Introduction
Prostate cancer is the most prevalent malignancy and
the second leading cause of cancer-related mortality in men
worldwide (1). A total of 233,000 new
cases and 29,480 associated mortalities are projected to occur in
the United States in 2014 (2).
Current therapy strategies for prostate cancer mainly include
radical prostatectomy, external beam radiation, cryotherapy,
chemotherapy or hormonal therapy. Among these, hormonal therapy is
the most commonly used method, while for androgen-independent
cancer, few effective treatment methods are available (3). Therefore, the requirement to develop
novel and effective anti-prostate cancer drugs is extremely
urgent.
Angiogenesis is an essential step for the formation,
progression and metastasis of numerous types of cancer, including
prostate cancer (4). Previous studies
have reported that the level of several angiogenic factors, such as
vascular endothelial growth factor (VEGF) (5), transforming growth factor-β (6), fibroblast growth factors (FGFs)
(7) and cyclooxygenase-2 (8), are upregulated in prostate cancer. An
increasing number of studies are now focusing on angiogenin, a
14.4-kDa angiogenic ribonuclease, which was firstly isolated from
the conditioned medium of human colon adenocarcinoma HT-29 cells
based on its angiogenic activity, and which plays a controlling
role in tumor angiogenesis (9).
Angiogenin can transfer to the nucleus to stimulate rRNA
transcription, which is an indispensable process in angiogenesis
and cell proliferation pathways, and is also a crossroad for
angiogenesis induced by acidic and basic FGFs (αFGF, βFGF), VEGF
and epidermal growth factor (10,11). In
addition, angiogenin was reported to have an anti-apoptotic effect
by targeting p53 and B-cell lymphoma 2, thereby accelerating cancer
development (12). Angiogenin is
upregulated in a number of cancers, including prostate cancer. It
has been demonstrated that the plasma angiogenin level is elevated
in prostate cancer patients, particularly in hormonal refractory
prostate cancer patients (13), and
that serum angiogenin may be used as a prostate cancer diagnostic
tool among candidates for biopsy (14). Katona et al (15) reported that angiogenin expression
increased as prostate cancer progressed from a benign phenotype to
invasive adenocarcinoma. Using immunohistochemistry, Yoshioka et
al (16) found that strong
staining for angiogenin was present in the extracellular matrix
(ECM), cytoplasm and nucleus of prostate cancer tissues, but that
no staining was present in the cytoplasm and nucleus of normal
prostate tissues, with strong staining in the ECM. Earlier studies
indicated that angiogenin antagonists could prevent HT-29 tumor
appearance and growth (17,18). It is conceivable that an inhibitor
targeted to angiogenin may achieve ideal effects for the treatment
of prostate cancer.
Neomycin, an aminoglycoside antibiotic, was firstly
found to have an inhibitory effect on angiogenin nuclear
translocation in human umbilical vein endothelial cells (HUVECs)
(19), but its use as a
chemotherapeutic agent is limited due to its nephrotoxicity and
ototoxicity. Notably, as a derivative of neomycin, neamine has
equivalent effects but less toxicity than neomycin. Previous
studies demonstrated that neamine could inhibit the proliferation
of human hepatoma H7402 cells (20)
and human oral cancer HSC-2 cells (21). Furthermore, it suppressed the growth
of the established A549 (22) HT-29,
MDA-MB-435, A431 (23), HSC-2 and SAS
(21) tumor transplants, and the
formation of Kaposi's sarcoma-associated herpesvirus-positive
primary effusion lymphoma (12).
The human prostate cell line PC-3, is a
hormone-independent cell line. Hormone therapy is the most commonly
used treatment for prostate cancer; however, chemotherapy is
available for androgen-independent cancer. Cis-platinum (DDP) is an
effective chemotherapeutic drug for prostate cancer, and there are
currently no small-molecule targeted inhibitors for prostate
cancer, so the present study selected DDP as a positive comparison.
The present study investigated the anti-prostate cancer activity of
neamine compared with DDP, and the mechanism behind this.
Materials and methods
Preparation of neamine
Neamine was obtained from neomycin through
alcoholysis (24). Briefly, 90 g
neomycin sulfate (Sanxia Pharmaceutical Factory, Yichang, Hubei,
China) was reflux in 3 liters of methanol with 1 mM HCl for 10 h.
