Introduction
Surgery is the mainstay of treatment for penile
squamous cell carcinoma (SCC). Partial or total penectomy (PE) has
historically been considered the standard treatment for invasive
disease (1). Although the local
control rate of PE is ~95% (1), it
has a significant negative impact on the patient's sexual function,
quality of life, social interactions, self-image and self-esteem
(2). During the past decade, there
has been a change in the management of primary tumors with an
emphasis on penile sparing surgery (PSS) (3). This change has been driven by an
improved understanding of the biology of the disease (4), quality improvements in pathological
evaluation and continuous refinements of surgical techniques
(5). PSS has been previously reported
to produce excellent cosmetic and functional results without
sacrificing oncological outcomes in certain patients with
early-stage penile tumors (3,6–9).
Accordingly, an organ-sparing approach has been recommended for
patients with stage T1 disease, according to the 2009 TNM clinical
and pathological classification system (10), in national and international
guidelines (11–13). Stage T1 penile tumors are classified
as tumors which have invaded the subepithelial connective tissue,
without invasion of the corpus spongiosum or corpora cavernosa
(10).
Despite the evolution of conservative surgery at
academic centers, the national practice pattern of surgical
treatment for penile SCC in the United States (US) is largely
unknown. Therefore, the aims of the current study were to examine
data from the most recent Surveillance, Epidemiology and End
Results (SEER) cancer registry (14),
and to elucidate whether there are disparities in the use of PSS.
Since only a limited number of reports exist regarding the
oncological safety of PSS, the current study aimed to examine the
penile cancer-specific survival following conservative surgery in a
population-based setting, and to compare oncological outcomes
between PSS and PE in stage T1 disease.
Materials and methods
Participants and variables
The SEER program was used to identify patients who
received surgical treatment for primary invasive penile SCC between
1998 and 2009. The population-based database included 18 cancer
registries and covered ~28% of the US population. Since the study
used a public set of identified data, it was exempted from
institutional review boards.
Case listing
Case listing was generated using codes specific for
primary site and morphology, which included the following: The
International Classification of Disease for Oncology 2nd edition
(ICD-O-2; codes, C60.0–60.9) and 3rd edition (ICD-O-3; codes,
8070–8076) for histological subtype (SCC type). The sample was
limited to patients with adequate information and a preliminary
cohort of 1,293 patients diagnosed with invasive penile SCC was
identified. A patient who underwent local tumor destruction was
excluded. Therefore, this process yielded a sample comprising 1,292
eligible patients.
Surgical procedures
The surgical procedures for primary disease were
identified and separated into two groups: Local tumor excision
(LTE) and PE. The SEER database was used to retrieve demographic
and disease characteristics, including age, ethnicity, marital
status, year of diagnosis, tumor stage (T-stage), primary tumor
size, SEER stage and grade. T-stages were assigned according to the
American Joint Committee on Cancer 6th staging system (15). SEER stage is a simplified version of
stage defining the extent of the disease (localized, regional and
distant). Survival time was measured as the interval from the date
of diagnosis until the date of mortality or until the last
follow-up. Mortalities from penile cancer were coded as penile
cancer-specific mortality (PCSM) and all other mortalities were
considered as other-cause mortality (OCM).
Statistical analysis
Continuous data are presented as the median
[interquartile range (IQR)] and categorical data are presented as
proportions. The χ2 test for trend was used to evaluate
whether there was a linear trend in the proportions. Multivariate
logistic regression analysis was used to evaluate the adjusted
associations between covariables and utilization of LTE.
A substantial proportion of patients with penile SCC
succumb to the disease as a result of competing causes of
mortality, such as ischemic heart disease, stroke and diabetes
(16). Competing risk analysis was
used to account for the effect of OCM and provide unbiased
estimates of PCSM. The cumulative incidence was plotted to
graphically depict PCSM and OCM. Gray's test was used to assess the
statistical significance of a prognostic factor in a cumulative
incidence analysis (17).
Multivariable competing risk regression analysis was used to
evaluate the adjusted effects of covariates on PCSM, as proposed by
Fine and Gray (18).
In order to enable balanced comparisons between LTE
and PE, propensity score matching was used to adjust for the
inherent selection bias within observational data (19). Using propensity score matching,
randomized trials can be statistically reproduced by balancing the
characteristics of different treatment groups. The propensity to
undergo LTE was calculated using a multivariable logistic
regression model that adjusted significant confounders. In
addition, the nearest neighbor method matching, with a caliper
width of 0.2 of the standard deviation of the logit, was used to
match cases. The standardized difference measure was also used to
assess how closely the PE patients matched the LTE cases.
All the analyses were performed using R software
(version 3.0.0; http://www.r-project.org.). P-values were two-tailed
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical characteristics of
patients
The descriptive characteristics of the 1,292
eligible patients with penile SCC are presented in Table I. The median age was 67 years, while
the majority of patients were Caucasian (85.7%), married (62.9%)
and diagnosed with T1 disease (54.1%). Of these patients, 313
(24.2%) underwent LTE and 979 (75.8%) received partial or total
PE.
 | Table I.Demographic and disease
characteristics of 1,292 patients with penile squamous cell
carcinoma (1998–2009). |
Table I.
