Introduction
Testicular germ cell tumors (TGCTs) are a type of
solid tumor that are most commonly observed in young men aged 15–35
years (1). There is a relatively
large difference in the incidence of TGCT among different regions
or different races, but the worldwide incidence of TGCT has
increased in the last 30–40 years (2,3). However,
>95% patients diagnosed at an early stage are cured (4). TGCT treatment is associated with chronic
side effects; patients with advanced-stage cancer are less
sensitive to comprehensive treatments, resulting in a poor
prognosis (5). The molecular biology
of TGCT remains largely unknown, but the onset and development of
the disease is a process that involves multiple genes (6). Increased understanding of the molecular
biology of TGCT is essential in order to define targeted treatments
and identify novel markers for diagnosis and prognosis.
Testis developmental related gene 1 (TDRG1;
GenBank ID, DQ168992) is specifically expressed in human testicular
spermatogenic cells, but not in non-reproductive tissues.
Furthermore, expression levels of TDRG1 are relatively
increased in young men aged 15–33 years (7). Notably, all TGCT originate from the
spermatogenic cells and the high-risk ages for the development of
TGCT are consistent with the expression pattern of TDRG1.
Previous studies have demonstrated the abnormal expression of TDRG1
protein in TGCT compared with non-malignant human testicular
tissues (8). Therefore, it is
possible that TDRG1 may be associated with the onset and
development of TGCT.
The use of short hairpin (sh)RNA to silence a target
gene has become a conventional and accurate method (9). In the present study, RNA interference
(RNAi) was used to downregulate the expression level of
TDRG1 in TGCT NTERA-2 cells. The effects of reduced
TDRG1 expression levels on NTERA-2 cells were observed. The
biological behavior of NTERA-2 cells with reduced TDRG1
expression was detected and compared with control groups and the
association between these biological behaviors and TDRG1 was
analyzed.
Materials and methods
Cell culture
The human TGCT NTERA-2 cell line was obtained from
the Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Shanghai, China) and cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS; Invitrogen Life
Technologies, Carlsbad, CA, USA) at 37°C in a humidified atmosphere
of 5% CO2.
TDRG1-targeting siRNA expression
vector
Two previously constructed recombinant plasmid
vectors were used in the present study (10). The recombinant plasmids were named
pGPU6/GFP/Neo-shRNA486 (psh486) and pGPU6/GFP/Neo-shRNA-control
(pshneg), respectively. The DNA concentration and purity of the
plasmids was detected by ultraviolet spectrophotometry (SmartSpec
3000; Bio-Rad Laboratories, Inc., Hercules, CA, USA) then they were
stored at −20°C. The shRNA486 sequence (the mRNA coding region of
486–506 is in upper case): Sense 5′-caccGC GCA GGA TCA A GC TAC AAT
G ttc aag aga CAT TGT AGC TTG ATC CTG CGC ttt ttt g-3′ and
antisense 5′-gat cca aaa aaG CGC AGG ATC AAG CTA CAA TGt ctc ttg
aaC ATT GTA GCT TGA TCC TGC GC-3′. The negative control sequence
(the target sequence that has no homology with the human gene is in
upper case): Sense, 5′-caccGT TCT CCG AAC GTG TCA CGTC caa gag aTT
ACG TGA CAC GTT CGG AGA Att ttt tg-3′ and antisense 5′-gat cca aaa
aaTTCT CCG AAC GTG TCA CGT AAt ctc tTG ACG TGA CAC GTT CGG AGA
Ac-3′.
Transfection and grouping
NTERA-2 cells were passaged with 0.25 g/l trypsin
(GE Healthcare Life Sciences, Logan, UT, USA) and seeded into
6-well plates at a density of 4.5×104 cells/well. When
the cells reached ≥70% confluence, they were transfected in 3
groups as follows: i) Control group (no transfection); ii) negative
control group (pshneg transfection); and iii) shRNA486 group
(psh486 transfection).
Following the Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA) manufacturer's instructions, the
plasmids described above and the liposome were diluted separately
with Opti-MEM (Invitrogen Life Technologies). The effective ratio
of plasmid:liposome was 1:2.5 (µg:µl). The diluted plasmid and
liposome were mixed and incubated at room temperature for 20 min to
allow complex formation. The transfection mixture was then added to
each well with 2 ml of FBS-free DMEM. Following 6 h incubation at
37°C, the mixture was replaced with complete medium. Fluorescence
microscopy (Nikon E800; Nikon Corporation, Tokyo, Japan) was used
to confirm successful transfection after 48 h.
