Introduction
Cancer is the leading cause of mortality in
economically developed countries and is the second leading cause of
mortality in developing countries. The burden of cancer is
increasing in economically developing countries as a result of
population aging and growth, in addition to an increasing adoption
of cancer-associated lifestyle choices, including smoking, physical
inactivity and a Western diet (1).
Based on the GLOBOCAN 2008 estimates (2), ~12.7 million cancer cases and 7.6
million cancer-associated mortalities are estimated to have
occurred in that year. Of these, 56% of the cases and 64% of the
mortalities occurred in the economically developing world (2). Colorectal cancer is the third most
commonly diagnosed cancer in males and the second most commonly
diagnosed cancer in females, with >1.2 million novel cancer
cases and 608,700 mortalities estimated to occur each year. Despite
considerable advances in modern therapeutic strategies, the overall
survival time of patients undergoing complete resection of
carcinomas is short (3). Therefore,
clarification of the molecular mechanisms of colorectal carcinoma
and the identification of a good biomarker to indicate the
carcinogenesis and subsequent progression of the carcinoma is of
considerable significance for the prevention, treatment and
evaluation of prognosis of this disease. A potential candidate
biomarker for colorectal carcinoma is mammalian target of rapamycin
(mTOR), a serine/threonine protein kinase that plays a key role in
regulating important cellular functions, including cell
proliferation, growth, survival and mobility, and angiogenesis
(4–11). In several non-colorectal tumors, the
activation of the mTOR pathway and overexpression of the mTOR
protein are associated with an increasingly aggressive clinical
course, and have been reported to be useful for targeted therapy
(12–14). In the present study, the role of the
mTOR/70 kDa ribosomal protein S6 kinase (P70S6K) signaling pathway
in the stepwise development of colorectal carcinoma was
investigated. The association between the expression of mTOR and
P70S6K and the clinical pathological factors of the carcinoma was
also investigated in the present study, as well as the importance
of the role of this pathway in colorectal carcinoma.
Materials and methods
A total of 111 patients with colorectal carcinoma
that underwent curative surgery without prior treatment at the
Binzhou Central Hospital (Binzhou, China) between June 2005 and
July 2013 were enrolled in the present study. These patients
consisted of 58 men and 53 women with ages ranging between 30 and
69 years. The carcinoma lesions were located in the colon in 79
patients and rectum in 32 patients. Of these patients, histological
grading resulted in 24 being classified as stage I, 28 being
classified as II, 45 being classified as stage III and 14 being
classified as stage IV, according to the tumor-node-metastasis
(TNM) staging system revised by the Union for International Cancer
Control (15). In addition, 40
samples from adenomatous polyps and 40 samples from normal colonic
mucosa were also obtained from the Binzhou Central Hospital. None
of the patients underwent chemotherapy or radiotherapy prior to
surgery and all patients provided consent for the use of tumor
tissue for clinical research. This study was approved by the Ethics
Committee of Binzhou Medical College (Binzhou, China).
Immunohistochemistry
The resected specimens were fixed in 10% formalin,
cut into 4-mm thick slices and mounted onto adhesive-coated slides.
The slides were deparaffinized in xylene twice for 10 min and
rehydrated through descending concentrations of ethanol. Antigen
retrieval was performed in 0.01 mol/l citrate buffer (pH 6.0) for 2
min and 30 sec at 100°C, using a microwave oven. Endogenous
peroxidase activity was blocked with 0.3% hydrogen peroxidase for
10 min. Subsequent to washing with phosphate-buffered saline (PBS),
the sections were incubated with blocking serum for 1 h. The p-mTOR
and p-P70S6K proteins were detected using primary polyclonal rabbit
antibodies against p-mTOR and p-P70S6K, respectively. Specimens
were incubated with the primary antibody overnight at 4°C. Using an
Olympus microscope (Olympus, Tokyo, Japan), the protein expression
was evaluated by three pathologists that were blinded to the
clinical data of the patients, and the values were then
averaged.