Next, 40 ml ammonia and 3 liters of methanol were added to conduct
ammonification for 2 h. The precipitate was washed with methanol,
purified by recrystallization in an ethanol:water mixture (9:1) and
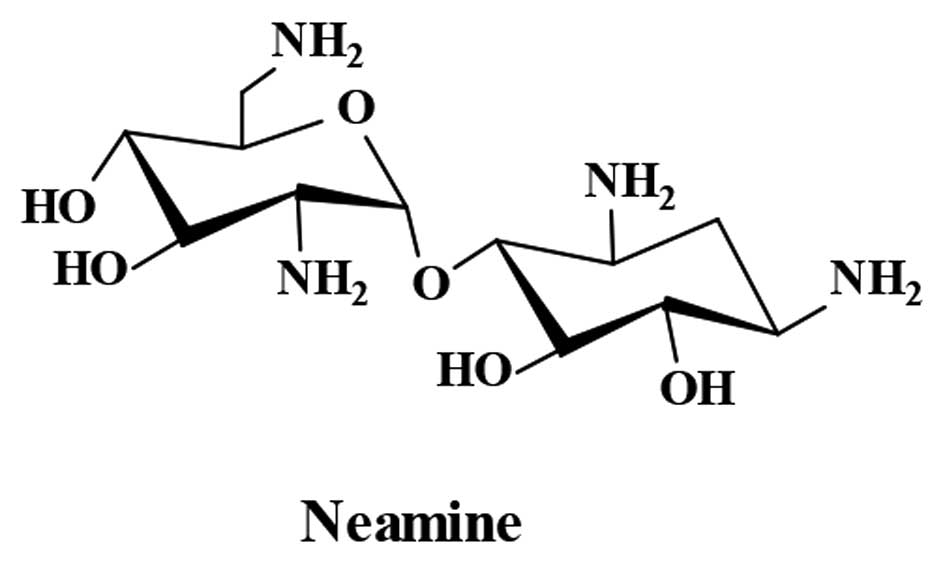
then dried in an oven; 13 g neamine was obtained. The structure of
neamine (Fig. 1) was confirmed by
nuclear magnetic resonance spectroscopy (Bruker Corporation,
Beijing, China) and the purity (99.67%) was determined by
evaporative light scattering detector-high performance liquid
chromatography (Waters Corporation, Milford, MA, USA).
Cell lines, reagents and mice
HUVECs and the human prostate cancer PC-3 cell line
were kindly provided by the Gynecological Oncology Center of Tongji
Hospital (Wuhan, China). The HUVECs were cultured in ECM medium
(ScienCell, Carlsbad, CA, USA) with 5% fetal bovine serum (FBS), 1%
endothelial cell growth supplement and 1% penicillin-streptomycin,
and the PC-3 cells were cultured in RPMI 1640 medium (GE
Healthcare, Logan, UT, USA) supplemented with 10% FBS. Angiogenin
and rabbit anti-human angiogenin polyclonal antibody (R113, 10
µg.ml) were donated by Professor Guo-Fu Hu (Molecular Oncology
Research Institute, Tufts Medical Center, Boston, MA, USA). Cluster
of differentiation (CD)31 rabbit polyclonal antibody (cat no.
ab28364; 1:2,000) and Ki-67 mouse monoclonal antibody (clone,
MIB-1; 1:2,000) were purchased from Abcam (Cambridge, UK) and Dako
(Glostrup, Denmark), respectively. DDP was produced by Qilu
Pharmaceutical Factory (Shandong, China). The 4-week-old, specific
pathogen-free (SPF), male Balb/c nude mice (certificate number,
43004700004854) were provided by the Human SJA Laboratory Animal
Co. Ltd., (Changsha, China) and maintained in the SPF-level
Experimental Animal Center of Huazhong University of Science and
Technology (Wuhan, China).
Nuclear translocation of
angiogenin
The HUVEC cells or PC-3 cells (1×104 per
well) were cultured in 24-well plates with slides for 24 h and
treated with 100 µmol neamine in the presence or absence of 1 µg/ml
angiogenin for 40 min at 37°C. The cells were then fixed with
methanol at −20°C for 15 min. The fixed cells were blocked with 5%
bovine serum albumin in phosphate-buffered saline (PBS) and then
incubated with 10 µg/ml rabbit anti-human angiogenin polyclonal
antibody R113 at 4°C overnight, washed three times with PBS and
incubated with fluorescein isothiocyanate-labeled goat anti-rabbit
immunoglobulin G (1:100) at room temperature for 1 h in the dark.
The cells were washed, dyed with Hoechst 33342, mounted with 50%
glycerol and observed under a confocal laser scanning microscope
(Nikon, Tokyo, Japan).
Cell viability assay
The HUVEC or PC-3 cells were seeded into 96-well
plates (5,000 cells/well) and treated with 1 µg/ml angiogenin in
the presence of various concentrations of neamine (0, 10, 25, 50
100 and 200 µmol) for 48 h. After 4 h of incubation with MTT, 150
µl DMSO was added to dissolve the crystals. The absorbance was
determined by a Synergy HT plate reader (Biotek Instruments Inc.,
Winooski, VT, USA) at 490 nm. The cell viability was calculated
relative to the control cells. The assay was repeated three
times.