RNA extraction and fluorescence
quantitative polymerase chain reaction (qPCR)
The specific primers designed to amplify
TDRG1 and GAPDH were designed and synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China). The following primer
sequences were used: TDRG1, sense 5′-GAA GAG GAG GGA GGC AGT CT-3′
and antisense 5′-GCC CAA TTC CTC TTG ACT GA-3′; GAPDH, sense 5′-ACC
ACA GTC CAT GCC ATC AC-3′ and antisense 5′- TCC ACC ACC CTG TTG CTG
TA-3′. Total RNA was extracted from NTERA-2 cells using TRIzol
reagent (Invitrogen Life Technologies). First strand cDNA synthesis
was performed with the RevertAid H Minus First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Fluorescence qPCR was performed in a total volume of 25 µl
containing 1 µl cDNA, 100 nM of each TDRG1 or GAPDH primer, 12 µl
2X SYBR Green PCR Master mix (Thermo Fisher Scientific, Inc.) and
ddH2O to a total volume of 25 µl. All the procedures
were performed according the manufacturer's instructions. The
reaction parameters were 95°C for 5 min (pre-denaturation),
followed by 20 sec each at 94°C (denaturation), 56°C (annealing)
and 72°C (elongation) for 40 cycles, then a final elongation step
for 5 min at 72°C. Preliminary experiments verified that the
amplification efficiency of the target gene was similar to that of
the reference gene GAPDH. The 2−ΔΔCt method was used for
relative quantification and statistical analysis (11). Each sample was set up as three
duplications and tested in triplicate.
Immunofluorescence
The cells cultured on glass coverslips were
transfected according to the groups outlined previously. At 1, 3 or
5 days post-transfection, the cells were washed twice with
phosphate-buffered saline (PBS) and fixed with ice cold 4%
paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) for 20 min at
room temperature, air-dried, and washed with PBS 3 times. The cells
were then exposed to 0.25% Triton X-100 (Sigma-Aldrich) for 20 min.
Normal goat serum blocking fluid (10%; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) was applied to the
fixed cells for 20 min at room temperature and then the blocking
fluid was removed. Mouse anti-human TDRG1 monoclonal antibody
(12) (1:1,000; ProMab
Biotechnologies, Inc., Richmond, CA, USA) was applied to the cells
and incubated overnight at 4°C (12).
The cells were then washed with PBS 3 times, the secondary antibody
Cy3-conjugated goat anti-mouse IgG (1:200; cat. no. A0521; Beyotime
Institute of Biotechnology, Shanghai, China) was applied to the
cells and the cells were incubated in the dark at 37°C for 40 min.
The cells were then washed with PBS 3 times, and stained with DAPI
(100 ng/ml; Beijing Solarbio Science & Technology Co., Ltd.),
and the cells were again rinsed with PBS 3 times. The cells were
observed using a fluorescence microscope (Nikon E800) and the
integrated optical density (IOD) of the TDRG1 protein expression in
10 randomly selected visual fields was measured using ‘Motic Fluo’
software, version 1.0 (Motic China Group Corporation, Ltd.,
Shenzhen, China).
Cell proliferation assay
The effect of TDRG1 on the proliferation of NTERA-2
cells was measured using MTT (Sigma-Aldrich) assay. The transfected
cells were cultured in 96-well plates at a density of
~1×104 cells/well in a volume of 100 µl. At time points
of 1, 3 or 5 days, 20 µl 5 mg/ml MTT solution was added to each
well and the cells were incubated for 4 h at 37°C. The supernatant
in each well was aspirated and discarded and 150 µl dimethyl
sulfoxide (Amresco, LLC, Solon, OH, USA) was added to dissolve the
formazan. A microplate reader (WD-2102A; Beijing Liuyi Instrument
Factory, Beijing, China) was used to detect the OD value for each
well at a wavelength of 570 nm. Each sample was tested in
triplicate.
Cell invasion assay
The in vitro invasion capability of the cells
was measured using the Transwell invasion assay. The transfected
cells were seeded at a density of ~1×105 cells/well in
the top chamber with a membrane (6-well insert, 8-µm pore size;
Corning Incorporated, New York, NY, USA) coated in Matrigel (1
mg/ml; BD Biosciences, Franklin Lakes, NJ, USA). The cells were
cultured in serum-free medium, and medium containing 10% FBS was
used as a chemoattractant in the lower chamber. Following 24-h
incubation, the chamber was removed, the Matrigel and the cells in
the upper chamber were wiped with a cotton swab and discarded. The
cells in the lower chamber were then fixed with 95% alcohol for
15–20 min and stained with 0.5% eosin (Beijing Solarbio Science
& Technology Co., Ltd.) for 10 min. The cells in 5 randomly
selected visual fields were counted under a light microscope
(Olympus IX70; Olympus Corporation, Osaka, Japan), and the average
value was recorded. This experiment was repeated 3 times.