Demographic and disease
characteristics of 1,292 patients with penile squamous cell
carcinoma (1998–2009).
| Characteristics | n (%) |
|---|
| Age, years (median,
interquartile range) | 67 (57–77) |
| Ethnicity |
|
|
Caucasian | 1,107 (85.7) |
| African
descent | 119 (9.2) |
|
Other | 66 (5.1) |
| Marital status |
|
|
Married | 813 (62.9) |
|
Single | 479 (37.1) |
| Year of
diagnosis |
|
|
1988–2000 | 250 (15.4) |
|
2001–2003 | 449 (27.7) |
|
2004–2006 | 434 (26.7) |
|
2007–2009 | 490 (30.2) |
| Tumor stage |
|
| T1 | 699 (54.1) |
| T2 | 381 (29.5) |
| T3-4 | 212 (16.4) |
| Primary tumor size,
cm |
|
|
<1 | 106 (8.2) |
|
1–1.9 | 259 (20.0) |
|
2–2.9 | 304 (23.5) |
|
3–3.9 | 261 (20.2) |
| ≥4 | 362 (28.0) |
| SEER stage |
|
|
Localized | 706 (54.6) |
|
Regional | 550 (42.6) |
|
Distant | 36 (2.8) |
| Tumor grade |
|
| Grade
I | 366 (28.3) |
| Grade
II | 639 (49.5) |
| Grade
III-IV | 287 (22.2) |
| Treatment of
primary disease |
|
| Local
tumor excision | 313 (24.2) |
| Partial
penectomy | 801 (62.0) |
| Total
penectomy | 178 (13.8) |
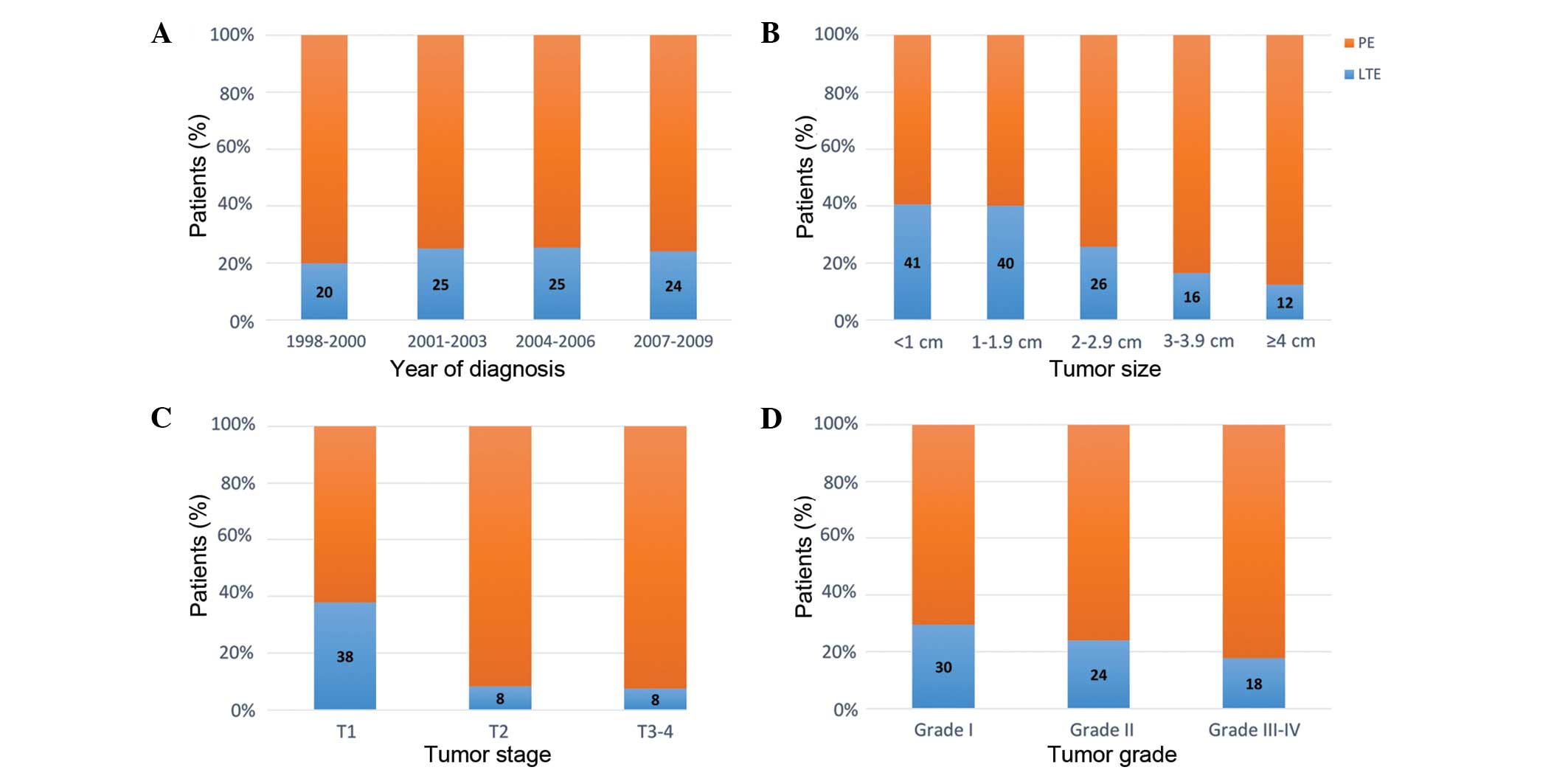
Distribution of LTE and PE
Fig. 1 illustrates the
distribution of LTE and PE stratified according to the year of
diagnosis, primary tumor size, T-stage and tumor grade. Increased
LTE utilization rates were observed in smaller tumors, lower
T-stage and lower tumor grade (all P<0.05). The overall use of
LTE in the general population was similar throughout the study
period (P=0.53).