Score evaluation
The intensity of staining was scored as follows: 0,
no expression, no brown staining; 1, weak expression, light brown
staining; 2, moderate expression, intermediate brown staining; and
3, strong expression, dark brown staining. The extent of staining
was scored based on the proportion of cells stained in the
respective lesions, as follows: 0, <5% of cells; 1, 5–25% of
cells; 2, 26–50% of cells; 3, 51–75%; and 4, >75% of cells. The
final score was determined by multiplying the intensity of staining
score by the extent of staining score, yielding a range between 0
and 12. Tissues that scored between 9 and 12 were defined as
exhibiting a preserved or strong staining pattern (++), 5–8 was
defined as a weak staining pattern (+) and 0–4 was defined as
markedly reduced or no expression (–). In particular,
under-expression was defined as no staining, or positive staining
in the tumor tissue that was decreased compared with the matched
normal tissue. Normal expression was defined as positive staining
that was similar to the matched normal tissue, and over-expression
was defined as positive staining that was increased compared with
the matched normal tissue.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The one-step RT-PCR system (Thermo Fisher
Scientific, Pittsburgh, PA, USA) was used to isolate the RNA from
tissues. The primer sequences used were as follows: mTOR sense,
5′-CTGGGACTCAAATGTGTGCAGTTC-3′ and antisense,
5′-GAACAATAGGGTGAATGATCCGGG-3′; and P70S6K sense,
5′-TACTTCGGGTACTTGGTAA-3′ and antisense, 5′-GATGAAGGGATGGTTTACT-3′.
A 302-bp β-actin fragment was amplified as an internal control. The
primers used for β-actin were as follows: forward,
5′-TCCTCCCTGGAGAAGAGCTA-3′ and reverse,
5′-TCAGGAGGAGCAATGATGTTG-3′. Subsequent to denaturation by heating
at 95°C for 1 min the samples were exposed to 30 cycles (β-actin,
25 cycles) at 95°C for 30 sec, 60°C for 30 sec and 68°C for 90 sec,
with a final extension at 68°C for 10 min.
Western blot analysis
Whole-cell lysates were prepared from human
colorectal cancer or normal colorectal tissue specimens. Standard
western blotting was performed using primary polyclonal rabbit
anti-mouse p-mTOR (1:1,000; cat. no. 5536S; Cell Signaling
Technology, Inc., Danvers, MA, USA) and p-P70S6K (1:1,000; cat. no.