Flow cytometric analysis
An annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) double-staining cell apoptosis test kit
(KeyGene Inc., Nanjing, China) was used to detect the rate of
apoptosis in each group. The cells were collected at 48 h following
transfection and resuspended in the binding buffer containing
annexin V-FITC and PI according to the manufacturer's instructions.
All the samples were then analyzed using a FACScan flow cytometer
(PT001374; BD Biosciences, San Jose, CA, USA) and the cells were
classified as living cells, early apoptotic cells, advanced
apoptotic cells and necrotic cells. The results were analyzed using
BD FACSDiva software, version 6.1.3 (BD Biosciences) and the
experiment was performed 3 times.
Statistical analysis
The results are expressed as the mean ± standard
deviation. SPSS software, version 13.0 (SPSS, Inc., Chicago, IL,
USA) was used to perform statistical analyses and the data were
analyzed by analysis of variance. P≤0.05 was considered to indicate
a statistically significant difference.
Results
TDRG1 expression levels were
downregulated following transfection
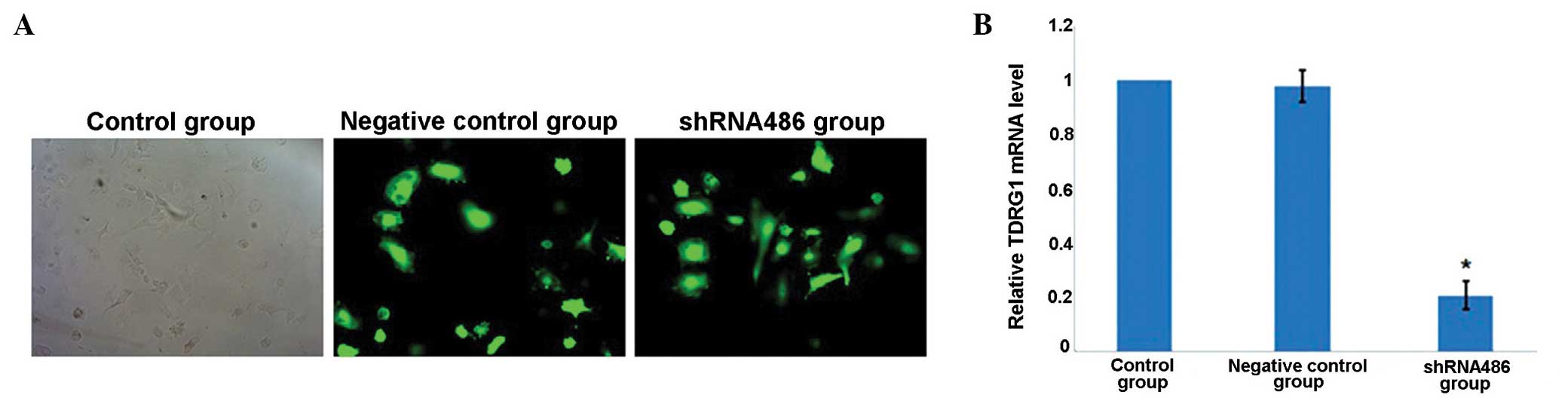
At 3 days post-transfection, the expression levels
of GFP were observed using the fluorescence microscope (Fig. 1A). Two types of recombinant vector
were successfully transfected into NTERA-2 cells. The relative
level of TDRG1 mRNA expression in the negative control group
was 0.99±0.04 (P>0.05 vs. the control group) on day 3, which was
greater than that of the shRNA486 group (0.21±0.03, P<0.05 vs.
the control group; Fig. 1B).
