Introduction
Since screening tests for prostate cancer using
prostate-specific antigen (PSA) were introduced, the proportion of
patients exhibiting locally advanced disease at diagnosis has
decreased in Japan (1). Accordingly,
the number of patients undergoing external-beam radiotherapy (EBRT)
as curative treatment has increased (2). Despite this, the mortality rate
associated with prostate cancer has continuously increased over the
past few decades (3); therefore, to
address this, radiotherapy combined with androgen deprivation
therapy (ADT) has been evaluated for the treatment prostate
cancer.
Various studies have demonstrated that EBRT combined
with ADT provides improved biochemical progression-free and overall
survival compared with EBRT alone (4–6). However,
the use of ADT has been reported to be a risk factor for
cardiovascular mortality (7) and
deterioration in the quality of life (8). Furthermore, Bolla et al (9) recently reported that EBRT combined with
six months of ADT resulted in shorter overall survival times than
EBRT combined with 36 months of ADT; however, it remains unclear
whether EBRT combined with an ADT duration of ≥36 months provides
any benefit for patients with prostate cancer. Thus, the present
study was performed to investigate survival rate and the incidence
of adverse events in high-risk localized prostate cancer patients
treated with EBRT combined with ADT administered over a short
(<36 months) or long (≥36 months) period.
Patients and methods
Patients
The present study retrospectively identified 173
patients with high-risk localized prostate cancer who were treated
using definitive EBRT between January 2001 and August 2011 at the
Hachioji Center of the Tokyo Medical University Hospital (Tokyo,
Japan). Written informed consent was obtained for all patients
prior to EBRT treatment. High-risk disease was diagnosed on the
basis of the presence of at least one of the following factors,
according to the classification utilized by Kuban et al
(10): A stage T3-4 tumor, a tumor
with a Gleason score of ≥8 or a serum PSA level of >20 ng/ml.
Additionally, the disease stage was determined according to the
sixth edition of the tumor-node-metastasis classification system of
the International Union Against Cancer 2002 (11).
Treatment strategy
Patients were divided into two groups according to
the duration of ADT, with one receiving a short-term course of ADT
(<36 months; n=54) and the other a long-term course of ADT (≥36
months; n=119).
EBRT treatment preparation and the actual procedure
were performed with the patient in a supine position and with a
full bladder. For treatment preparation, all patients underwent
pelvic computed tomography at a 2.5-mm slice thickness. Typically,
the EBRT treatment included prostate and pelvic lymph node
irradiation using anteroposterior opposite ports or a box technique
at a dose of 40 Gy. An additional dose of 30 Gy was administrated
to the prostate and the proximal portion of the seminal vesicle
using the lateral and anterior ports. Subsequently, all patients
were treated with photons of 10 MV and 1.8 or 2.0 Gy, once a day,
five days a week.
Follow-up procedure
After the completion of EBRT, clinical assessments,
laboratory tests for toxicity and PSA measurements were performed
every three months for five years and every six months thereafter.
Biochemical progression was defined as a rise in PSA levels of 2
ng/ml above the PSA nadir according to the American Society for
Therapeutic Radiation and Oncology consensus guidelines (12); progression-free survival was defined
as the time from commencing EBRT to the time of biochemical
progression, clinical progression or mortality from any cause; and
overall survival was defined as the time from commencing EBRT to
the time of mortality from any cause.
Statistical analysis
The survival rate was calculated using the
Kaplan-Meier method, the difference in survival was assessed by
performing the log-rank test, and hazard ratio and confidence
intervals were estimated using Cox's proportional hazards model.
Furthermore, statistical analysis was performed using Stata
statistical software (version 12; StataCorp, College Station, TX,
USA) and the Common Terminology Criteria for Adverse Events
(version 3.0) (13) was used to
assess toxicities. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients
The characteristics of the 173 patients with
histologically-proven adenocarcinoma included in the current
analysis are summarized in Table I.
The median age of the patients was 74 years (range, 56–87 years)
and all patients had an Eastern Cooperative Oncology Group
performance status of 0 or 1 (14).