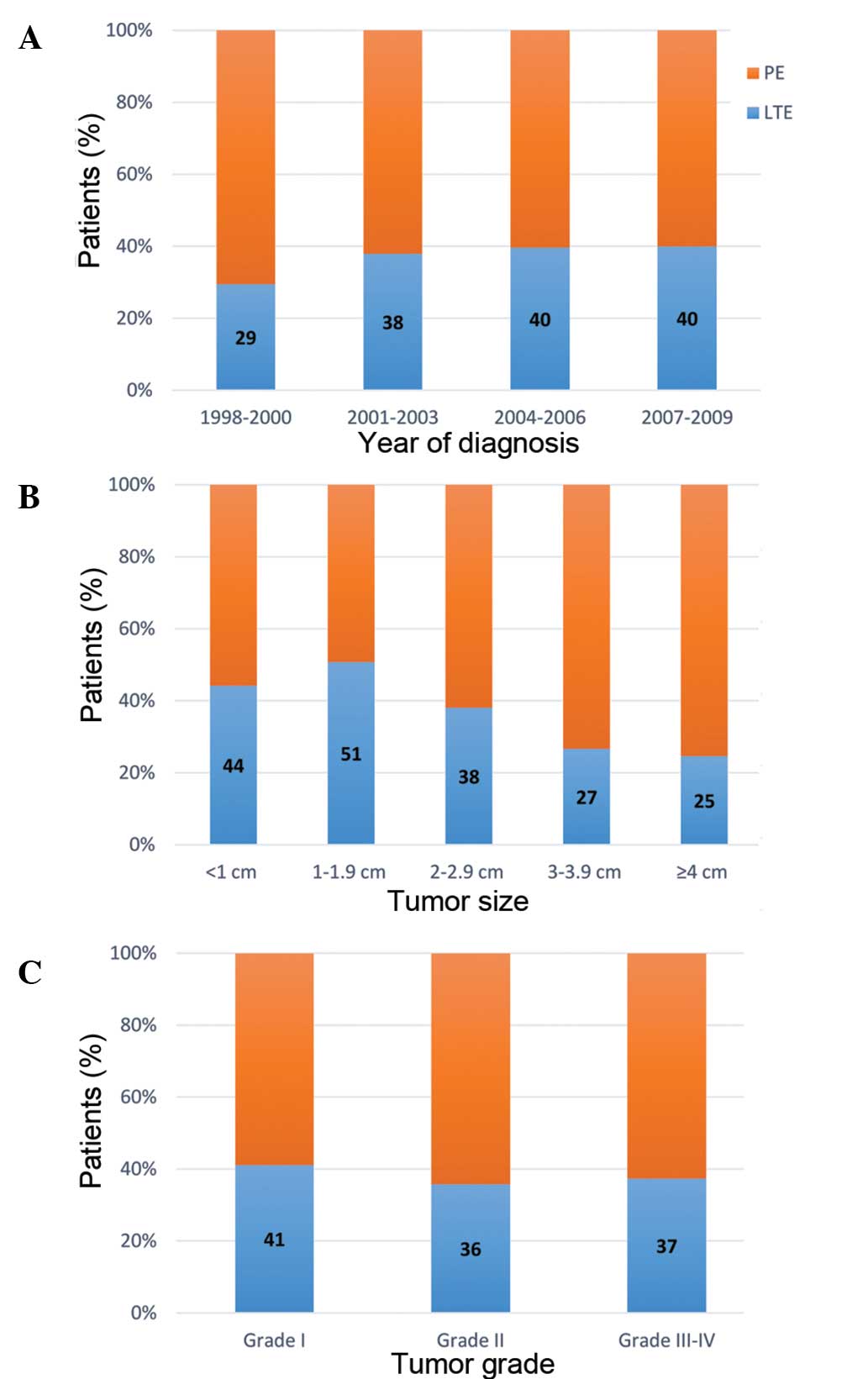
Rate of LTE within stage T1 disease
patients
The rate of LTE within patients with stage T1
disease was further investigated (Fig.
2). Among the 699 stage T1 patients, 265 (37.9%) were treated
with LTE. The rate of LTE increased moderately from 29 to 40% over
the study period, however this increase was not statistically
significant (P=0.10; Fig. 2A). In
addition, patients with smaller tumors were more likely to receive
LTE (P<0.01; Fig. 2B). However,
lower tumor grade was not associated with a higher rate of LTE
(P=0.56; Fig. 2C).
Using multivariate analyses, the adjusted
associations between individual characteristics and the use of LTE
was assessed. Table II demonstrates
that patients treated with LTE were younger, more often of African
descent, with tumor size of <3 cm and with stage T1 disease (all
P<0.01). Unmarried men were more likely to select PE treatment
than LTE, whereas married men were more likely to receive LTE than
PE, however, no signficant difference was identified (P=0.08). By
contrast, SEER stage and tumor grade were not independent
predictors of conservative surgery.
 | Table II.Multivariate analyses of predictors
for the receipt of local tumor excision in patients with penile SCC
(n=1292). |
Table II.
Multivariate analyses of predictors
for the receipt of local tumor excision in patients with penile SCC
(n=1292).