In vivo study on the growth of PC-3
cell tumor xenografts
This study was conducted at the SPF-level
Experimental Animal Center of Huazhong University of Science and
Technology. All animal protocols were in accordance with the
Chinese animal protection laws and guidelines for the use of living
animals for scientific purposes and were approved by the Ethical
Committees of Tongji Medical College, Huazhong University of
Science and Technology. All mice were allowed to acclimate to new
surroundings for 5 days prior to the experiments. A total of
5×106 PC-3 cells resuspended in 200 µl serum-free RPMI
1640 medium and 50% Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA) were injected subcutaneously into the male Balb/c nude mice.
When the average tumor volume reached ~100 mm3, the mice
were randomly divided into three groups (n=6/group) and treated
with an injection of saline into the tail vein, DDP (2 mg/kg, every
other day, 6 times) and neamine (15 mg/kg, every day, 12 times).
The body weights of the mice, and the length and width of the
tumors were measured every 3 days. The tumor volume (V) was
calculated according to the formula: V = length × width2
/ 2, while the body weight change rate was expressed by
Wn / W1 × 100, in which Wn and W1 represented
the body weights measured at the corresponding day and the first
day, respectively‥ After 12 days of continuous administration, the
mice were sacrificed sacrificed by cervical vertebra dislocation,
and the tumors were isolated and weighted.
Immunohistochemistry
Immunohistochemistry was performed as previously
described (25), with the following
modifications, sections were incubated with 10 µg/ml R113 antibody,
CD31 (1:2,000) polyclonal antibody and Ki-67 monoclonal antibody