Furthermore, 66% of tumors were classified as T3 or T4 and 60% of
tumors were assigned a Gleason score of ≥8 (15). The median pretreatment PSA level was
24 ng/ml (range, 0.2–400 ng/ml) and the median follow-up duration
of the patients was 53 months (range, 8–143 months).
 | Table I.Patient and disease
characteristics. |
Table I.
Patient and disease
characteristics.
|
| Duration of ADT,
months |
|
|---|
|
|
|
|
|---|
| Parameter | <36 (n=54) | ≥36 (n=119) | P-value |
|---|
| Age, years |
|
|
|
| Median
(range) | 74 (83–58) | 74 (87–56) | 0.73 |
| T stage, n (%) |
|
|
|
| T1 | 12 (22) | 18 (15) |
|
| T2 | 9 (17) | 20 (17) |
|
| T3 | 31 (57) | 74 (62) |
|
| T4 | 2 (4) | 7 (6) | 0.67 |
| Gleason score, n
(%) |
|
|
|
| ≤6 | 6 (11) | 11 (9) |
|
| 7 | 14 (26) | 38 (32) |
|
| ≥8 | 34 (63) | 70 (59) | 0.71 |
| PSA
levela, n (%) |
|
|
|
|
<10.0 | 15 (28) | 20 (17) |
|
|
10.0–20.0 | 7 (13) | 27 (23) |
|
|
≥20.0 | 32 (59) | 72 (60) | 0.14 |
| Irradiated site, n
(%) |
|
|
|
| Prostate
only | 3 (6) | 14 (12) |
|
| Pelvic
lymph node and prostate | 51 (94) | 105 (88) | 0.28 |
Treatment
In total, five patients (3%) underwent orchiectomy