| Variables | Odds ratio (95%
CI) | P-value |
|---|
| Age | 0.99
(0.98–1.00) | <0.01 |
|
Ethnicitya |
|
|
| African
descent | 1.72
(1.07–2.75) | 0.02 |
|
Other | 0.9
(0.46–1.75) | 0.75 |
| Marital
statusb |
|
|
|
Unmarried | 1.30
(0.97–1.75) | 0.08 |
| Primary tumor
sizec, cm |
|
|
|
1–1.9 | 1.20
(0.74–1.96) | 0.46 |
|
2–2.9 | 0.70
(0.43–1.14) | 0.15 |
|
3–3.9 | 0.44
(0.25–0.75) | <0.01 |
| ≥4 | 0.37
(0.21–0.63) | <0.01 |
| Tumor
staged |
|
|
| T2 | 0.18
(0.10–0.31) | <0.01 |
|
T3-T4 | 0.16
(0.08–0.31) | <0.01 |
| SEER
stagee |
|
|
|
Regional | 1.09
(0.68–1.74) | 0.71 |
|
Distant | 1.16
(0.40–3.37) | 0.78 |
| Tumor
gradef |
|
|
| Grade
II | 0.92
(0.67–1.26) | 0.60 |
| Grade
III-IV | 0.77
(0.50–1.17) | 0.21 |
| Year of
diagnosisg |
|
|
|
2001–2003 | 1.31
(0.81–2.14) | 0.28 |
|
2004–2006 | 1.46
(0.90–2.37) | 0.12 |
|
2007–2009 | 1.38
(0.86–2.21) | 0.18 |
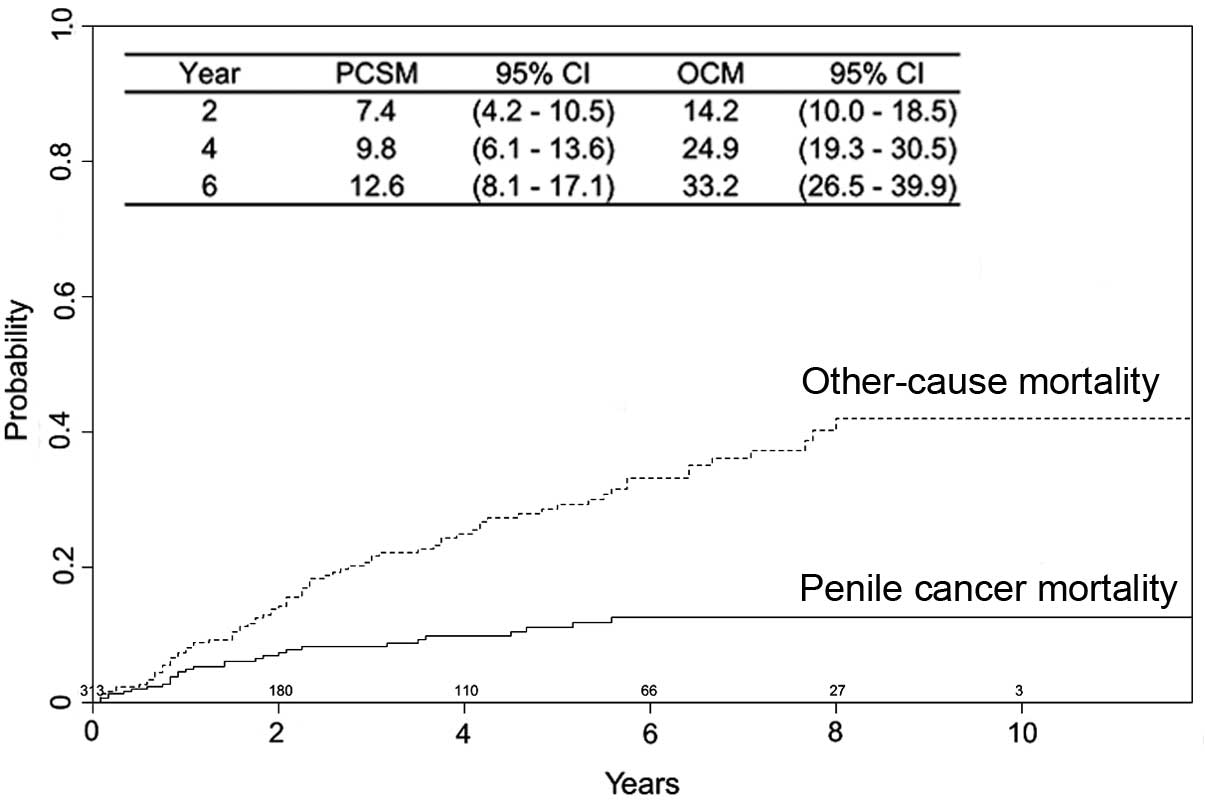
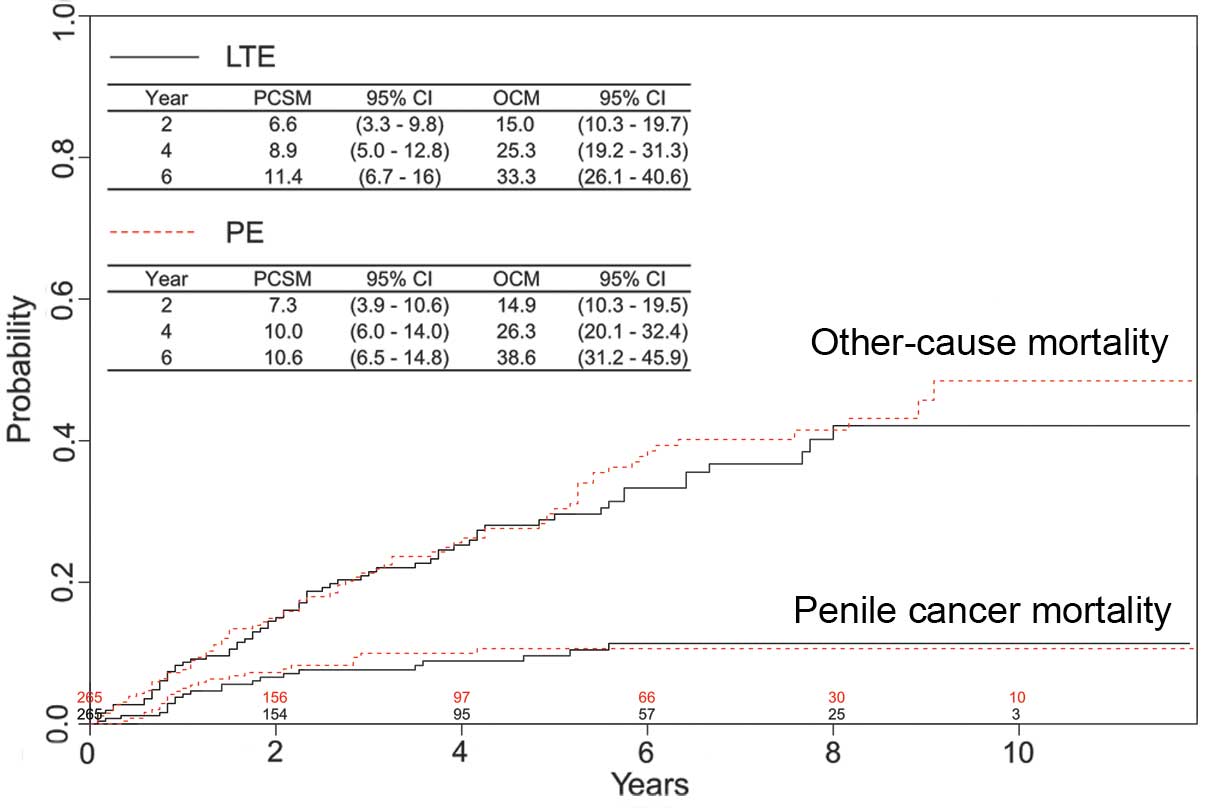
Survival outcomes
The survival outcomes in 313 patients who received
LTE were further evaluated. During a median follow-up of 55 months
(IQR, 45–65 months), 29 patients (9.3%) succumbed to penile cancer
and 79 patients (25.2%) succumbed to another cause. Estimates of
PCSM and OCM are presented in Fig. 3.