(1:200). Angiogenin was stained with R113 antibody, neovessels were
stained with CD31 and proliferating cells were stained with Ki-67
monoclonal antibody. The mean positive staining density was
analyzed in 5 randomly selected areas in each section at x400
magnification using image analysis software (Image-Pro plus 7.0;
Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All tests were performed using the SPSS 20.0
statistical software package (IBM SPSS, Armonk, NY, USA). Data are
presented as the mean ± standard deviation. Analysis of variance
and Student's t-test were used to evaluate statistical
significance, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Neamine blocks the translocation of
angiogenin
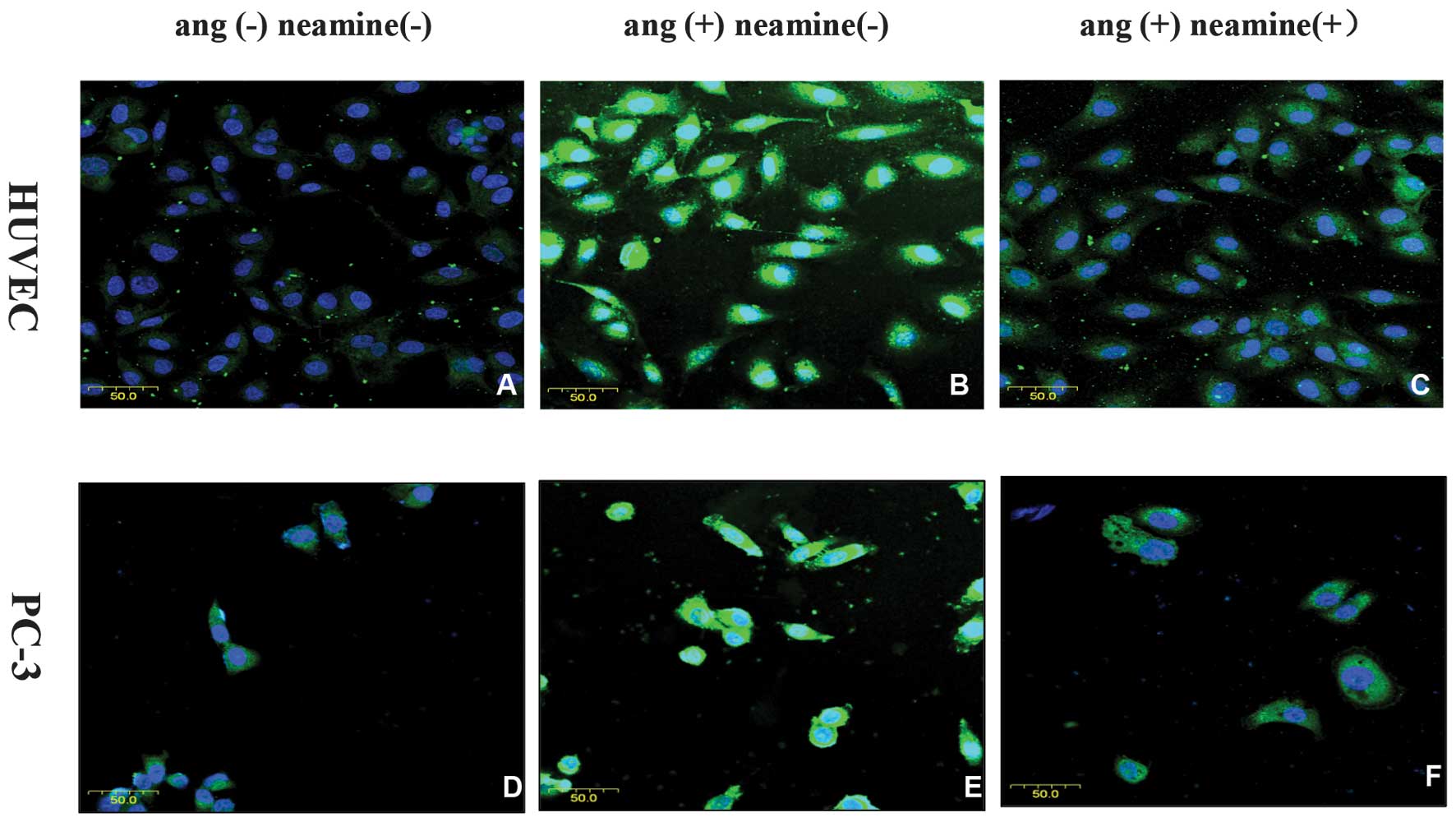
Immunofluorescence was used to observe the effects
of neamine on the translocation of angiogenin. As shown in Fig. 2, it presented stronger staining of
angiogenin in the nucleus of the HUVECs (Fig. 2B) and PC-3 cells (Fig. 2E) when exogenous angiogenin was added
compared with the control group without exogenous angiogenin
stimulation (Fig. 2A and D). In the
presence of 100 µmol neamine (Fig. 2C and
F), the nuclear expression of angiogenin was markedly
decreased.
Neamine reduces cell viability in
HUVEC and PC-3 cells
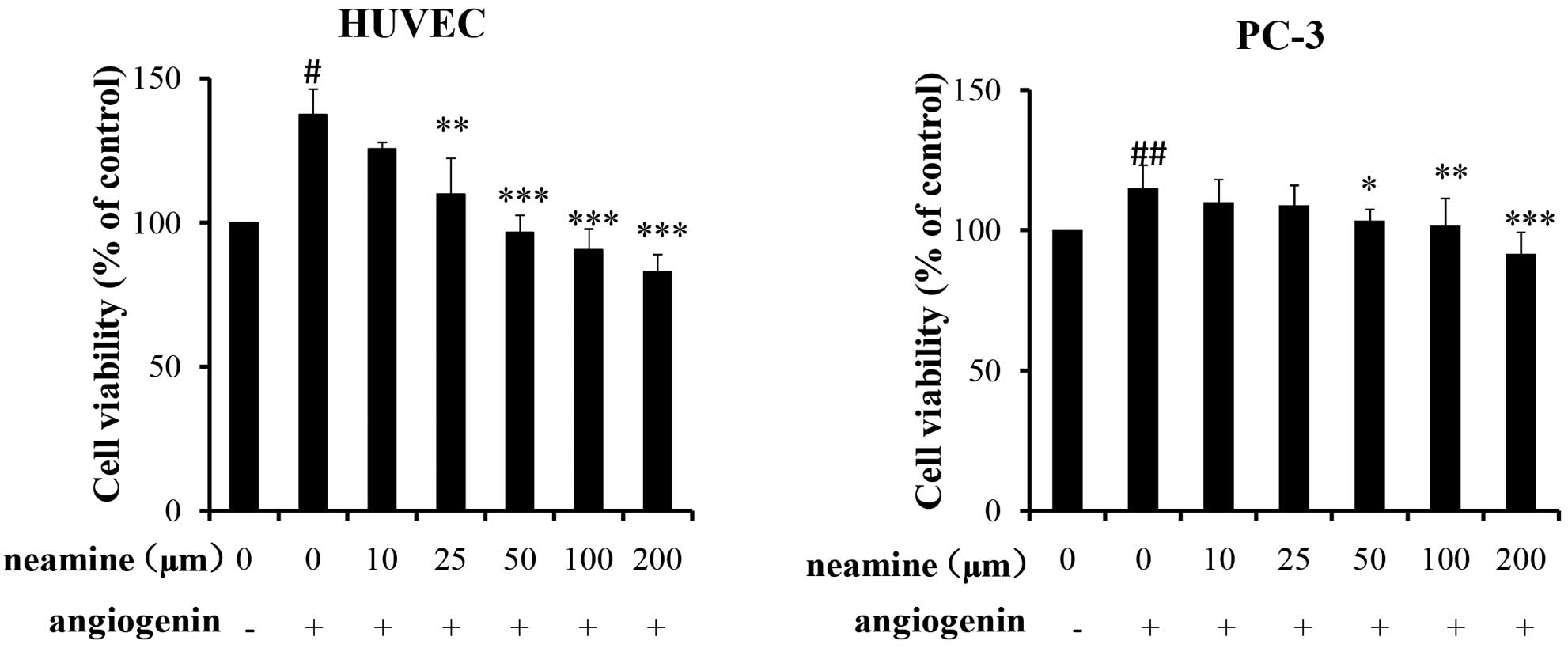
As shown in Fig. 3, it
was demonstrated that exogenous angiogenin promoted HUVEC and PC-3
cell proliferation, and that neamine inhibited angiogenin-mediated
HUVEC and PC-3 cell proliferation and viability in a dose-dependent
manner in the range of 10–200 µmol. With regard to the HUVECs,
exogenous angiogenin promoted 37.4% cell proliferation compared
with the control wells (without angiogenin), and 50 µmol neamine
completely inhibited angiogenin-induced cell proliferation.