and 151 patients (87%) were treated with a luteinizing
hormone-releasing hormone (LH-RH) agonist (3.75 mg subcutaneously
per month) and bicalutamide (80 mg/day) immediately after
histological confirmation of prostate cancer. A total of 14 (8%)
and three (2%) patients received only LH-RH or bicalutamide,
respectively. The median duration of ADT was 47 months (range,
3–144 months); 54 patients (31%) received a short-term course of
ADT (<36 months; median, 24.5 months) and the remaining 119
patients (69%) received a long long-term course (≥36 months;
median, 58 months). Furthermore, neoadjuvant ADT was administered
to 71 patients (41%) for a median duration of four months.
All patients underwent definitive EBRT, however, 17
patients (10%) underwent prostate irradiation only. The total
median dose received was 69.6 Gy (range, 65.6–74 Gy) and the median
duration of EBRT was 53 days (range, 45–66 days).
Survival outcomes
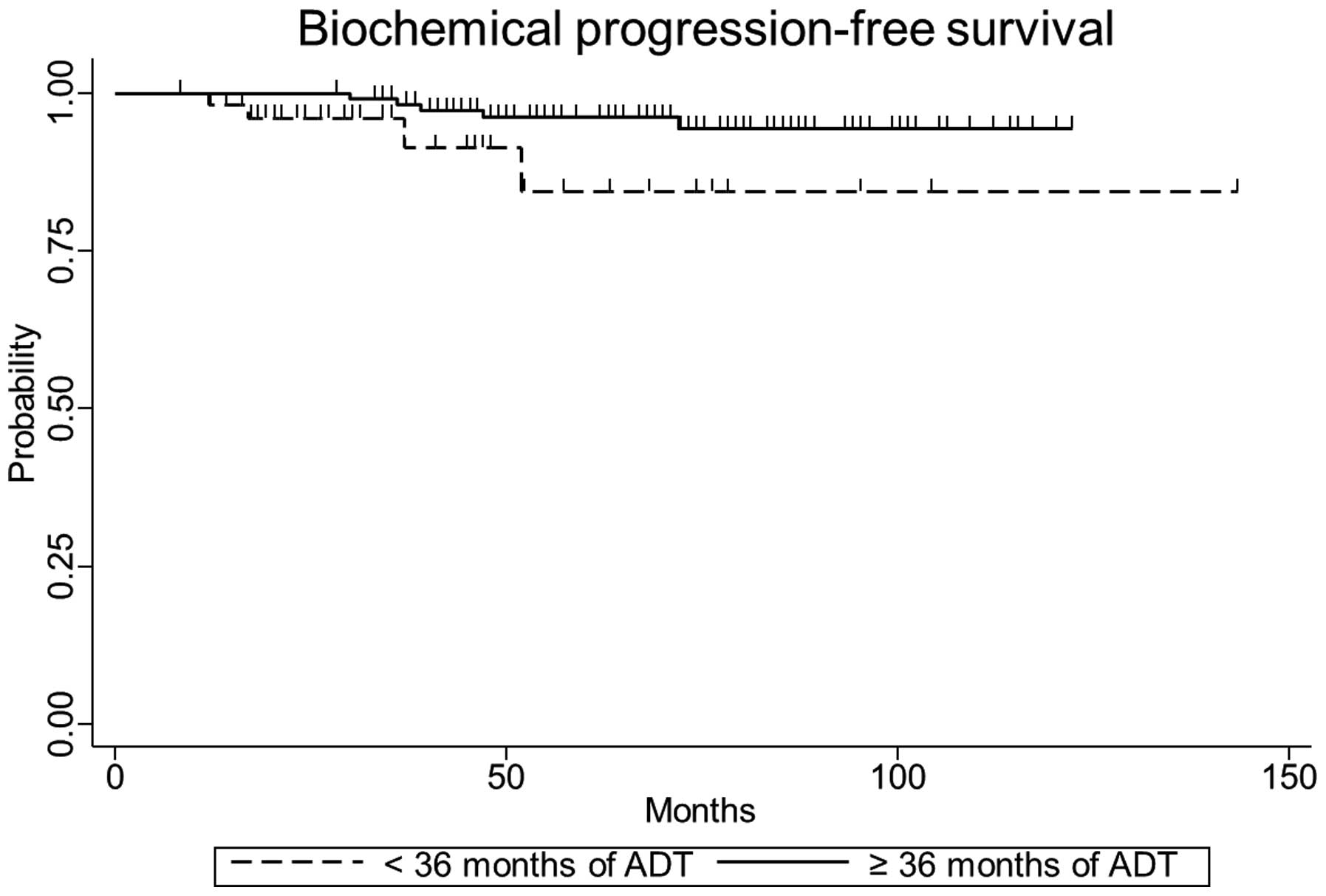
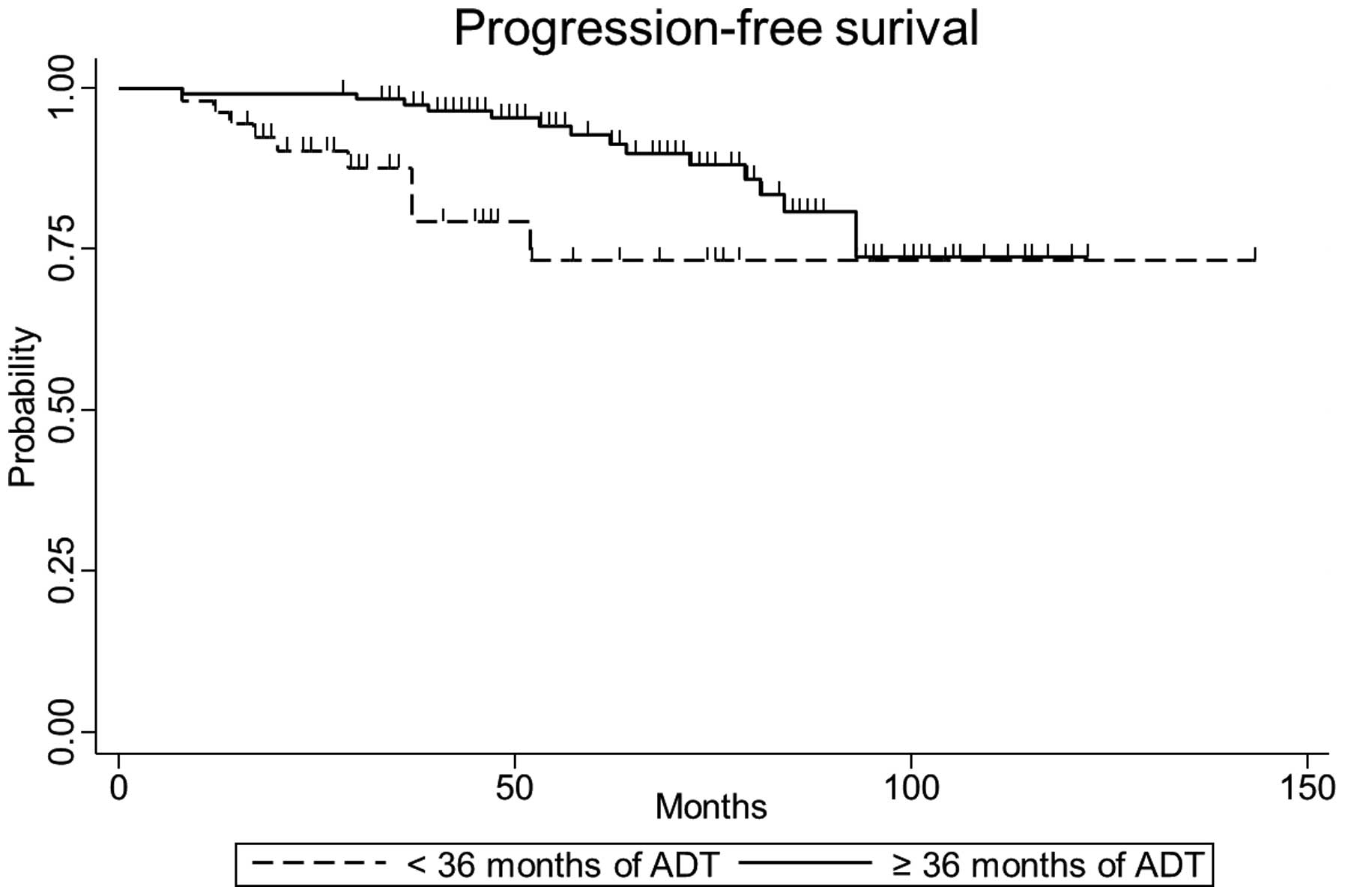
The five-year biochemical progression-free, clinical
progression-free and overall survival rates were 84.0% [95%
confidence interval (CI), 58.0–94.6], 72.9% (95% CI, 50.9–86.2) and
86.8% (95% CI, 70.0–94.6), respectively, for patients in the
short-term ADT group, and 96.2% (95% CI, 90.2–98.6), 92.8% (95% CI,
85.3–96.6) and 94.4% (95% CI, 86.9–97.7), respectively, for
patients in the long-term ADT group. The differences in five-year
biochemical (P=0.04) and clinical progression-free survival
(P<0.01) were statistically significant between the short- and
long-term treatment groups, however, the difference in overall
survival (P=0.16) was not. The biochemical and clinical
progression-free survival curves according to the duration of ADT
are indicated in Figs. 1 and 2. Only the duration of ADT was identified as
a significant prognostic factor for progression-free survival in
high-risk localized prostate cancer, according to multivariate
analysis (hazard ratio, 0.30; 95% CI, 0.12–0.75; P=0.01; Table II).
 | Table II.Prognostic factors for
progression-free survival. |
Table II.
Prognostic factors for
progression-free survival.
| Prognostic
factor | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Age, <75 vs. ≥75
years | 0.82 | 0.35–1.93 | 0.64 |
| T stage, T1,2 vs.