Mortality in patients treated with LTE was not usually a result of
the penile cancer. The risk factors associated with PCSM were
investigated in this cohort (Table
III). Patients who were older, with a tumor size of 3–4 cm and
with regional or distant disease (SEER stage) had a significantly
increased likelihood of mortality associated with penile cancer
(all P<0.05). Multivariate analyses were also performed to
identify risk factors in males that underwent PE (n=979). By
contrast, only tumor grade and SEER stage were independent
predictors of PCSM (data not shown).
 | Table III.Multivariate analyses of predictors
of penile cancer-specific mortality in patients treated with local
tumor excision (n=313). |
Table III.
Multivariate analyses of predictors
of penile cancer-specific mortality in patients treated with local
tumor excision (n=313).
| Variables | Hazard ratio (95%
CI) | P-value |
|---|
| Age | 1.03
(1.00–1.05) | 0.03 |
|
Ethnicitya |
|
|
| African
descent/Other | 0.15
(0.02–1.21) | 0.08 |
| Marital
statusb |
|
|
|
Single | 0.75
(0.31–1.80) | 0.52 |
| Primary tumor
sizec, cm |
|
|
|
1–1.9 | 1.00
(0.19–5.17) | 1.00 |
|
2–2.9 | 2.41
(0.50–11.52) | 0.27 |
|
3–3.9 | 6.79
(1.32–35.07) | 0.02 |
| ≥4 | 1.99
(0.35–11.32) | 0.44 |
| Tumor
staged |
|
|
|
T2-T4 | 0.49
(0.15–1.55) | 0.22 |
| SEER
stagee |
|
|
|
Regional/Distant | 4.83
(1.74–13.38) | <0.01 |
| Tumor
gradef |
|
|
| Grade
II | 0.76
(0.29–2.00) | 0.58 |
| Grade
III-IV | 0.94
(0.27–3.23) | 0.92 |
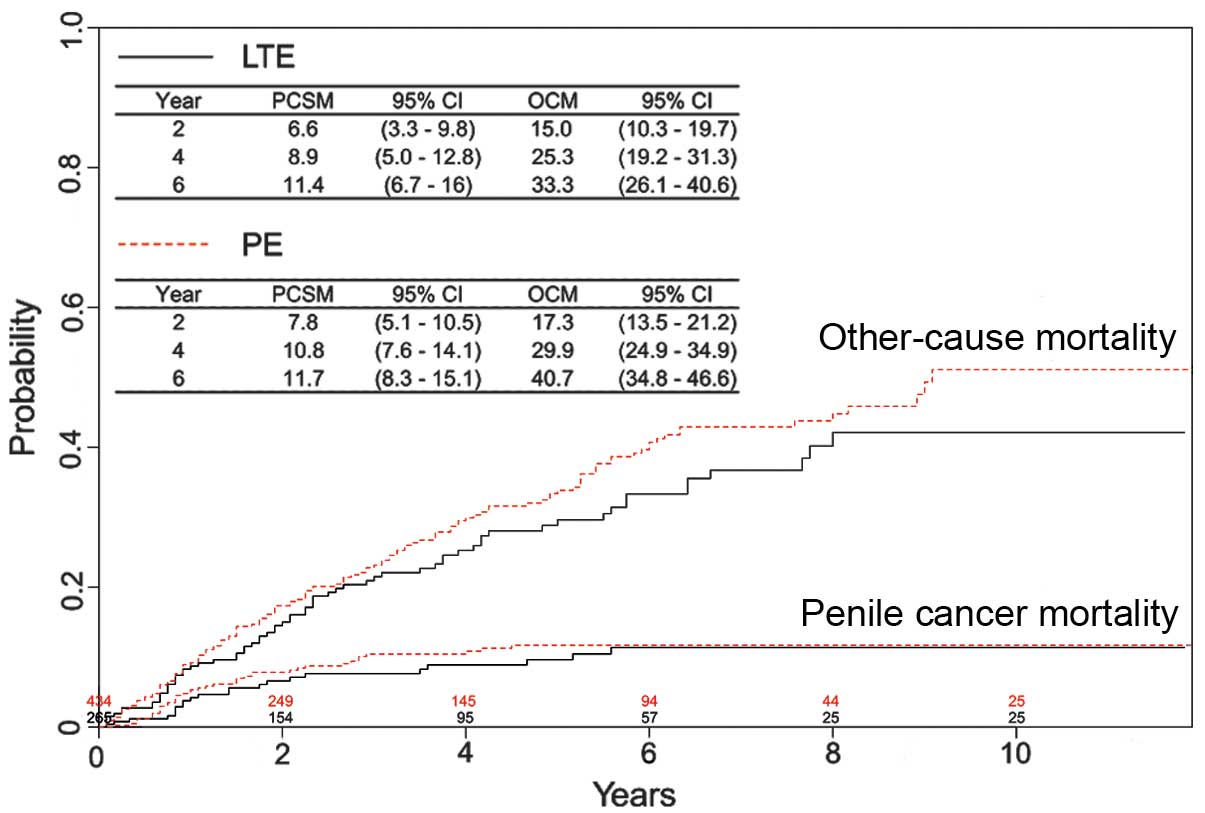
Risk of PCSM in T1 disease
patients
Whether LTE was associated with a higher risk of
PCSM was investigated in the T1 disease subgroup. Of these
patients, 265 (37.9%) and 434 (62.1%) were treated with LTE or PE,
respectively. During a median follow-up period of 59 months (IQR,
54–66 months), penile cancer was identified as the cause of
mortality in 22 patients (8.3%) treated with LTE and 41 patients
(9.4%) treated with PE. As shown in Fig.