However, for the PC-3 cells, in the presence of angiogenin, there
was only a 15% cell proliferation promotion rate, which was lower
than that in the HUVECs. Additionally, 50 µmol neamine inhibited
angiogenin-induced proliferation activity by 75%, and the full
inhibition was achieved at 200 µmol neamine.
Neamine has a comparative antitumor
effect, but lower toxicity (weight loss), compared with DDP in PC-3
xenograft models
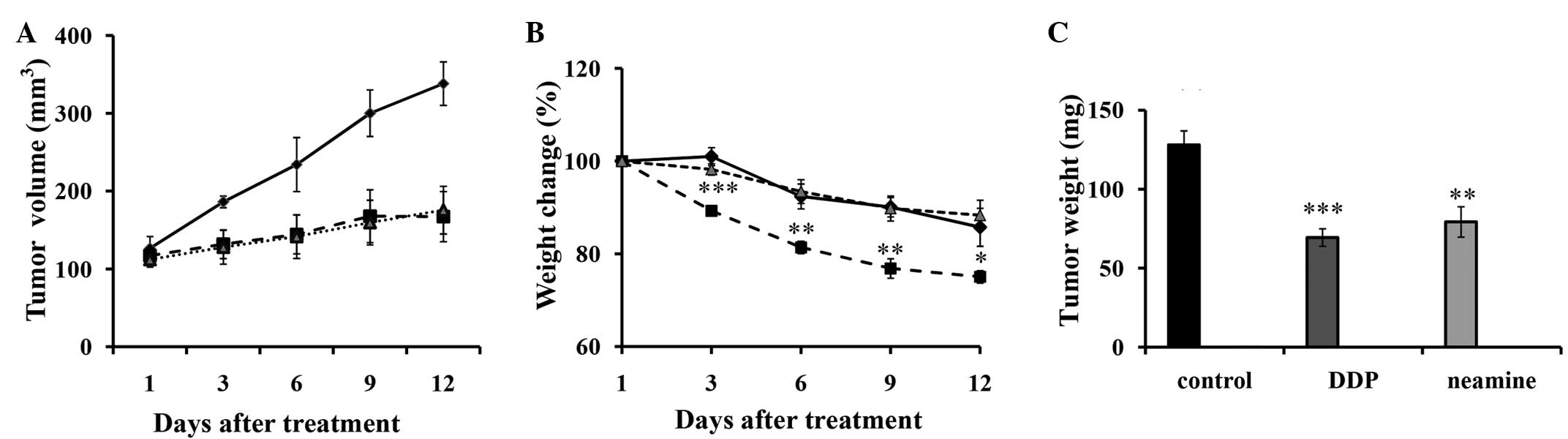
As shown in Fig. 4A,
continuous intravenous administration of DDP and neamine
significantly suppressed the tumor size and volume from 3 days
post-treatment until the end of the experiment. As indicated in
Fig. 4B, an irregular decrease in
body weight was presented in all groups, and there was a
significant difference between DDP and the saline control group
during the whole process, while no marked difference was observed
between the neamine group and the saline control group. The average
weight of the harvested tumors in the saline group was 128±8.86 mg
(Fig. 4C), whereas the average tumor
weights in the DDP and neamine groups were 69.38±5.51 and
79.26±9.59 mg, respectively. There was no marked difference in
tumor growth and weight between the neamine and DDP groups, but
lower toxicity (weight loss) was found in the neamine group
compared with the DDP.