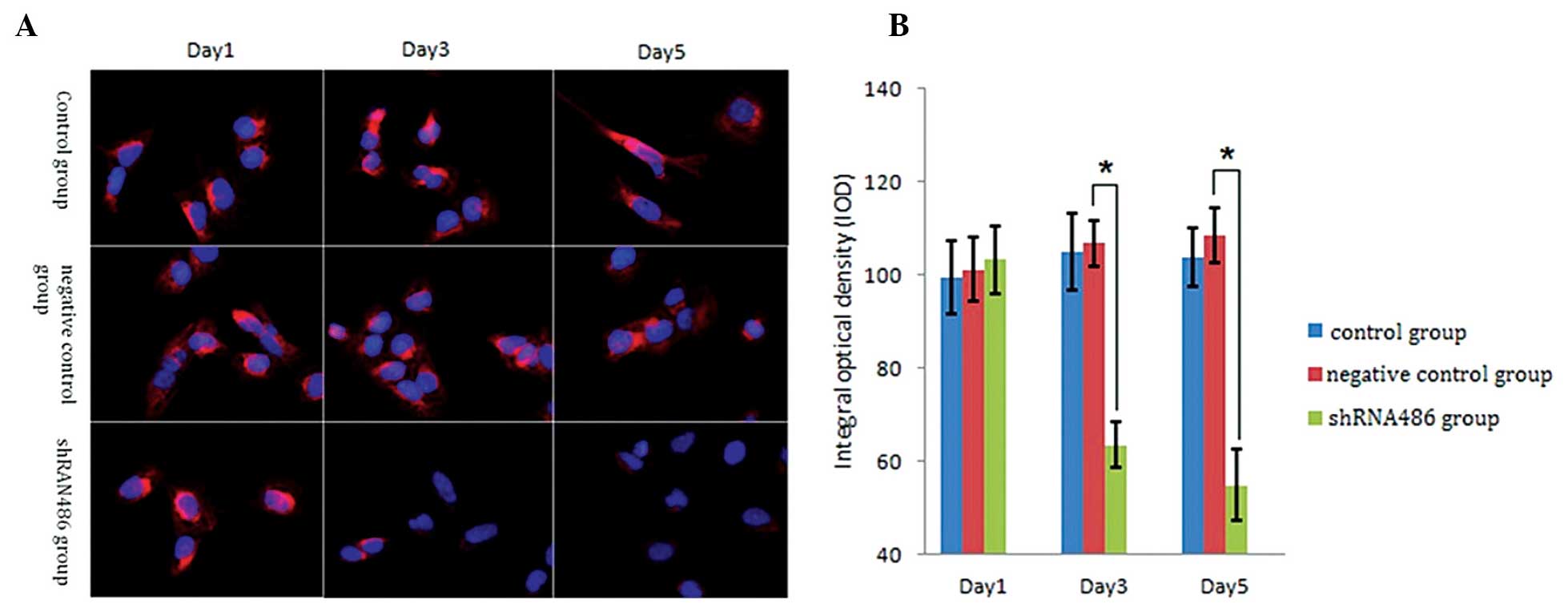
Immunofluorescence detection was used to observe TDRG1 protein
expression following shRNA transfection (Fig. 2A). At 1 day post-transfection, the IOD
values of the TDRG1 protein did not significantly differ among the
three groups (Fig. 2B; P>0.05):
Control group, 99.41±7.76; negative control group, 101.19±8.18; and
shRNA486 group, 103.31±6.17. However, at day 3 and 5 subsequent to
transfection, the IOD value of the TDRG1 protein in the shRNA486
group was significantly lower compared with the other two groups
(Fig. 2B; P<0.05). At 3 days
post-transfection: Control group, 104.93±6.83; negative control
group, 106.85±5.00; and shRNA486 group, 63.46±6.00. At 5 days
post-transfection: Control group, 103.87±7.32; negative control
group, 108.52±4.89; and shRNA486 group, 54.92±7.59. Notably, the
IOD value of the TDRG1 protein in the negative control group was
not significantly different compared with the control group
(P>0.05).
TDRG1 silencing reduces the
proliferation ability of NTERA-2 cells
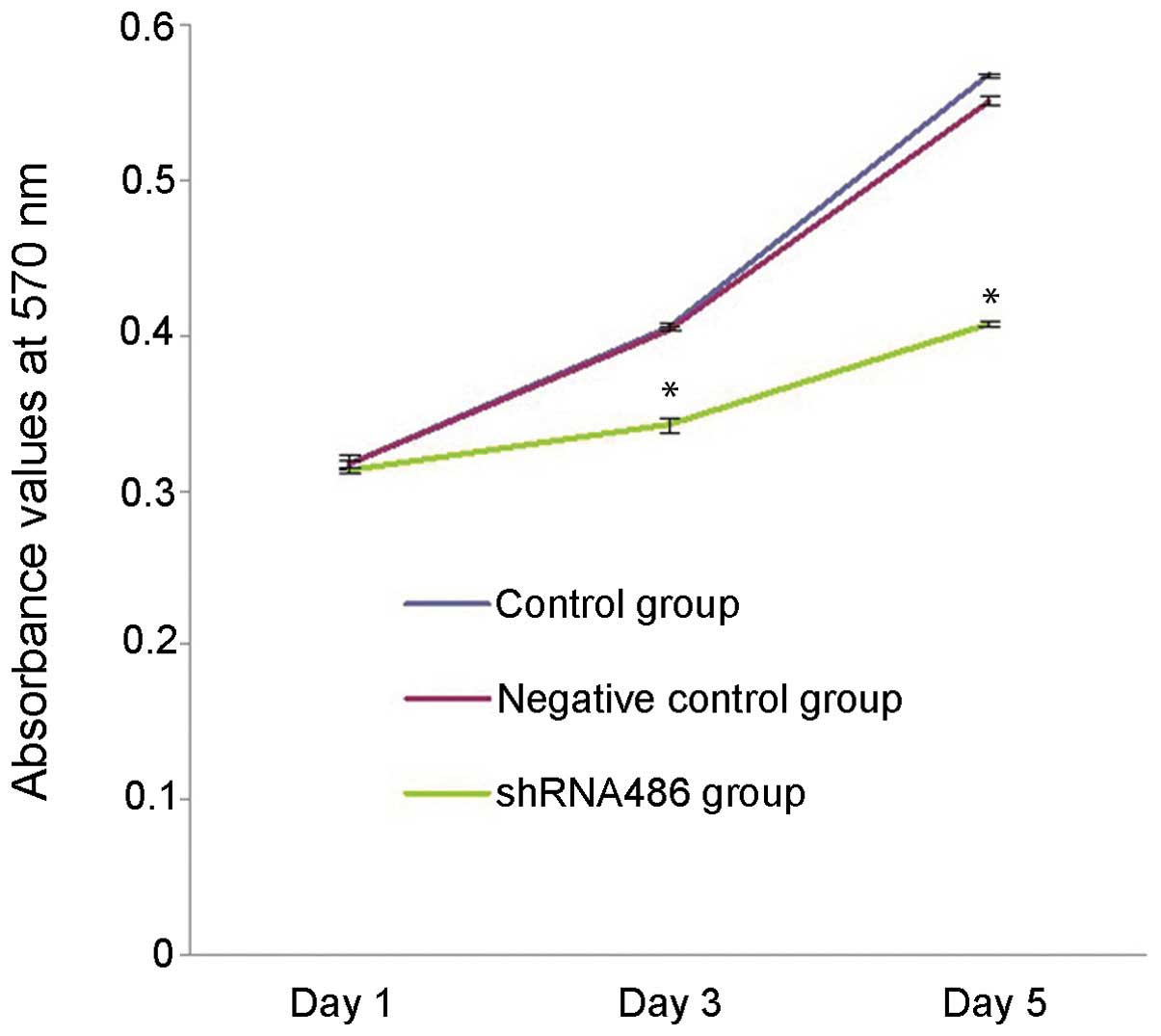
At 1, 3 and 5 days post-transfection, the in
vitro proliferation ability of NTERA-2 cells in each group was
assessed using MTT assay (Fig. 3).