T3,4 | 1.63 | 0.63–4.23 | 0.32 |
| Gleason score, <8
vs. ≥8 | 0.95 | 0.10–2.23 | 0.90 |
| PSA, <20 vs. ≥20
ng/ml | 1.37 | 0.55–3.41 | 0.50 |
| Duration of ADT,
<36 vs. ≥36 months | 0.30 | 0.12–0.75 | 0.01 |
| Irradiated volume, PO
vs. WP | 0.57 | 0.17–1.87 | 0.35 |
Toxicity
With regard to the treatment-associated toxicities
of grade II or above, diarrhea was significantly more common
amongst patients treated with long-term ADT than among patients who
underwent short-term ADT (P=0.02; Table
III). By contrast, cardiovascular toxicity was significantly
more common amongst patients treated with short-term ADT compared
with those treated with long-term ADT (P<0.01; Table III).
 | Table III.Number of toxicities grade II or above
in groups treated with ADT for <36 (n=54) or ≥36 (n=119)
months. |
Table III.
Number of toxicities grade II or above
in groups treated with ADT for <36 (n=54) or ≥36 (n=119)
months.
|
| Duration of ADT,
months |
|
|---|
|
|
|
|
|---|
| Toxicity | <36, n (%) | ≥36, n (%) | P-value |
|---|
| Gastrointestinal |
|
|
|
|
Diarrhea | 2 (4) | 19 (16) | 0.02 |
| Rectal
bleeding | 1 (2) | 0 (0) | 0.31 |
| Genitourinary | 8 (15) | 26 (22) | 0.42 |
| Cardiovascular | 5 (9) | 0 (0) | <0.01 |
Discussion
In the present retrospective study, it was
identified that high-risk prostate cancer patients treated with
EBRT in combination with ADT for ≥36 months demonstrated improved
progression-free survival rates compared with patients treated with
EBRT in combination with ADT for <36 months. Over the median
53-month follow-up period, overall survival was not significantly
different between the two groups, although a number of patients
undergoing short-term ADT exhibited cardiovascular toxicity.
For high-risk prostate cancer, three previously
conducted clinical trials have compared the outcomes of patients
treated with long-term ADT alone and those treated with long-term
ADT in combination with EBRT (16–18). These
trials demonstrated improved biochemical progression-free (16) and overall (17,18)
survival amongst patients who underwent combined long-term ADT and
EBRT; however, the patient cohorts in these studies were inherently
heterogeneous and different clinical stages were included. For
example, the Scandinavian Prostate Cancer Group Study-7 and Swedish
Association for Urological Oncology-3 (SPCG-7/SAUO-3) trial
included a higher proportion of patients with favorable
characteristics (18). By contrast,
trials conducted in France (16), as
well as in Canada and the UK [conducted by the National Cancer
Institute of Canada Clinical Trial Group (PR.3) and the Medical
Research Council (PR07; NCIC/MRC)] (17) enrolled patients with clinical stage T3
or T4 tumors, and >60% of patients had a PSA level of >20 mg.
Similarly, ~70% of patients in the present study had a T3 or T4
stage tumor and 60% of patients had a PSA level of ≥20 mg. Overall,
the patient characteristics in the current study were similar to
those in the two aforementioned trials, and five-year
progression-free survival was comparable between the current study
and the NCIC/MRC trial. However, the outcome of the patients in the
French study was apparently inferior to those in the present study
and NCIC/MRC trials, despite the use of similar radiation doses and
the administration of LH-RH.
With regard to the Gleason score, tumors in the
present study were assigned a higher Gleason score compared with
the SPCG-7/SAUO-3 and NCIC/MRC trials. However, Albertsen et
al (19) reported that
pathologists have recently tended to assign higher Gleason scores,
with tumors diagnosed between 2002 and 2004 being assigned higher
scores than those diagnosed between 1990 and 1992. As a result,
prostate cancer mortality rates for these patients artificially
improved from 2.08 to 1.50 mortalities per 100 individuals when the
Gleason score was standardized (19).
Therefore, greater care may be required when comparing current data
to that reported in previous studies.
In addition to the combination of ADT followed by
EBRT, the administration of EBRT followed by ADT has been
demonstrated to provide improved clinical outcomes compared with
EBRT alone (4–6). However, these results were reported in
trials that enrolled patients with more favorable prostate cancer
risk factors compared with other trials of combined ADT and EBRT.