4, no statistically significant difference in PCSM was observed
between the treatment groups (P=0.66). In addition, OCM was
comparable for patients treated with LTE or PE (P=0.21). In order
to reduce selection bias in the assignment of treatments, matched
groups were generated using the propensity score matching method.
Table IV demonstrates that
significant variations of covariates were diminished following
statistical processing. In the matched series, the rate of PCSM did
not differ significantly between patients treated with LTE or PE,
with four-year PCSM rates of 8.9 and 10.0%, respectively (P=0.93;
Fig. 5).
 | Table IV.Propensity score matching of surgical
procedures in patients with stage T1 disease. |
Table IV.
Propensity score matching of surgical
procedures in patients with stage T1 disease.
|
|
| Prior to
matching | Subsequent to
matching |
|---|
|
|
|
|
|
|---|
| Variables | Tumor excision | Penectomy | P-value | Penectomy | P-value |
|---|
| Total number,
n | 265 | 434 |
| 265 |
|
| Mean age,
years | 64.7 | 67.7 | <0.01 | 65.0 | 0.84 |
| Ethnicity |
|
| 0.48 |
| 0.21 |
|
Caucasian | 222 | 375 |
| 229 |
|
| African
descent | 30 | 37 |
| 19 |
|
|
Other | 13 | 22 |
| 17 |
|
| Marital status |
|
| 0.53 |
| 0.23 |
|
Married | 167 | 285 |
| 181 |
|
|
Single | 98 | 149 |
| 84 |
|
| Tumor grade |
|
| 0.42 |
| 0.33 |
| Grade
I | 100 | 143 |
| 85 |
|
| Grade
II | 122 | 219 |
| 138 |
|
| Grade
III-IV | 43 | 72 |
| 42 |
|
| Primary tumor size,
cm |
|
| <0.01 |
| 0.92 |
|
<1 | 38 | 48 |
| 44 |
|
|
1–1.9 | 97 | 94 |
| 88 |
|
|
2–2.9 | 67 | 109 |
| 68 |
|
|
3–3.9 | 33 | 91 |
| 33 |
|
| ≥4 | 30 | 92 |
| 32 |
|
| SEER stage |
|
| 0.29 |
| 0.66 |
|
Localized | 242 | 384 |
| 238 |
|
|
Regional/Distant | 23 | 50 |
| 27 |
|
| Mean propensity
score | 0.59 | 0.64 | <0.01 | 0.59 | 0.55 |
Discussion
Over the past decade, growing evidence has indicated
the safety, efficacy and benefit of PSS over PE for certain
patients with early-stage penile tumors. A total of 10 studies
reported in the literature were identified, which investigated the
role of conservative surgery in invasive penile cancer (Table V; 1,3,6–8,20–23). Local
disease control was achieved in 82.2% of the cases following PSS.
Although PSS was associated with an increased risk of local
failure, it did not appear to compromise long-term cancer-specific
survival. The five-year cancer-specific survival rate following
local recurrence was 92% in two large studies (1,6). In
addition, sexual function, micturition and cosmetic results were
generally well maintained following conservative surgery (3,6–8,20–23). Accordingly, the use of PSS has risen
dramatically at tertiary care centers and has been recommended by
certain guidelines, including the European Association of Urology
(EAU) Guidelines Group on Penile Cancer, as a standard surgical
approach (11–13).
 | Table V.Literature review of oncological
outcomes following penile-sparing surgery. |
Table V.
Literature review of oncological
outcomes following penile-sparing surgery.
| First author, year
(ref) | Number of patients,
n | Tumor stage ≥T1,
% | Follow-up,
months | Local recurrence
rate, % |
|---|
| Smith, 2007
(3) | 72 | 100.0 | 27 (mean) | 4.2 |
| Leijte, 2008
(1) | 415 | 69.4 | 60.6 (median) | 27.7 |
| Morelli, 2009
(20) | 17 | 88.2 | 36 (mean) | 0.0 |
| Feldman, 2011
(21) | 56 | 50.0 | 60
(median)a | 21.4 |
| Li, 2011 (8) | 32 | 78.1 | 26.5 (median) | 9.3 |
| O'Kane, 2011
(22) | 25 | 76.0 | 28 (mean) | 4.0 |
| Li, 2012 (23) | 12 | 25.0 | 62.5 (mean) | 8.3 |
| Philippou, 2012
(6) | 179 | 100.0 | 39 (median) | 8.9 |
| Veeratterapillay,
2012 (7) | 65 | 76.9 | 40 (median) | 6.2 |
| Total | 873 | 77.8 |
| 17.8 |
The surgical treatment paradigm for primary disease
emphasizes the underuse of PSS in the USA. In the present study,
≥50% of patients with a T1 tumor of <2 cm were identified to
have undergone traditional radical surgery. Although the results
identified that the utilization rate of LTE has increased from 29
to 40% in stage T1 disease over the last decade, PE remains the
most commonly performed type of surgery for early-stage penile
tumors. The reasons for this are multifactorial and may include the
lack of substantial evidence for oncological safety and the
technical challenges associated with PSS. The long-term outcomes of
PSS have not previously been available to prove that conservative
surgery provided comparable cancer-specific survival as
conventional procedures (1,6). Furthermore, there may be a requirement
for additional training of surgical skills to safely and
effectively perform PSS. Since there is no centralized management
of this rare disease in North America, treatment standardization
and gain of experience are slow in the community setting.