9234S; Cell Signaling Technology, Inc.) antibodies. Polyclonal
rabbit anti-human β-actin antibody (1:1,000; cat. no. sc-130656;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was used as the
loading control. Horseradish peroxidase-conjugated goat anti-rabbit
IgG (1:2,000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) was
used as the secondary antibody.
Statistical analysis
SPSS software, version 17.0, (SPSS, Inc., Chicago,
IL, USA) was employed to analyze all data. Differences between
groups were compared using the χ2 test or Pearson's
product-moment correlation. P<0.05 was considered to indicate a
statistically significant difference.
Results
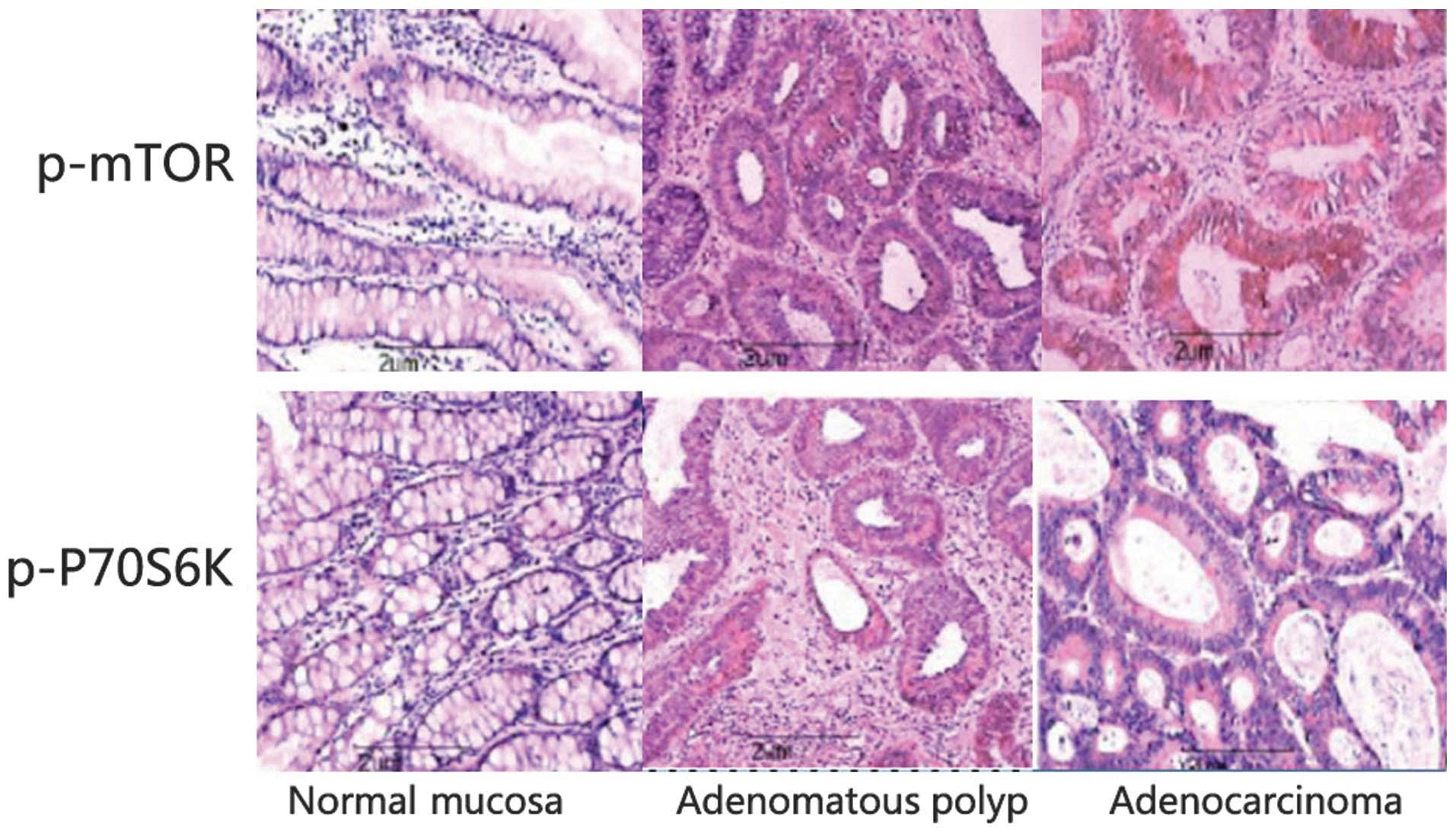
Association between p-mTOR and
p-P70S6K expression and colorectal carcinoma
As shown in Table I,
four out of 40 normal colonic mucosa tissues (10.0%) demonstrated
weak p-mTOR expression. By contrast, p-mTOR was weakly to
moderately expressed in 11 out of 40 tissues samples from
adenomatous polyps (27.5%) and over-expressed in 67 out of 111
colorectal adenocarcinoma tissue samples (60.4%) (P<0.001). In
normal colonic mucosa samples, only two out of 40 tissue samples
(5.0%) demonstrated weak p-P70S6K expression. By contrast, p-P70S6K
was weakly to moderately expressed in eight out of 40 adenomatous
polyp tissue samples (20.0%) and over-expressed in 73 out of 111
colorectal adenocarcinoma tissues (65.8%) (Table II). Increased expression of p-mTOR
and p-P70S6K was observed in the membrane and cytoplasm of tumor
cells. The χ2 test indicated that the expression of
p-mTOR and p-P70S6K was significantly associated with colorectal
adenocarcinoma tissues (P<0.001; Tables I and II; Fig.
1).
 | Table I.Expression of p-mTOR in
adenocarcinomas, adenomatous polyps and normal mucosa. |
Table I.
Expression of p-mTOR in
adenocarcinomas, adenomatous polyps and normal mucosa.
|
|
| Expression of
p-mTORa |
|
|---|
|
|
|
|
|
|---|
| Tissue type | Total, n | Yes, n (%) | No, n (%) | χ2 |
|---|
| Adenocarcinoma | 111 | 67 (60.4) | 44 (39.6) |
|
| Adenomatous
polyps | 40 | 11(27.5) | 29 (72.5) |
|
| Normal mucosa | 40 | 4 (10.0) | 36 (90.0) | 85.8 |
 | Table II.Expression of p-P70S6K in
adenocarcinomas, adenomatous polyps and normal mucosa. |
Table II.
