment planning using
the same CT images was performed for the most recently treated
SCC-ES case in this series. The dose-volume histograms of tumor and
normal tissues, including the optic nerve, chiasm, brain and brain
stem, from 3D-CRT and IMRT were then compared with those from PBT
in order to assess the physical advantages of using proton beams
for the treatment of SCC-ES.
Case report
Patients
A total of 7 patients with SCC originating from the
ethmoid sinus were reviewed retrospectively in this study. The
patients were treated with definitive PBT at the University of
Tsukuba Hospital (Ibaraki, Japan) between January 1997 and December
2012. Patients with recurrent disease or tumors originating from
other regions were excluded. Details of the patients'
characteristics are presented in Table
I. The median age of the patients was 63 years, ranging from
46–79 years. Written informed consent was obtained from all
subjects prior to the initiation of treatment.
 | Table I.Characteristics of the 7 patients. |
Table I.
Characteristics of the 7 patients.
| No. | Age | Gender | Radiation
therapy | Dose
fractionation | Chemotherapy (cycles,
n) |
|---|
| 1 | 79 | Female | X-ray and PBT | 70.4 GyE/38 F | No |
| 2 | 58 | Male | X-ray and PBT | 70.4 GyE/38 F | No |
| 3 | 46 | Male | PBT | 72.0 GyE/36 F | No |
| 4 | 53 | Female | PBT | 72.0 GyE/36 F | No |
| 5 | 63 | Female | PBT | 74.0 GyE/37 F | No |
| 6 | 71 | Male | PBT | 74.0 GyE/37 F | Induction FP (2) |
| 7 | 64 | Female | PBT | 76.0 GyE/38 F | Concurrent FP
(2) |
Pretreatment evaluation was performed by physical
examination, nasopharyngeal endoscopy, computed tomography (CT) and
magnetic resonance imaging (MRI). All tumors evaluated in the
present study were categorized as T4bN0M0 based on sections of the
nasal cavity and paranasal sinuses, according to the 7th edition of
the tumor-node-metastasis classification of the International Union
Against Cancer (2009) (12).
Proton beam therapy
The RT treatment policy for the present study has
been described previously (13). PBT
planning was performed on a 3D CT planning system with a 5-mm slice
thickness. Patients were immobilized in the supine position using a
thermoplastic mask. In principle, the clinical target volume (CTV)
included the gross tumor volume and bilateral ethmoid sinuses, and
the initial planning target volume was determined by adding margins
of 8–10 mm to the CTV. To spare the surrounding normal tissues and
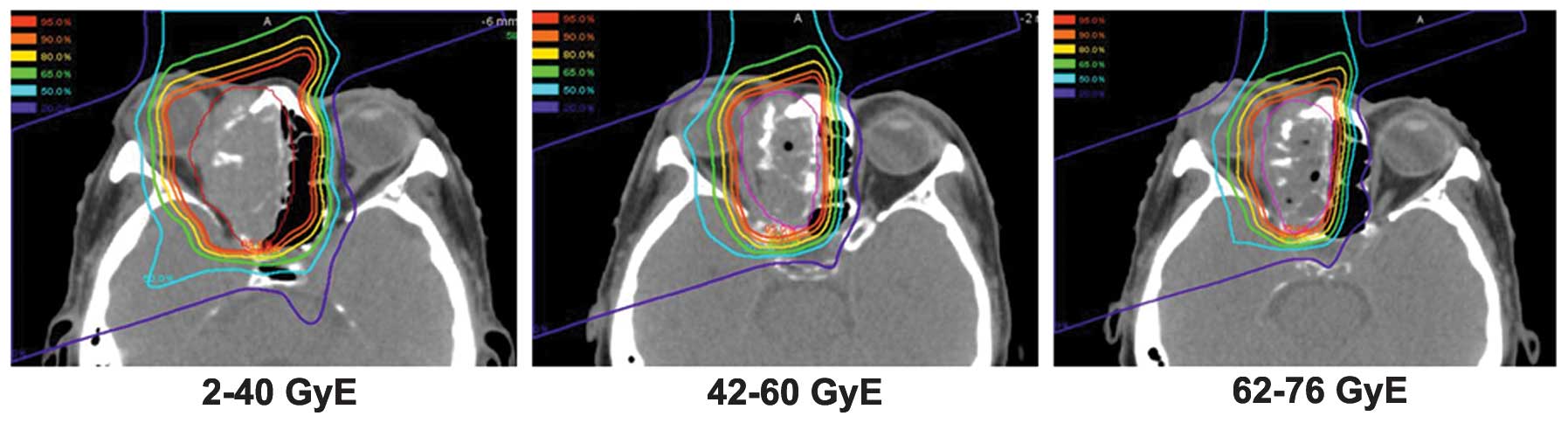
to adapt the planning target volume to the tumor volume reduction,
treatment plans were commonly changed twice to the total doses of
30–40 Gy equivalents (GyE) and 60 GyE (Fig. 1). Dose constraints for the organs at