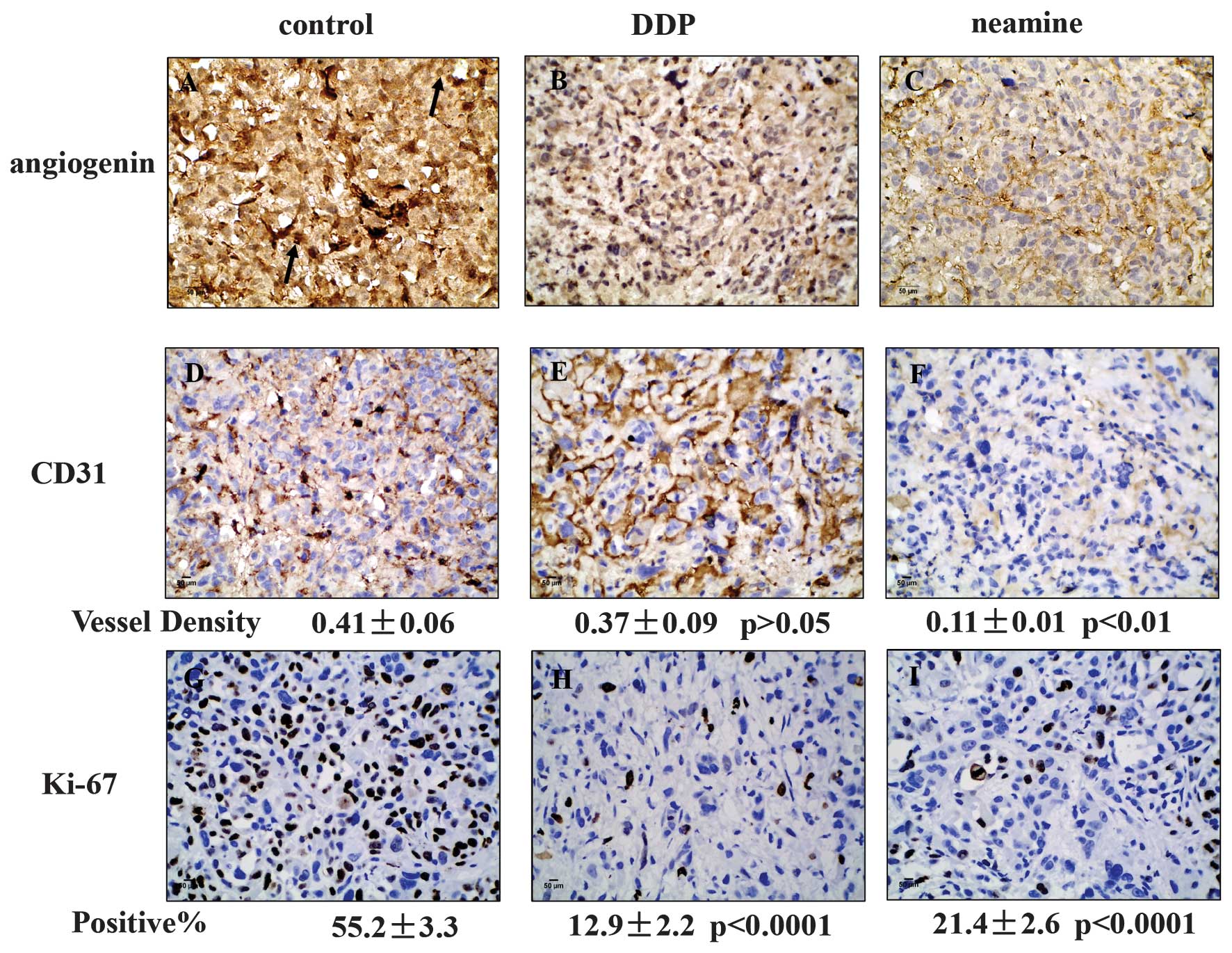
Expression of angiogenin, CD31 and
Ki-67 in tumors in the DDP and neamine groups
As shown in Fig. 5,
strong angiogenin staining was present in the nucleus and cytoplasm
of the tumors from the saline control group; the expression was
focused mainly in the cytoplasm, with little in the nucleus
following neamine-treatment, but strong nuclear expression was
still present in the tumors of the DDP group, showing that neamine
effectively restrained the nuclear translocation of angiogenin. The
mean density of CD31 in the saline control, DDP and neamine groups
was 0.41±0.06, 0.37±0.09 (P=0.512 vs. control) and 0.11±0.01
(P=0.002 vs. control; P=0.003 vs. DDP), respectively, indicating
that neamine decreased tumor angiogenesis, but that DDP exhibited
no significant effect on tumor angiogenesis. The percentage of
Ki-67-positive cells was 55.23±3.36% in the saline control group,
12.97±2.21% (P﹤0.001 vs. control, P=0.009 vs. neamine) in the DDP
group and 21.43±2.59% (P﹤0.001 vs. control) in the neamine group,
which represented a 76.5 and 61.2% decrease in tumor cell
proliferation in the DDP and neamine groups, respectively.