Furthermore, the duration of ADT varied, lasting for four (6), six (5) and
36 (4) months. Whilst these studies
demonstrated that EBRT plus long-term androgen suppression may
improve the survival of prostate cancer patients, no evidence was
provided with regard to the optimal duration of androgen
suppression.
The Radiation Therapy Oncology Group (RTOG) 92–02
(20) and European Organization for
Research and Treatment of Cancer (EORTC) 22961 (9) trials addressed the issue of the optimal
duration of ADT. The RTOG 92–02 trial compared disease-free and
overall survival times between patients treated with ADT for 4 or
24 months. The latter resulted in significantly longer disease-free
survival times, but not overall survival times. However, a
statistically significant difference in overall survival time was
observed in a subset analysis of patients with tumors of Gleason
scores of 8–10. The EORTC 22961 trial investigated whether six
months of ADT was as efficacious as 36 months of ADT with respect
to overall survival (9). The results
indicated that survival associated with six months of ADT combined
with EBRT was inferior to survival with 36 months of ADT combined
with EBRT. These results are consistent with the findings of the
present study and indicate that long-term ADT is an appropriate
treatment strategy, particularly for high-risk prostate cancer
patients.
Treatment with ADT should be adopted with caution,
as it may induce a number of morbidities, including myocardial
infarction (7). For example, five
patients in the short-term ADT group of the present study
experienced exacerbated cardiovascular disease; however,
retrospective data of >5,000 patients has demonstrated that ADT
was not associated with induced cardiovascular disease if patients
had no existing comorbidities (21).
D'Amico et al (7) reported
that the duration of ADT was not associated with myocardial
infarction, but that it was associated with older age, indicating
that ADT may be used with caution for the treatment of older
patients or those exhibiting cardiovascular disease, regardless of
ADT duration.
Based on a previous study conducted at the Fox Chase
Cancer Center, Feigenberg et al (8) reported that the use of long-term ADT
increased the incidence of late gastrointestinal and genitourinary
morbidity of grade II or over. Furthermore, the RTOG 92–02 trial
indicated that long-term ADT marginally increased the rate of late
toxicities (20). With respect to
increased toxicities in patients treated with long-term ADT, the
present findings were consistent with these previous studies.
In the current study, irradiation to the pelvic node
was the most frequent type of EBRT (90% of patients), followed by
irradiation to the prostate only; however, multivariate analysis
revealed that long-term ADT was the only significant prognostic
factor for the progression-free survival of high-risk prostate
cancer patients. Numerous trial protocols for high-risk prostate
cancer patients have specified the irradiation of pelvic lymph
nodes at a dose of 45 or 50 Gy (4,5,9,17,18,20),
however, the RTOG 94–13 trial did not report improved disease
outcomes upon using pelvic lymph node and prostate irradiation
compared with irradiation of the prostate alone (22). Thus, pelvic lymph node irradiation was
not determined to provide any clinical benefit.
In conclusion, in the present study, EBRT combined
with ≥36 months of ADT for patients with high-risk localized
prostate cancer resulted in prolonged biochemical and
progression-free survival compared to EBRT combined with a shorter
duration of ADT, and exhibited an acceptable toxicity profile.
References
|
1
|
Watanabe H: Mass screening program for
prostatic cancer in Japan. Int J Clin Oncol. 6:66–73.
2001.PubMed/NCBI
|
|
2
|
Ogawa K, Nakamura K, Onishi H, et al:
Japanese Patterns of Care Study Working Subgroup of Prostate
Cancer: Radical external beam radiotherapy for prostate cancer in
Japan: results of the 1999–2001 patterns of care process survey.
Jpn J Clin Oncol. 36:40–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marugame T and Mizuno S: Comparison of
prostate cancer mortality in five countries: France, Italy, Japan,
UK and USA from the WHO mortality database (1960–2000). Jpn J Clin
Oncol. 35:690–691. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bolla M, Van Tienhoven G, Warde P, et al:
External irradiation with or without long-term androgen suppression
for prostate cancer with high metastatic risk: 10-year results of
an EORTC randomised study. Lancet Oncol. 11:1066–1073. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Amico AV, Chen MH, Renshaw AA, Loffredo
M and Kantoff PW: Androgen suppression and radiation vs. radiation
alone for prostate cancer: a randomized trial. JAMA. 299:289–295.