risk were as follows: Optic nerve of the healthy side/chiasm, 50
GyE; optic lens, 10 GyE; and brainstem surface, 50 GyE. However, in
certain cases where the tumor was adjacent to these organs, it was
not possible to follow these constraints. A median total total dose
of 72 GyE (range, 70.4–76 GyE) was delivered at a fractional dose
of 1.8–2.0 GyE. Patient 6 underwent two 28-day cycles of induction
chemotherapy with 5-fluorouracil (5-FU; 800 mg/m2, days,
1–5) and cisplatin (CDDP; 60 mg/m2, day 1) prior to PBT
treatment. Patient 7 underwent two 28-day cycles of chemotherapy
with 5-FU (700 mg/m2, days 1–5) and cisplatin (70
mg/m2, day 6), which was administered concurrently with
PBT (Table I).
Follow-up, treatment efficacy and
toxicity evaluation
The last follow-up was performed in February 2014,
and the median follow-up time was 3.6 years. The follow-up included
a physical examination and nasopharyngeal endoscopy, which was
performed at 1–2 month intervals during the first year following
completion of PBT and at 2–3 month intervals thereafter.
The initial treatment effect was evaluated using the
RECIST 1.0 criteria (14) based on
diagnostic imaging by CT and/or MRI at 2–3 months following the
completion of PBT. The diagnostic imaging was repeated routinely
every 6 months thereafter. The toxicities were graded according to
the Common Terminology Criteria for Adverse Events version 4.0
(15).
Results
Efficacy and failure pattern
The details of the treatment results are presented
in Table II and Fig. 2. Evaluation of the initial treatment
response demonstrated a complete response (CR) in 2 patients (29%)
and a partial response (PR) in the remaining 5 patients. The median
survival time of all patients was 43 months (range, 12–62
months).
 | Table II.Treatment outcome and observed
toxicity in the 7 patients. |
Table II.
Treatment outcome and observed
toxicity in the 7 patients.
| No. | Initial effect | Recurrence
(time) | Status | Survival time,
months | Late toxicity
(grade) |
|---|
| 1 | CR | None | NED | 62 | Cataracts (3),
contralateral optic nerve damage (3) |
| 2 | PR | Local (5 months) | DWD | 12 | None |
| 3 | PR | None | DOD | 53 | Brain necrosis
(1) |
| 4 | PR | Local (22
months) | DWD | 43 | Cataracts (3) |
| 5 | PR | Local+LN (1
month) | DWD | 12 | None |
| 6 | PR | Local (2 months) | DWD | 47 | None |
| 7 | CR | None | DOD | 20 | Brain necrosis
(2) |
Recurrences were observed in 4/5 (80%) patients in
the PR group according to the initial tumor response 1–22 months
after the completion of PBT. The initial recurrence sites were the
primary tumor lesion in 4 patients and the regional lymph nodes in
1 patient, respectively, but no distant metastases were detected.