Discussion
Angiogenin, as a key angiogenic factor, can transfer
into the cell nucleus and then bind to the rRNA gene to stimulate
rRNA transcription (11), which is
essential for angiogenesis and cell proliferation. The elevated
expression of angiogenin has been reported in a number of cancer
types, and prostate cancer is no exception. Previous studies
indicated that the expression of angiogenin increased along with
the progression of prostate cancer (15), and that the plasma angiogenin level
was elevated in prostate cancer patients, particularly in hormonal
refractory prostate cancer patients (13). Neomycin and neamine were found to
inhibit the nuclear translocation of angiogenin (26), but neamine had much less toxicity than
neomycin (27,28). PC-3 cells are a type of
hormone-independent prostate cancer cell, and in the present study,
it was demonstrated that neamine blocked the translocation of
angiogenin in the PC-3 cells, with an effect that was comparable to
DDP in PC-3 xenografts, but with much lower toxicity. Therefore,
neamine may hold great potential as a potent agent against prostate
cancer, particularly the hormone-independent type.
In theory, the translocation of angiogenin could
enhance rRNA transcription and further promote cell proliferation.
The cell viability results in the present study indicated that
angiogenin-stimulated cell proliferation of the HUVECs and PC-3
cells rather than basal level cell proliferation was inhibited by
neamine, and also that the HUVECs were more sensitive to neamine
than the prostate cancer PC-3 cell line. Thus, it may be concluded
that neamine may not induce drug tolerance and cytotoxicity based
on its main pertinence for endothelial cells and angiogenin
upregulated cancer cells.
In the present study, it was confirmed that
exogenous angiogenin underwent nuclear translocation in the PC-3
cells and HUVECs, and that neamine effectively blocked this
process. The nuclear expression of angiogenin was significantly
decreased in the neamine-treated tumors compared with the saline
controls. Treatment with neamine decreased CD31 expression to a
greater extent than DDP, but Ki-67 expression to a lesser extent
than DDP. This proved that neamine exhibited a dual effect by
suppressing tumor angiogenesis and cancer cell proliferation, but
that DDP exhibited no marked effect on angiogenesis.
As observed in the present animal experiments,
treatment with neamine or DDP inhibited the progression of
established PC-3 transplanted tumors in Balb/c nude mice. However,
although DDP achieved better results, it exhibited a bigger adverse
impact on body weight and more side-effects compared with neamine
in the whole process.
Angiogenesis is a major step for the growth, spread
and metastasis of solid tumors, therefore anti-angiogenesis agents
may have great potential in targeting malignancy. A variety of
anti-angiogenesis drugs have been used in clinical or pre-clinical
research, with the greatest success being Avastin, which has been
approved to be used in the treatment of metastatic colorectal
cancer (29). The present results
showing the significant effect of neamine against prostate cancer
also highlight the potential of neamine as an anti-angiogenesis
drug. However, the combination of an anti-angiogenesis drug and a
chemotherapeutic drug may be more effective in halting the
progression of cancer. Just as indicated in Fig. 5, neamine exhibited a better effect on
angiogenesis, but a weaker effect on cell proliferation compared
with DDP, while DDP exhibited a better effect on cell
proliferation, but a weaker effect on angiogenesis compared with
neamine. Thus, future clinical experiments will investigate the
synergistic effect of neamine and DDP against prostate cancer.
Acknowledgements
This study was supported by the National Major
Special Project Foundation of China Ministry of Science and
Technology (no. 2012ZX09103101047), the National Natural Science
Foundation of China (no. 81373873) and the Central College Basic
Scientific Research Business Special Fund (no. 2014QN129).
References
|
1
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Santos AF, Huang H and Tindall DJ: The
androgen receptor: A potential target for therapy of prostate
cancer. Steroids. 69:79–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Izawa JI and Dinney CP: The role of
angiogenesis in prostate and other urologic cancers: A review.
CMAJ. 164:662–670. 2001.PubMed/NCBI
|
|
5
|
Ferrer FA, Miller LJ, Andrawis RI,
Kurtzman SH, Albertsen PC, Laudone VP and Kreutzer DL: Vascular
endothelial growth factor (VEGF) expression in human prostate
cancer: In situ and in vitro expression of VEGF by human prostate
cancer cells. J Urol. 157:2329–2333. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wikström P, Bergh A and Damber JE:
Transforming growth factor-beta1 and prostate cancer. Scand J Urol
Nephrol. 34:85–94. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Acevedo VD, Gangula RD, Freeman KW, et al:
Inducible FGFR-1 activation leads to irreversible prostate
adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer
Cell. 12:559–571. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uotila P, Valve E, Martikainen P,
Nevalainen M, Nurmi M and Härkönen P: Increased expression of
cyclooxygenase-2 and nitric oxide synthase-2 in human prostate
cancer. Urol Res. 29:23–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fett JW, Strydom DJ, Lobb RR, et al:
Isolation and characterization of angiogenin, an angiogenic protein
from human carcinoma cells. Biochemistry. 24:5480–5486. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moroianu J and Riordan JF: Nuclear
translocation of angiogenin in proliferating endothelial cells is
essential to its angiogenic activity. Proc Natl Acad Sci USA.