The proliferation ability of NTERA-2 cells transfected with the
psh486 was significantly reduced compared with the cells in the
control group on day 3 (15.4% reduction, P<0.001) and day 5
(26.1% reduction, P<0.001) post-transfection. No significant
differences in proliferation capacity were observed at days 1, 3 or
5 (P>0.05) between the control and negative control group.
TDRG1 silencing reduced the invasion
ability of NTERA-2 cells
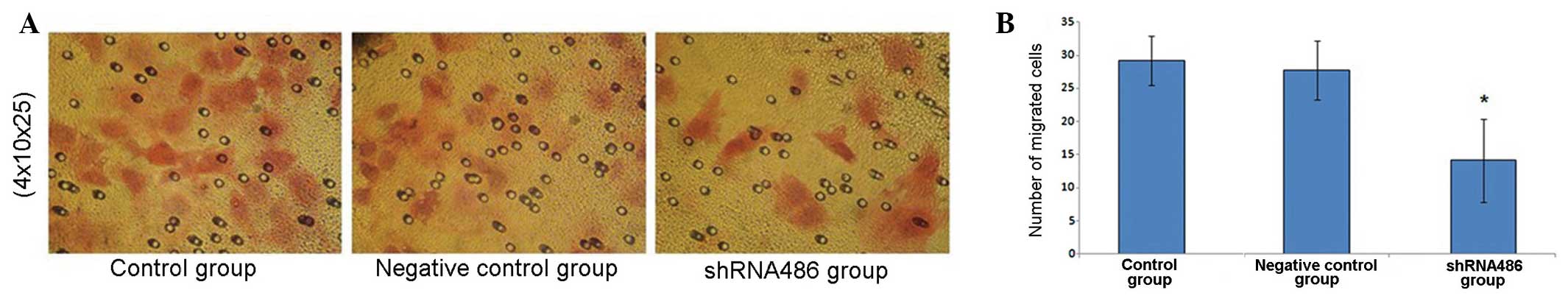
The cells that penetrated the filter membrane were
stained with eosin and counted under the light microscope
(magnification 4×10×25, Fig. 4A). The
number of cells penetrating the filter membrane in the shRNA486
group was 14.13±6.24, compared with the numbers in the control and
negative control groups, which were 29.20±3.70 and 27.73±4.47
cells, respectively (Fig. 4B). No
significant difference was observed between the number of cells
penetrating the filter membrane in the two control groups
(P=0.728). There was a significant reduction in the number of cells
penetrating the filter membrane in the shRNA486 group compared with
the two control groups (P=0.010 vs. the control group and P=0.015
vs. the negative control group).