In addition, there were no recurrences in the CR group.
Toxicity
Prior to the start of PBT, 3 patients presented with
visual impairment, and another patient presented with ipsilateral
visual loss due to the disease. These conditions were therefore not
included among the toxicities induced by the treatment in the
present study. There was no grade 3 or severe acute toxicity.
Regarding late toxicity, grade 3 contralateral optic nerve damage
and cataracts were observed in 2 and 1 patient, respectively, grade
1 brain necrosis was observed in 1 patient, and 2 brain necrosis
was observed in 1 patient. In the brain necrosis case, the
irradiation field included the brain tissues surrounding the tumor,
and a total dose of 70 GyE was delivered, even though the shrinking
field technique used for boost therapy was adopted. For all 3 cases
with visual impairment prior to the start of PBT, their symptoms
did not improve following treatment.
Discussion
The reported treatment outcomes, including quality
of life, have been disappointing for patients with SCC-ES, even if
they were candidates for radical surgery. Despite RT being an
alternative curative treatment method, it is difficult to
administer a high irradiation dose to the tumor without severe
complications (16,17). Consequently, comparing the superiority
of treatment modalities for SCC-ES, such as surgery and RT combined
with or without chemotherapy, is made difficult by the lack of
availability of information concerning SCC-ES treatment outcomes,
mainly due to the rarity of this disease.
Waldron et al (5) reported that the 5-year survival rates
and the local tumor control with RT alone were affected by the
tumor T-stage and histological type. In particular, local
recurrences were observed in 8/11 (72%) patients with T4 SCC-ES,
and almost all recurrences developed within a year of treatment. In
addition, the median overall survival time for the 11 patients was
≤12 months. However, in the present study, local tumor control was
achieved in 3/7 (43%) patients with T4 SCC-ES, and the median