The disparities in the use of LTE in the general
practice pattern were also investigated in the present study. As
expected, tumor size and T-stage were strongly associated with the
use of LTE. Furthermore, LTE was more frequently performed in
younger patients or those of African descent. The reason for the
disparities in the use of LTE in different ages and ethnicities
remains unclear. However, the same phenomenon was observed in
patients choosing to receive a penile implant following treatment
of prostate cancer (24). In
multivariate analysis, predictors of penile implant surgery were
younger and of African-American descent. Therefore, the authors
speculated that physicians may have a better appreciation of the
impact of PSS in young male patients and those of African-American
descent (24). Further investigation
of these biases is warranted, and increased education of physicians
and patients may alleviate the discrepancy of care.
In the population-based cohort, the six-year PCSM
rate following LTE was 12.6%, which is consistent with other large
studies (1,6). On the contrary, mortality in long-term
survivors were more likely from a cause other than penile cancer.
Accounting for the influence of competing risk, the factors that
predict mortality from penile cancer following conservative surgery
were examined in the present study. Notably, old age and large
tumor size were independent predictors of PCSM in patients treated
with LTE. Therefore, these two factors may indicate an increased
risk of failure to the specific treatment. Mohs et al
(25) previously reported the
five-year follow-up of penile cancer patients treated with
micrographic surgery. Initial tumor size appeared to be an
important factor in local control with 0% recurrence in males with
lesions <2 cm and 50% recurrence in those with lesions >3 cm.
As the average length of the glans is ~4 cm (26), local excision of tumors with a size
>3 cm may result in a tumor-free margin of <1 cm. Agrawal
et al (4) observed an absence
of grade 1–2 penile SCC that extended 1 cm beyond the gross tumor
margin; however, 25% of grade 3 lesions were histologically
positive at 1 cm (4). In these
patients with larger tumors, glansectomy with reconstruction is a
better alternative to achieve satisfactory oncological and
functional outcomes (27). The reason
why age was a negative predictor in LTE patients is not clear. One
explanation is that elderly patients may be subject to suboptimal
follow-up, which is critically important following PSS. In
addition, other causes of mortality may be misattributed as
mortality due to penile cancer in older patients. Furthermore, the
natural disease course of penile SCC may be more aggressive in the
elderly. Epidemiology studies of vulval cancer have demonstrated a
bimodal age distribution; human papillomavirus (HPV)-associated
cancer manifests at an earlier age compared with HPV-unrelated
cancer (28). Bezerra et al
(29) reported a significantly lower
HPV infection rate in elderly patients. It is possible that penile
cancer in elderly patients may arise from a carcinogenesis pathway
with a high degree of genetic alterations not driven by HPV
(30). Since detailed follow-up data
regarding tumor recurrences were not available and the SEER
database does not contain further information on tumor
characteristics beyond stage and grade, the explanations proposed
in the present study remain speculative.
Although stage T1 disease is recommended as an
appropriate indication for PSS (11–13), no
direct comparison exists between LTE and PE in these patients. In
the SEER database, 37.9 and 62.1% of stage T1 disease patients
underwent conservative surgery or PE, respectively. Although no
randomized assignment of treatment existed in the observational
study, statistical processing may aid in the adjustment for
differences of baseline characteristics and reduce the inherited
selection bias. Therefore, the propensity score matching method was
employed in the current study to mimic random allocation of
treatment in stage T1 penile SCC. The comparisons of PCSM between
matched cohorts concluded that penile cancer-specific survival
following PSS was not reduced. Corroborating the findings from
tertiary care centers, the results of the present study provide
considerable evidence to support the generalization of conservative
surgery on a large scale. In stage T1 disease, the utilization rate
of LTE has improved from 29 to 40% over the last decade. Although
encouraging, the changing trend remains slow compared with the
similar situation in breast-sparing surgery (31). The accumulating evidence may
accelerate the dissemination of PSS in early-stage penile
tumors.
However, the current study is not devoid of
limitations. Although the SEER program provides the largest and
most comprehensive cancer registry in the USA, there are other
variables not routinely collected, including anatomical features,
comorbid conditions and patient preferences. These factors may
account for some of the observed disparities in the use of PSS.
Since the SEER program only records the first course of treatment
received, adjuvant therapy for primary disease and its effect on
PCSM may not be evaluated from this data. The conclusions of the
present study may be biased due to excluding a proportion of
patients from analyses as a result of missing information.
Furthermore, there are inherent difficulties in accurately
determining the types of LTE based on ambiguity in coding. Despite
these limitations, the current study represents a comprehensive and
contemporary analysis of the surgical treatments for primary
lesions of penile cancer in the USA. The results may add value to
existing knowledge since the majority of previous case studies were
single-center based and thus more likely to have a selection bias.
Furthermore, accumulating large cohorts from contemporary practice
is challenging for rare cancer types.
In conclusion, the underuse of PSS is pronounced in
the general community with significant disparities in age and
ethnicity. The present population-based study provides evidence
supporting the oncological safety of PSS as compared with PE in
early-stage disease. Awareness of these issues may improve the
quality of health care in penile cancer patients.
Acknowledgements
The authors would like to thank all the study
participants, urologists and study coordinators for participating
in the study.