Expression of p-P70S6K in
adenocarcinomas, adenomatous polyps and normal mucosa.
|
|
| Expression of
p-P70S6Ka |
|
|---|
|
|
|
|
|
|---|
| Tissue type | Total, n | Yes, n (%) | No, n (%) | χ2 |
|---|
| Adenocarcinoma | 111 | 73 (65.8) | 38 (34.2) |
|
| Adenomatous
polyps | 40 | 8 (20.0) | 32 (80.0) |
|
| Normal mucosa | 40 | 2 (5.0) | 38 (95.0) | 102.4 |
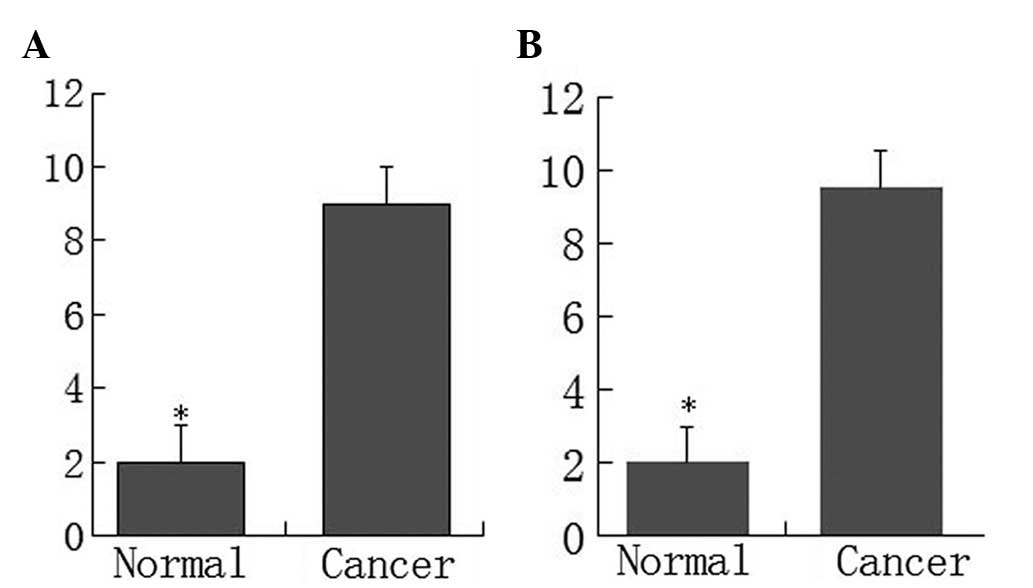
Additional analysis indicated that the p-mTOR and
p-P70S6K proteins were overexpressed in the primary tumor tissue
compared with the normal colorectal tissue. The scores for p-mTOR
and p-P70S6K expression in adenocarcinomas were significantly
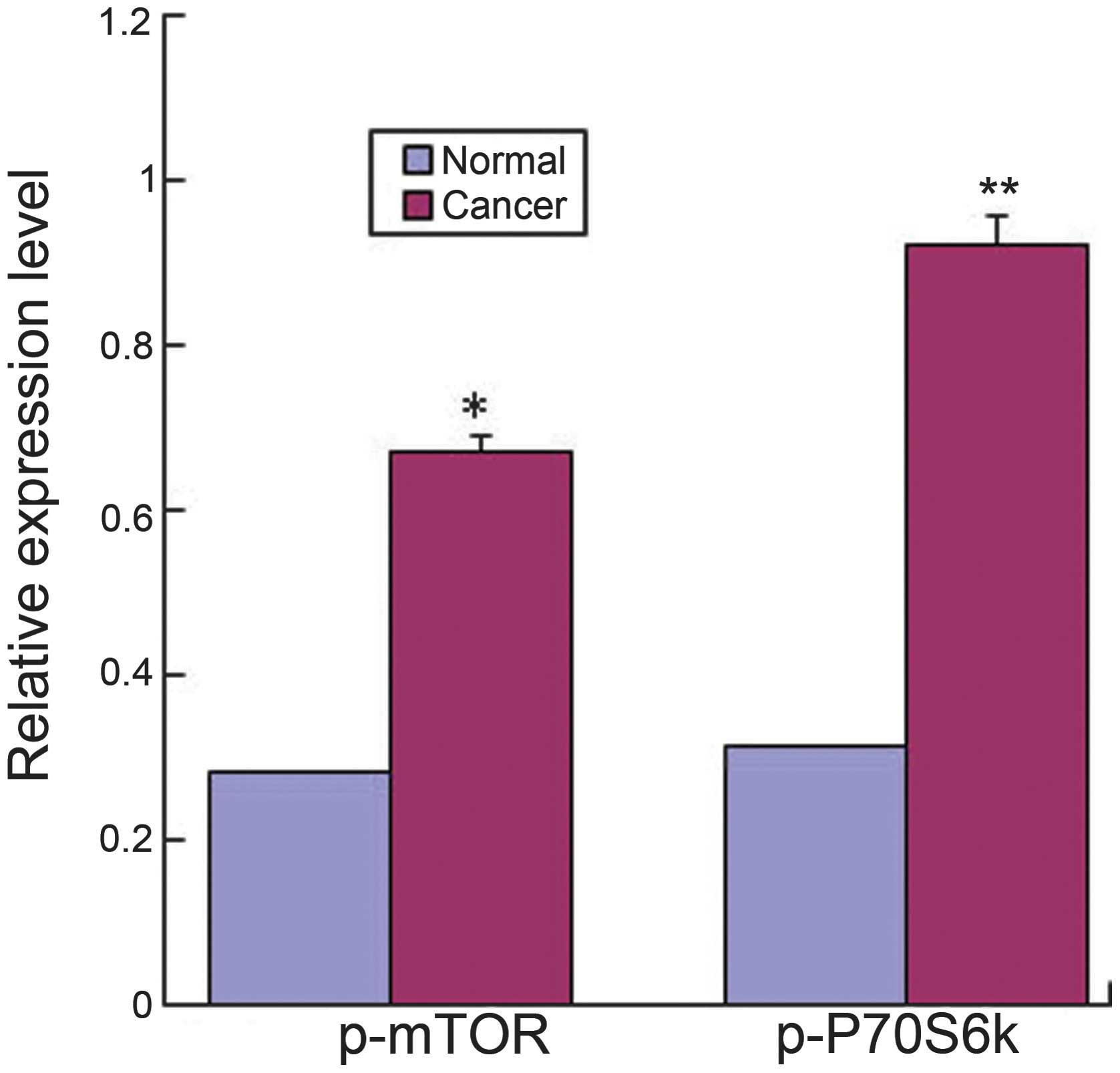