91:1677–1681. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kishimoto K, Liu S, Tsuji T, Olson KA and
Hu GF: Endogenous angiogenin in endothelial cells is a general
requirement for cell proliferation and angiogenesis. Oncogene.
24:445–456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bottero V, Sadagopan S, Johnson KE, Dutta
S, Veettil MV and Chandran B: Kaposi's sarcoma-associated
herpesvirus-positive primary effusion lymphoma tumor formation in
NOD/SCID mice is inhibited by neomycin and neamine blocking
angiogenin's nuclear translocation. J Virol. 87:11806–11820. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Majumder PK, Yeh JJ, George DJ, et al:
Prostate intraepithelial neoplasia induced by prostate restricted
Akt activation: The MPAKT model. Proc Natl Acad Sci USA.
100:7841–7846. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pina F, Botelho F, Lopes T, et al: Can
serum angiogenin be used to improve the diagnostic performance in
prostate cancer screening. Eur J Cancer Prev. 23:166–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katona TM, Neubauer BL, Iversen PW, Zhang
S, Baldridge LA and Cheng L: Elevated expression of angiogenin in
prostate cancer and its precursors. Clin Cancer Res. 11:8358–8363.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshioka N, Wang L, Kishimoto K, Tsuji T
and Hu GF: A therapeutic target for prostate cancer based on
angiogenin-stimulated angiogenesis and cancer cell proliferation.
Proc Natl Acad Sci USA. 103:14519–14524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olson KA, Fett JW, French TC, Key ME and
Vallee BL: Angiogenin antagonists prevent tumor growth in vivo.
Proc Natl Acad Sci USA. 92:442–446. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Olson KA, French TC, Vallee BL and Fett
JW: A monoclonal antibody to human angiogenin suppresses tumor
growth in athymic mice. Cancer Res. 54:4576–4579. 1994.PubMed/NCBI
|
|
19
|
Hu GF: Neomycin inhibits
angiogenin-induced angiogenesis. Proc Natl Acad Sci USA.
95:9791–9795. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao J, Wang YC, Yang LY, Yu DH, Pan PT
and Wang L: Neamine inhibits cell proliferation, migration, and
invasion in H7402 human hepatoma cells. Saudi Med J. 31:1309–1314.
2010.PubMed/NCBI
|
|
21
|
Kishimoto K, Yoshida S, Ibaragi S,
Yoshioka N, Hu GF and Sasaki A: Neamine inhibits oral cancer
progression by suppressing angiogenin-mediated angiogenesis and
cancer cell proliferation. Anticancer Res. 34:2113–2121.
2014.PubMed/NCBI
|
|
22
|
Yuan Y, Wang F, Liu XH, Gong DJ, Cheng HZ
and Huang SD: Angiogenin is involved in lung adenocarcinoma cell
proliferation and angiogenesis. Lung Cancer. 66:28–36. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirukawa S, Olson KA, Tsuji T and Hu GF:
Neamine inhibits xenografic human tumor growth and angiogenesis in
athymic mice. Clin Cancer Res. 11:8745–8752. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Majumdar MK and Majumdar SK: Isolation and
characterization of three phosphoamido-neomycins and their
conversion into neomycin by Streptomyces fradiae. Biochem J.
120:271–278. 1970.PubMed/NCBI
|
|
25
|
Deng SR, Li J, Zhang ZQ, et al: DS147
improves pregnancy in mice with embryo implantation dysfunction
induced by controlled ovarian stimulation. J Huazhong Univ Sci
Technolog Med Sci. 33:573–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirukawa S, Olson KA, Tsuji T and Hu GF:
Neamine inhibits xenografic human tumor growth and angiogenesis in
athymic mice. Clin Cancer Res. 11:8745–8752. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Williams PD, Bennett DB, Gleason CR and
Hottendorf GH: Correlation between renal membrane binding and
nephrotoxicity of aminoglycosides. Antimicrob Agents Chemother.
31:570–574. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Au S, Weiner N and Schacht J: Membrane
perturbation by aminoglycosides as a simple screen of their
toxicity. Antimicrob Agents Chemother. 30:395–397. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang B and Wang JJ: Industry News: Avastin
approved for metastatic colorectal cancer. Discov Med. 4:79–80.
2004.PubMed/NCBI
|