TDRG1 silencing induces the apoptosis
of NTERA-2 cells
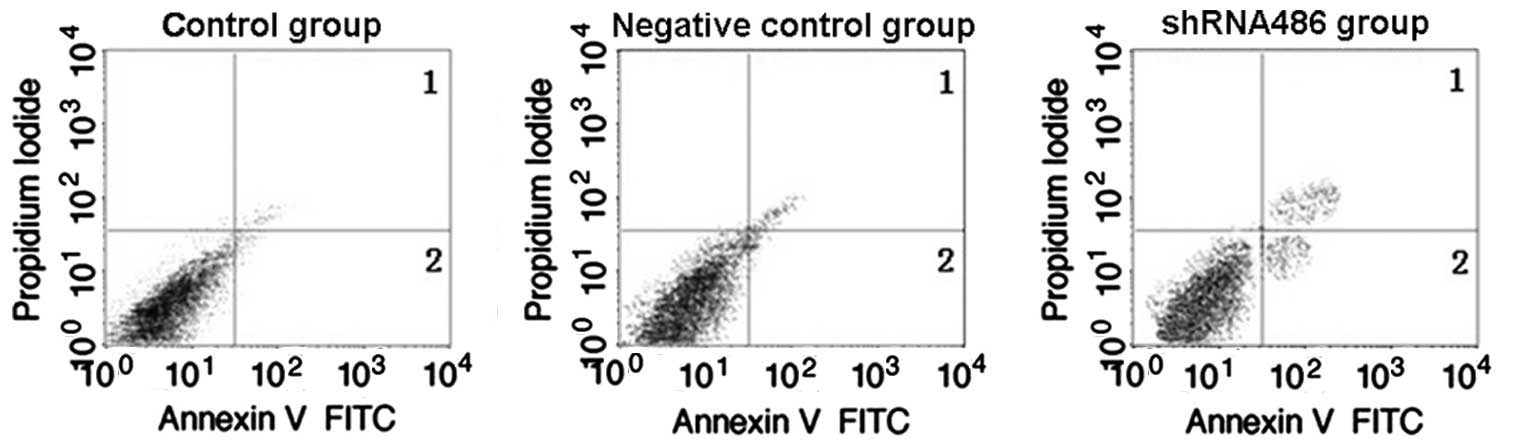
At 3 days post-transfection, the proportion of
apoptotic cells in each group was detected using flow cytometry
(Fig. 5). The proportions of early
apoptotic cells present in each group were as follows: shRNA486,
4.52±0.87%; control, 2.49±0.54%; and negative control, 2.58±0.88%.
The proportions of total apoptotic cells present in each group were
as follows: shRNA486, 9.72±1.37%; control, 5.97±0.96%; and negative
control, 6.28±1.26%. The proportions of early apoptotic cells and
total apoptotic cells were significantly increased in the shRNA486
group compared with the other two groups (P=0.019 vs. the negative
control group and P=0.009 vs. the control group). No significant
difference was demonstrated between the control and negative
control groups (P>0.05).
Discussion
The majority of cases of testiculoma occur in young
adults and the incidence is increasing; thus it is important to
study its pathogenesis (13). The
histological characteristics of testiculomas are complex. Germ cell
tumors account for ~95% of cases, primarily consisting of seminoma
and non-seminoma (4). Seminomas
account for ~50% of TGCT cases and closely resemble intratubular
germ cell neoplasia of an unspecified type (ITGCNU), which is the
first step in the development of all TGCTs. Therefore, human
seminoma cell lines may be the best cellular models for in
vitro studies (14). However,
with the exception of primary tumor samples, few tools are
available to study the molecular pathogenesis of seminoma (15). To the best of our knowledge and
according to the literature, only 3 cell lines (TCam-2, JKT-1 and
SEM-1) originate from seminoma (16–18).
Furthermore, the origin of these cell lines is not certain;
therefore it remains controversial to describe these cell lines as
seminoma cell lines (19,20). Taking these reasons into account, the
present study used the recognized embryonal carcinoma NTERA-2 cell
line as a cell model of TGCT (21,22).
Due to the poor differentiation characteristics and
the multiple differentiation potential of ITGCNU, which is the
common precursor of the majority of TGCT (23), the composition of TGCT is more closely
associated with the choice of therapy compared with other
urological neoplasms. Therefore, to determine the causes of
differentiation in different types of tumor, studies at the gene
and protein levels are particularly important. The onset and
development of TGCT are complex processes involving multiple
factors. Numerous proto-oncogenes and anti-oncogenes, including p53
and c-kit; multiple cell apoptosis genes, including
Fas/Fas-L; the expression of the telomerase RNA component; and gene
polymorphisms are all implicated in the process of TGCT development
(24–28).