2008.PubMed/NCBI
|
|
6
|
Jones CU, Hunt D, McGowan DG, et al:
Radiotherapy and short-term androgen deprivation for localized
prostate cancer. N Engl J Med. 365:107–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Amico AV, Denham JW, Crook J, et al:
Influence of androgen suppression therapy for prostate cancer on
the frequency and timing of fatal myocardial infarctions. J Clin
Oncol. 25:2420–2425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feigenberg SJ, Hanlon AL, Horwitz EM, Uzzo
RG, Eisenberg D and Pollack A: Long-term androgen deprivation
increases Grade 2 and higher late morbidity in prostate cancer
patients treated with three-dimensional conformal radiation
therapy. Int J Radiat Oncol Biol Phys. 62:397–405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bolla M, de Reijke TM, Van Tienhoven G, et
al: EORTC Radiation Oncology Group and Genito-Urinary Tract Cancer
Group: Duration of androgen suppression in the treatment of
prostate cancer. N Engl J Med. 360:2516–2527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuban DA, Thames HD, Levy LB, et al:
Long-term multi-institutional analysis of stage T1-T2 prostate
cancer treated with radiotherapy in the PSA era. Int J Radiat Oncol
Biol Phys. 57:915–928. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin LH and Wittekind CH; International
Union Against Cancer (UICC). TNM Classification of Malignant
Tumors. 6th. Wiley; New York, NY: 2002
|
|
12
|
Roach M III, Hanks G, Thames H Jr, et al:
Defining biochemical failure following radiotherapy with or without
hormonal therapy in men with clinically localized prostate cancer:
recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int
J Radiat Oncol Biol Phys. 65:965–974. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oken MM, Creech RH, Tormey DC, et al:
Toxicity and response criteria of the Eastern Cooperative Oncology
Group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL: ISUP Grading Committee: The 2005 International Society
of Urological Pathology (ISUP) Consensus Conference on Gleason
Grading of Prostatic Carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mottet N, Peneau M, Mazeron JJ, Molinie V
and Richaud P: Addition of radiotherapy to long-term androgen
deprivation in locally advanced prostate cancer: an open randomised
phase 3 trial. Eur Urol. 62:213–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Warde P, Mason M, Ding K, et al: NCIC CTG
PR.3/MRC UK PR07 Investigators: Combined androgen deprivation
therapy and radiation therapy for locally advanced prostate cancer:
a randomised, phase 3 trial. Lancet. 378:2104–2111. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Widmark A, Klepp O, Solberg A, et al:
Scandinavian Prostate Cancer Group Study 7; Swedish Association for
Urological Oncology 3: Endocrine treatment, with or without
radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3):
an open randomised phase III trial. Lancet. 373:301–308. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albertsen PC, Hanley JA, Barrows GH, et
al: Prostate cancer and the Will Rogers phenomenon. J Natl Cancer
Inst. 97:1248–1253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Horwitz EM, Bae K, Hanks GE, et al:
Ten-year follow-up of radiation therapy oncology group protocol
92–02: a phase III trial of the duration of elective androgen
deprivation in locally advanced prostate cancer. J Clin Oncol.
26:2497–2504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nanda A, Chen MH, Braccioforte MH, Moran
BJ and D'Amico AV: Hormonal therapy use for prostate cancer and
mortality in men with coronary artery disease-induced congestive
heart failure or myocardial infarction. JAMA. 302:866–873. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lawton CA, DeSilvio M, Roach M III, et al:
An update of the phase III trial comparing whole pelvic to prostate
only radiotherapy and neoadjuvant to adjuvant total androgen
suppression: updated analysis of RTOG 94–13, with emphasis on
unexpected hormone/radiation interactions. Int J Radiat Oncol Biol
Phys. 69:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|