higher compared with the normal mucosa (P<0.05) (Fig. 2). The results from RT-PCR also
indicated that the mRNAs for p-mTOR and p-P70S6K in colorectal
adenocarcinomas were significantly higher compared with the normal
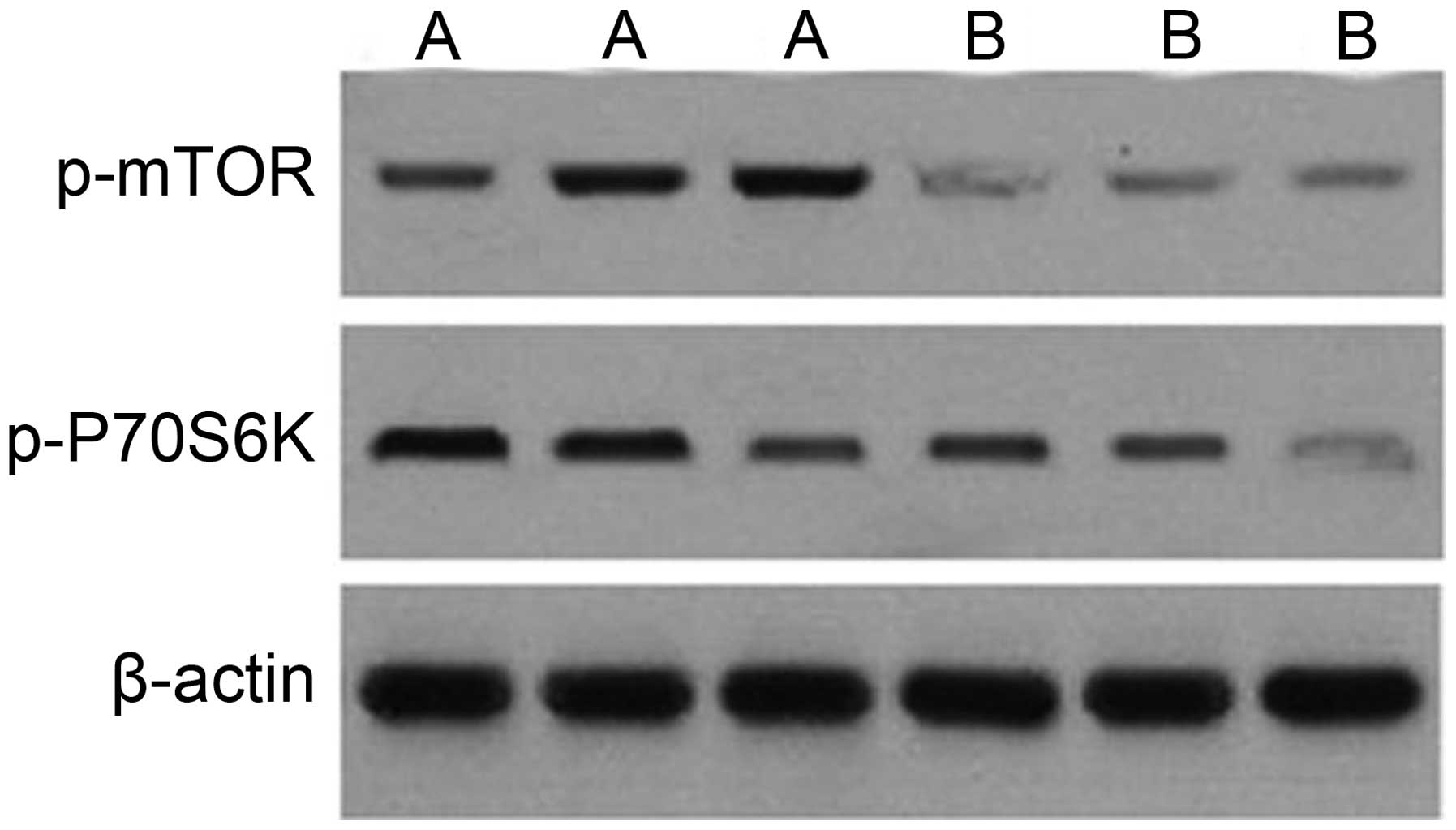
tissues (Fig. 3). In addition,
western blot analysis indicated that the expression of the p-mTOR
and p-P70S6K proteins was significantly higher in adenocarcinoma
tissues compared with normal tissues (Fig. 4).
Association between the expression of
p-mTOR and p-P70S6K and clinicopathological characteristics of
colorectal adenocarcinoma
By evaluating the clinical significance of p-mTOR
and p-P70S6K overexpression, it was found that overexpression of
the p-mTOR protein was significantly associated with the TNM stage
(P<0.001), occurrence of lymph node metastasis (P=0.021),
occurrence of distant metastasis (P=0.029) and degree of
differentiation (P=0.006) in colorectal adenocarcinoma tissues. In
addition, p-P70S6K overexpression was also associated with the TNM
stage (P<0.001), incidence of lymph node metastasis
(P<0.001), occurrence of distant metastasis (P=0.034) and degree
of differentiation (P=0.002) in colorectal adenocarcinoma (Table III).
 | Table III.Differences in the overexpression of
p-mTOR and p-70S6k in association with clinicopathology parameters
of colorectal cancers patients. |
Table III.