TDRG1 is a novel gene that was identified in
a previous study by our research group through screening and
cloning with digital differential display (7). Reverse transcription-polymerase chain
reaction of multiple tissues demonstrated that TDRG1 was
expressed in human testes but not in other tissues, such as the
heart, liver, brain, epididymis, lung, kidney and spleen.
Immunohistochemical staining using a mouse anti-human TDRG1
monoclonal antibody (12)
demonstrated that TDRG1 was expressed solely in the
testicular seminiferous tubules and spermatogenic cells. No
expression was observed in the basal membrane or spermoblasts and a
relatively higher expression level was observed in the testes of
men between the ages of 15 and 34 years (7). Following analysis of TDRG1 protein
expression levels under pathological states, including testiculoma,
tuberculocele and testicular atrophy, using tissue microarray
technology, another previous study demonstrated that the expression
level of TDRG1 in the tissues of TGCT, such as seminoma and
teratoma, was significantly different compared with non-malignant
human testis (8). Furthermore,
significant differences were not observed in TDRG1
expression levels between the tuberculocele and testicular atrophy
groups (8). The characteristics of
TDRG1 expression levels in age, anatomical localization and
pathological patterns indicated that TDRG1 may be associated with
the oncogenesis, progression or transformation of TGCT.
RNAi degrades target mRNA by introducing exogenous
or endogenous double stranded RNA into cells, resulting in
inhibition of the corresponding gene (29). In studying the function of a target
gene via upregulating or downregulating its expression, it is
important to minimize alterations to the functions of other genes
through off-target effects. shRNA-mediated RNAi technology is a
targeted method for altering the expression of a specific gene
(30). In our previously published
work, four TDRG1-targeting shRNA recombinant plasmid vectors
were successfully constructed using the pGPu6/GFP/Neo (10). psh486 was selected for use in the
current study, as it was previously demonstrated the most
successful at blocking the expression of the TDRG1 gene.
This recombinant vector was successfully transfected into NTERA-2
cells in the present study, and reduced the expression of
TDRG1 mRNA in the cells by 79% at 3 days post-transfection.
The expression levels of the TDRG1 protein were also significantly
reduced. Therefore, the TDRG1 gene was silenced by psh486 in
NTERA-2 cells with high efficiency. NTERA-2 cells display various
biological characteristics of tumor cells. At 3 and 5 days
following TDRG1 gene silencing by psh486, the in
vitro proliferation ability of NTERA-2 cells was inhibited by
15.4 and 26.1%, respectively. Furthermore, at 3 and 5 days, the
early apoptotic potential and total apoptotic potential increased
by 75 and 54.8%. In addition, the invasion process of NTERA-2 cells
was simulated in vitro and demonstrated that the invasive
ability of NTERA-2 cells was inhibited by 49.1% in cells
transfected with psh486 compared with untransfected cells.
In conclusion, the present study indicated that the
biological behavior of NTERA-2 cells may be closely associated with
TDRG1, as the gene may promote the growth and invasion ability of
these cells. The gene may be functional in TGCT and can be
investigated further as a potential candidate gene in the
development of TGCT. However the specific underlying molecular
mechanism remains unclear and may involve interactions with other
genes. Future studies are required to investigate these remaining
questions in addition to the function of TDRG1 in cell lines
derived from other testicular tumors.
Acknowledgements
The present study was supported by grant no.
81372181 from the National Natural Science Foundation of China
(www.nsfc.gov.cn).
References
|
1
|
Horwich A, Shipley J and Huddart R:
Testicular germ-cell cancer. Lancet. 367:754–765. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chia VM, Quraishi SM, Devesa SS, Purdue
MP, Cook MB and McGlynn KA: International trends in the incidence
of testicular cancer, 1973–2002. Cancer Epidemiol Biomarkers Prev.
19:1151–1159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huyghe E, Matsuda T and Thonneau P:
Increasing incidence of testicular cancer worldwide: a review. J
Urol. 170:5–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winter C and Albers P: Testicular germ
cell tumors: pathogenesis, diagnosis and treatment. Nat Rev
Endocrinol. 7:43–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Albers P, Albrecht W, Algaba F, et al:
European Association of Urology: EAU guidelines on testicular
cancer: 2011 update. Eur Urol. 60:304–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McIntyre A, Gilbert D, Goddard N,
Looijenga L and Shipley J: Genes, chromosomes and the development
of testicular germ cell tumors of adolescents and adults. Genes
Chromosomes Cancer. 47:547–557. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang X, Li D, Yang J, et al:
Characterization of a novel human testis-specific gene: testis
developmental related gene 1 (TDRG1). Tohoku J Exp Med.