survival time was 43 months. It appears that the higher local
control rate in the present study was mainly due to the high
irradiation doses administered to the primary tumor, although the
number of subjects was small. Since a previous study demonstrated
that a total dose of ≥65 GyE is a preferable factor for improvement
of local tumor control and overall survival (16), and since all recurrences in the
present study actually developed at the primary tumor site,
intensification of the local treatment may be important to improve
the outcomes of SCC-ES.
Nevertheless, there are certain issues with
administering high irradiation doses to the SCC-ES primary tumor.
In particular, there are a number of vulnerable organs in this
region, including the brain, brain stem and optic nerve, and it is
potentially difficult to deliver ≥65 Gy to the tumor using
conventional RT methods without inducing severe late complications,
such as optic nerve damage and brain necrosis (16,17). To
reduce the irradiation dose to organs at risk, intensity-modulated
radiation therapy (IMRT) and PBT are often used, and Mock et
al (18) reported that PBT
reduces the amount of normal tissue exposed to irradiation compared
with photon-based treatment, particularly at low- and mid-dose
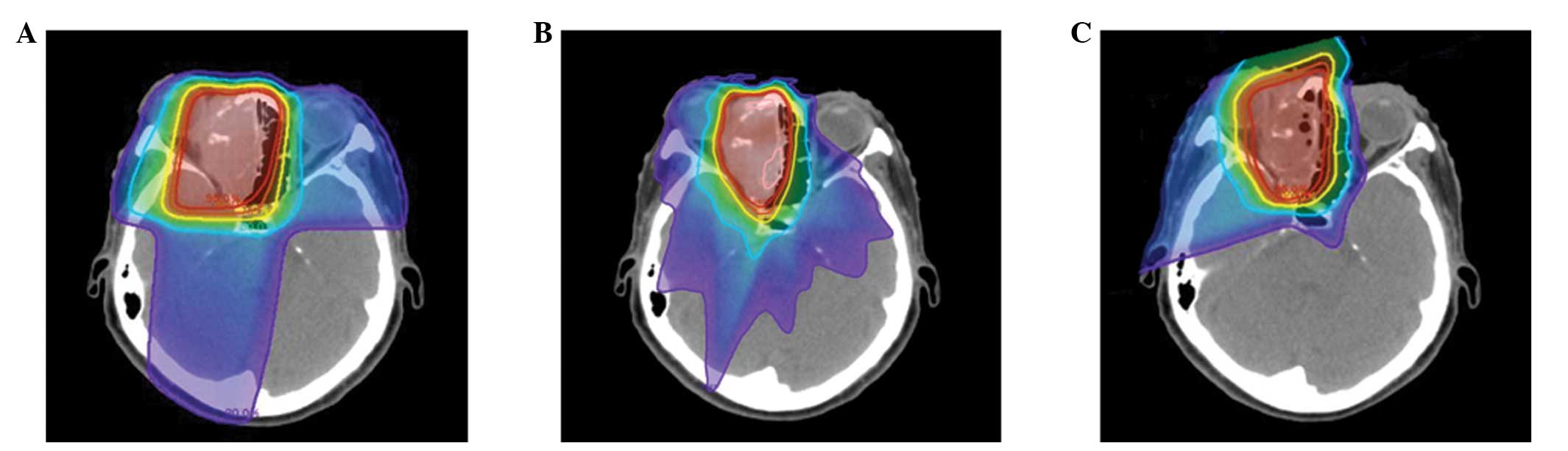
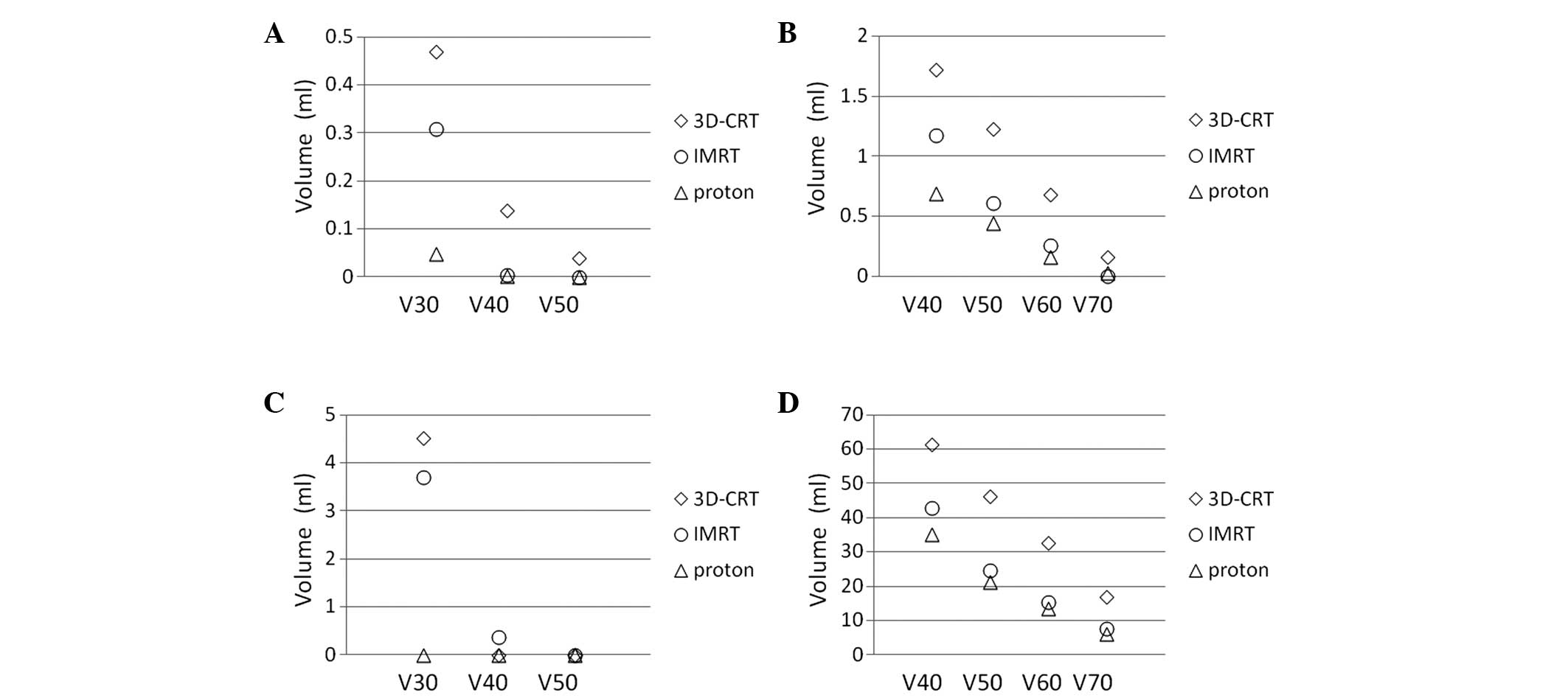
levels. Therefore, 3D-CRT and IMRT treatment planning was performed
for the most recently treated case in the present study using the
same CT images, in order to compare dose-volume histograms of the
normal tissues, including the contralateral optic nerve, chiasm,
brain and brain stem; the results are presented in Figs. 3 and 4.
PBT improves the dose conformation compared with photon-based
delivery methods due to the physical advantages of charged particle
beams (6). Since PBT has the
potential to reduce the irradiation dose to the organs at risk and
to provide high dose irradiation to the tumor, tumor control and
morbidity rates may be improved with this method (7–10).
However, the optimal dose required to control T4