References
|
1
|
Leijte JA, Kirrander P, Antonini N,
Windahl T and Horenblas S: Recurrence patterns of squamous cell
carcinoma of the penis: Recommendations for follow-up based on a
two-centre analysis of 700 patients. Eur Urol. 54:161–168. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maddineni SB, Lau MM and Sangar VK:
Identifying the needs of penile cancer sufferers: A systematic
review of the quality of life, psychosexual and psychosocial
literature in penile cancer. BMC Urol. 9:82009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith Y, Hadway P, Biedrzycki O, Perry MJ,
Corbishley C and Watkin NA: Reconstructive surgery for invasive
squamous carcinoma of the glans penis. Eur Urol. 52:1179–1185.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agrawal A, Pai D, Ananthakrishnan N, Smile
SR and Ratnakar C: The histological extent of the local spread of
carcinoma of the penis and its therapeutic implications. BJU Int.
85:299–301. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hegarty PK, Shabbir M, Hughes B, et al:
Penile preserving surgery and surgical strategies to maximize
penile form and function in penile cancer: Recommendations from the
United Kingdom experience. World J Urol. 27:179–187. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Philippou P, Shabbir M, Malone P, et al:
Conservative surgery for squamous cell carcinoma of the penis:
Resection margins and long-term oncological control. J Urol.
188:803–808. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Veeratterapillay R, Sahadevan K, Aluru P,
Asterling S, Rao GS and Greene D: Organ-preserving surgery for
penile cancer: description of techniques and surgical outcomes. BJU
Int. 110:1792–1795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Zhu Y, Zhang SL, et al:
Organ-sparing surgery for penile cancer: complications and
outcomes. Urology. 78:1121–1124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lont AP, Gallee MP, Meinhardt W, van
Tinteren H and Horenblas S: Penis conserving treatment for T1 and
T2 penile carcinoma: Clinical implications of a local recurrence. J
Urol. 176:575–580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th; Wiley-Blackwell;
Oxford: 2009
|
|
11
|
Solsona E, Bahl A, Brandes SB, et al: New
developments in the treatment of localized penile cancer. Urology.
76:(Suppl 1). S36–S42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pizzocaro G, Algaba F, Horenblas S, et al:
European Association of Urology (EAU) Guidelines Group on Penile
Cancer: EAU penile cancer guidelines 2009. Eur Urol. 57:1002–1012.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Comprehensive Cancer Network, .
Clinical practice guidelines in oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.aspAccessed.
December 2–2013
|
|
14
|
National Cancer Institute, . SEER*Stat
Databases. November;2011.Submission. http://seer.cancer.gov/data/seerstat/nov2011/Accessed.
February 4–2014
|
|
15
|
Greene FL, Page DL, Fleming ID, Fritz A,
Haller DG and Morrow M: AJCC cancer staging manual. 6th.
Springer-Verlag; Berlin, Germany: 2002, View Article : Google Scholar
|
|
16
|
Thuret R, Sun M, Abdollah F, et al:
Competing-risks analysis in patients with T1 squamous cell
carcinoma of the penis. BJU Int. 111:E174–E179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gray RJ: A class of K-sample tests for
comparing the cumulative incidence of a competing risk. Ann Stat.
16:1141–1154. 1988. View Article : Google Scholar
|
|
18
|
Fine JP and Gray RJ: A proportional
hazards model for the subdistribution of a competing risk. J Am
Stat Assoc. 94:496–509. 1999. View Article : Google Scholar
|
|
19
|
Rosenbaum PR and Rubin DB: Reducing bias
in observational studies using subclassification on the propensity
score. J Am Stat Assoc. 79:516–524. 1984. View Article : Google Scholar
|
|
20
|
Morelli G, Pagni R, Mariani C, et al:
Glansectomy with split-thickness skin graft for the treatment of
penile carcinoma. Int J Impot Res. 21:311–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feldman AS and McDougal WS: Long-term
outcome of excisional organ sparing surgery for carcinoma of the
penis. J Urol. 186:1303–1307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Kane HF, Pahuja A, Ho KJ, Thwaini A,
Nambirajan T and Keane P: Outcome of glansectomy and skin grafting
in the management of penile cancer. Adv Urol. 2011:2408242011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li P, Song N, Yin C, et al:
Glans-preserving surgery for superficial penile cancer. J Androl.
33:435–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tal R, Jacks LM, Elkin E and Mulhall JP:
Penile implant utilization following treatment for prostate cancer:
analysis of the SEER-Medicare database. J Sex Med. 8:1797–1804.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mohs FE, Snow SN, Messing EM and Kuglitsch
ME: Microscopically controlled surgery in the treatment of
carcinoma of the penis. J Urol. 133:961–966. 1985.PubMed/NCBI
|
|
26
|
Spyropoulos E, Borousas D, Mavrikos S,
Dellis A, Bourounis M and Athanasiadis S: Size of external genital
organs and somatometric parameters among physically normal men
younger than 40 years old. Urol. 60:485–491. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palminteri E, Berdondini E, Lazzeri M,
Mirri F and Barbagli G: Resurfacing and reconstruction of the glans
penis. Eur Urol. 52:893–898. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joura EA: Epidemiology, diagnosis and
treatment of vulvar intraepithelial neoplasia. Curr Opin Obstet
Gynecol. 14:39–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bezerra AL, Lopes A, Santiago GH, Ribeiro
KC, Latorre MR and Villa LL: Human papillomavirus as a prognostic
factor in carcinoma of the penis: Analysis of 82 patients treated
with amputation and bilateral lymphadenectomy. Cancer.
91:2315–2321. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rubin MA, Kleter B, Zhou M, et al:
Detection and typing of human papillomavirus DNA in penile
carcinoma: Evidence for multiple independent pathways of penile
carcinogenesis. Am J Pathol. 159:1211–1218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morris CR, Cohen R, Schlag R and Wright
WE: Increasing trends in the use of breast-conserving surgery in
California. Am J Public Health. 90:281–284. 2000. View Article : Google Scholar : PubMed/NCBI
|