225:311–318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen HY, Wen JM, Xiao XW, et al:
Expression of human testis development related gene 1 in testicular
cancer detected by tissue microarray. Zhonghua Nan Ke Xue.
16:883–886. 2010.(In Chinese). PubMed/NCBI
|
|
9
|
Lambeth LS and Smith CA: Short hairpin
RNA-mediated gene silencing. Methods Mol Biol. 942:205–232.
2013.PubMed/NCBI
|
|
10
|
Peng S, Yang J, Chen H, et al:
Construction of TDRG1 shRNA expression vector and interfering
effect of TDRG1 shRNA expression vector on NTERA-2 cells. Zhong Nan
Da Xue Xue Bao Yi Xue Ban. 37:979–982. 2012.(In Chinese).
PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen J, Jiang X, Tang Y, Yang J, Chen H and
Liu Z: Preparation and identification of monoclonal antibody
against human testis development related gene 1. Zhong Nan Da Xue
Xue Bao Yi Xue Ban. 35:230–235. 2010.(In Chinese). PubMed/NCBI
|
|
13
|
Rosen A, Jayram G, Drazer M and Eggener
SE: Global trends in testicular cancer incidence and mortality. Eur
Urol. 60:374–379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Emerson RE and Ulbright TM: Intratubular
germ cell neoplasia of the testis and its associated cancers: the
use of novel biomarkers. Pathology. 42:344–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olie RA, Boersma AW, Dekker MC, Nooter K,
Looijenga LH and Oosterhuis JW: Apoptosis of human seminoma cells
upon disruption of their microenvironment. Br J Cancer.
73:1031–1036. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kinugawa K, Hyodo F, Matsuki T, et al:
Establishment and characterization of a new human testicular
seminoma cell line, JKT-1. Int J Urol. 5:282–287. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizuno Y, Gotoh A, Kamidono S and Kitazawa
S: Establishment and characterization of a new human testicular
germ cell tumor cell line (TCam-2). Nihon Hinyokika Gakkai Zasshi.
84:1211–1218. 1993.(In Japanese). PubMed/NCBI
|
|
18
|
Russell SM, Lechner MG, Mokashi A, et al:
Establishment and characterization of a new human extragonadal germ
cell line, SEM-1, and its comparison with TCam-2 and JKT-1.
Urology. 81:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Jong J, Stoop H, Gillis AJ, et al:
JKT-1 is not a human seminoma cell line. Int J Androl. 30:350–365.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eckert D, Nettersheim D, Heukamp LC,
Kitazawa S, Biermann K and Schorle H: TCam-2 but not JKT-1 cells
resemble seminoma in cell culture. Cell Tissue Res. 331:529–538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Andrews PW, Damjanov I, Simon D, et al:
Pluripotent embryonal carcinoma clones derived from the human
teratocarcinoma cell line Tera-2. Differentiation in vivo and in
vitro. Lab Invest. 50:147–162. 1984.PubMed/NCBI
|
|
22
|
Hasibeder A, Venkataramani V, Thelen P,
Radzun HJ and Schweyer S: Phytoestrogens regulate the proliferation
and expression of stem cell factors in cell lines of malignant
testicular germ cell tumors. Int J Oncol. 43:1385–1394.
2013.PubMed/NCBI
|
|
23
|
Di Vizio D, Cito L, Boccia A, et al: Loss
of the tumor suppressor gene PTEN marks the transition from
intratubular germ cell neoplasias (ITGCN) to invasive germ cell
tumors. Oncogene. 24:1882–1894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gutekunst M, Oren M, Weilbacher A, et al:
p53 hypersensitivity is the predominant mechanism of the unique
responsiveness of testicular germ cell tumor (TGCT) cells to
cisplatin. PLoS One. 6:e191982011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biermann K, Göke F, Nettersheim D, et al:
c-KIT is frequently mutated in bilateral germ cell tumours and
down-regulated during progression from intratubular germ cell
neoplasia to seminoma. J Pathol. 213:311–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baldini E, Ulisse S, Marchioni E, et al:
Expression of Fas and Fas ligand in human testicular germ cell
tumours. Int J Androl. 32:123–130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Orlando C, Gelmini S, Selli C and Pazzagli
M: Telomerase in urological malignancy. J Urol. 166:666–673. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ruark E, Seal S, McDonald H, et al: UK
Testicular Cancer Collaboration (UKTCC): Identification of nine new
susceptibility loci for testicular cancer, including variants near
DAZL and PRDM14. Nat Genet. 45:686–689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moore CB, Guthrie EH, Huang MT and Taxman
DJ: Short hairpin RNA (shRNA): design, delivery, and assessment of
gene knockdown. Methods Mol Biol. 629:141–158. 2010.PubMed/NCBI
|