SCC-ES tumors remains unknown (13).
It is important to determine the appropriate total dose carefully
while monitoring complications and escalating the total dose, which
was achieved in the present study by gradually increasing the
irradiation dose from 70 to 76 GyE. As a result, there was not a
clear association between the irradiation dose and tumor control in
the present study, mainly due to the small sample size and
different tumor characteristics. A total of 4/7 patients survived
for ≥3 years, and no lethal brain damage was observed irrespective
of the irradiation dose. Therefore, patients with SCC-ES are
currently being treated with PBT at a total dose of 76 GyE combined
with concurrent chemotherapy at the University of Tsukuba Hospital.
On the other hand, grade 3 optic nerve damage and cataracts
developed in 1 and 2 patients, respectively, who received PBT.
Previous studies have demonstrated the usefulness for spot-scanning
proton therapy to significantly reduce the integral dose to head
and neck critical structures (19,20), and
prospective studies are underway to determine if this reduced dose
translates to an improved quality of life in patients.
In conclusion, 7 unresectable SCC-ES patients were
treated with PBT with or without concurrent chemotherapy, and local
tumor control was achieved in 3/7 patients (43%), while 4 patients
survived for ≥3 years. Furthermore, no lethal morbidity developed
in the long-term survivors. As all recurrences developed locally,
it appears that intensifying local treatment effects without
increasing severe morbidities in critical organs may be important
in order to improve treatment outcomes. PBT is therefore a
potentially effective and curative RT method for unresectable
SCC-ES.
Acknowledgements
A portion of the data obtained in the present study
was presented at the 16th Asian Research Symposium in Rhinology
held in Tokyo in 2013, and this study was supported by
Grants-in-Aid for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology (no. 24591832)
of Japan.
References
|
1
|
Blanco AI, Chao KS, Ozyigit G, et al:
Carcinoma of paranasal sinuses: Long-term outcomes with
radiotherapy. Int J Radiat Oncol Biol Phys. 59:51–58. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jansen EP, Keus RB, Hilgers FJ, et al:
Does the combination of radiotherapy and debulking surgery favor
survival in paranasal sinus carcinoma? Int J Radiat Oncol Biol
Phys. 48:27–35. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dulguerov P, Jacobsen MS, Allal AS,
Lehmann W and Calcaterra T: Nasal and paranasal sinus carcinoma:
Are we making progress? a series of 220 patients and a systematic
review. Cancer. 92:3012–3029. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang GL, Morrison WH, Garden AS, et al:
Ethmoid sinus carcinomas: Natural history and treatment results.
Radiother Oncol. 49:21–27. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waldron JN, O'Sullivan B, Warde P, et al:
Ethmoid sinus cancer: Twenty-nine cases managed with primary
radiation therapy. Int J Radiat Oncol Biol Phys. 41:361–369. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lomax AJ, Goitein M, Adams J, et al:
Intensity modulation in radiotherapy: Photons versus protons in the
paranasal sinus. Radiother Oncol. 66:11–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hojo H, Zenda S, Akimoto T, et al: Impact
of early radiological response evaluation on radiotherapeutic
outcomes in the patients with nasal cavity and paranasal sinus
malignancies. J Radiat Res. 53:704–709. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Truong MT, Kamat UR, Liebsch NJ, et al:
Proton radiation therapy for primary sphenoid sinus malignancies:
Treatment outcome and prognostic factors. Head Neck. 31:1297–1308.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pommier P, Liebsch NJ, Deschler DG, et al:
Proton beam radiation therapy for skull base adenoid cystic
carcinoma. Arch Otolaryngol Head Neck Surg. 132:1242–1249. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zenda S, Kohno R, Kawashima M, et al:
Proton beam therapy for unresectable malignancies of the nasal
cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys.
81:1473–1478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suit H and Urie M: Proton beams in
radiation therapy. J Nat Cancer Inst. 84:155–164. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sobin LH, Wittekind C and Gospodarowicz M:
Nasal cavity and paranasal sinuses. TNM Classification of Malignant
Tumors. 7th. Wiley-Blackwell; New York, NY: pp. 46–50. 2009
|
|
13
|
Fukumitsu N, Okumura T, Mizumoto M, et al:
Outcome of T4 (International Union Against Cancer Staging System,
7th edition) or recurrent nasal cavity and paranasal sinus
carcinoma treated with proton beam. Int J Radiat Oncol Biol Phys.
83:704–711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European organization for research and treatment of cancer,
national cancer institute of the United States, national cancer
institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Cancer Institute, . Common
Terminology Criteria for Adverse Events (CTCAE) v4.0 2010.
http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5′7.pdfAccessed.
April 24–2014
|
|
16
|
Hoppe BS, Nelson CJ, Gomez DR, et al:
Unresectable carcinoma of the paranasal sinuses: Outcomes and
toxicities. Int J Radiat Oncol Biol Phys. 72:763–769. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Padovani L, Pommier P, Clippe SS, et al:
Three-dimensional conformal radiotherapy for paranasal sinus
carcinoma: Clinical results for 25 patients. Int J Radiat Oncol
Biol Phys. 56:169–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mock U, Georg D, Bogner J, Auberger T and
Pötter R: Treatment planning comparison of conventional, 3d
conformal and intensity-modulated photon (IMRT) and proton therapy
for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys.
58:147–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kandula S, Zhu X, Garden AS, et al:
Spot-scanning beam proton therapy vs intensity-modulated radiation
therapy for ipsilateral head and neck malignancies: A treatment
planning comparison. Med Dosim. 38:390–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flynn RT, Bowen SR, Bentzen SM, Rockwell
Mackie T and Jeraj R: Intensity-modulated x-ray (IMXT) versus
proton (IMPT) therapy for theragnostic hypoxia-based dose painting.
Phys Med Biol. 53:4153–4167. 2008. View Article : Google Scholar : PubMed/NCBI
|