Differences in the overexpression of
p-mTOR and p-70S6k in association with clinicopathology parameters
of colorectal cancers patients.
|
|
| p-mTOR
overexpression | p-P70S6K
overexpression |
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | Total, n | n | P-value | n | P-value |
|---|
| Gender |
|
|
|
|
|
|
Male | 58 | 40 |
| 39 |
|
|
Female | 53 | 29 | 0.098 | 29 | 0.211 |
| Age, years |
|
|
|
|
|
|
<60 | 42 | 24 |
| 26 |
|
|
≥60 | 69 | 43 | 0.404 | 42 | 0.979 |
| Location |
|
|
|
|
|
|
Colon | 79 | 47 |
| 48 |
|
|
Rectum | 32 | 21 | 0.459 | 20 | 0.771 |
| TNM stage |
|
|
|
|
|
|
I/II | 52 | 26 |
| 21 |
|
|
III/IV | 59 | 43 | <0.001 | 47 | <0.001 |
| Lymph node
metastasis |
|
|
|
|
|
| No | 59 | 19 |
| 26 |
|
|
Yes | 52 | 36 | 0.021 | 40 | <0.001 |
| Distant
metastasis |
|
|
|
|
|
| No | 99 | 56 |
| 49 |
|
|
Yes | 12 | 10 | 0.029 | 11 | 0.034 |
|
Differentiation |
|
|
|
|
|
|
Well | 11 | 4 |
| 5 |
|
|
Moderate | 58 | 27 |
| 25 |
|
|
Poor | 42 | 35 | 0.006 | 36 | 0.002 |
Discussion
mTOR is a serine/threonine kinase that is involved
in multiple intracellular signaling pathways, promoting tumor
growth. In the presence of sufficient nutrients, mTOR is
phosphorylated through the phosphoinositide 3-kinase (PI3K)/Akt
signaling pathway, resulting in the transmittance of a positive
signal to P70S6K, and therefore participates in the inactivation of
the eukaryotic translation initiation factor 4E inhibitor. The
PI3K/Akt/mTOR signaling pathway, in particular, is frequently
altered in non-colorectal cancers, including gastric cancer
(16), biliary tract adenocarcinoma
(17), pancreatic ductal
adenocarcinoma (18), lung carcinoma
(19), urinary bladder carcinoma
(20), prostate cancer (21), cervical carcinoma (22), breast cancer (23) and renal cell carcinoma (24). Activated p-mTOR has been demonstrated
to be associated with tumors in numerous cancer tissues. It has
been found that the expression of the p-mTOR protein is elevated in
extrahepatic cholangiocarcinoma (25)
and the expression of the p-P70S6K protein is increased in
high-grade squamous intraepithelial lesions and cervical squamous
cell carcinoma compared with the normal cervical epithelium
(22). In the present study, the rate
of the positive expression of p-P70S6K and p-mTOR was detected to
be significantly higher in colorectal cancer tissues compared with
normal tissues. Furthermore, the p-P70S6K and p-mTOR expression
levels were found to be higher in colorectal adenocarcinoma tissue
samples compared with normal colonic mucosa, which indicates that
activated mTOR is highly associated with colorectal cancer and
plays a key role in tumor carcinogenesis.
It has been suggested that the expression of
p-P70S6K and p-mTOR is associated with various clinicopathological
variables in certain non-colorectal tumors. For example, Yu et
al reported that overexpression of the mTOR protein was
significantly associated with tumor differentiation, T1 and 2
tumors and stage I-III disease, whereas p-mTOR overexpression was
significantly associated with the occurrence of lymph node
metastasis and all stages of disease (26). Wang et al reported that the
expression of mTOR and p-mTOR may play an important role in
colorectal carcinogenesis, with an association between the
expression of mTOR and the degree of differentiation, invasiveness
and metastatic ability of the lesions (27). No et al found that the
expression of mTOR was highly associated with old age and
menopausal status, but not with other clinicopathological
characteristics (28). Dobashi et
al identified that the p-mTOR expression in lung adenocarcinoma
specimens was associated with the grade of histological
differentiation, whereas the expression of p-mTOR was associated
with lymph node metastasis in squamous cell carcinoma specimens
(19). Although Herberger et
al (17)found that p-mTOR was
expressed in 56 out of 88 biliary tract carcinoma samples, no
association was identified between the expression and any
clinicopathological variables. However, the expression did predict
the survival time of the patients (17).
Activation of P70S6K is achieved through the
phosphorylation of multiple serine/threonine residues by
stimulation with growth factors, including epidermal growth factor,
thrombin and lysophosphatidic acid (29,30). Li
et al reported that the expression of p-P70S6K was found to
be inversely associated with the tumor size, depth of invasion,
lymph node metastasis and Union for International Cancer Control
staging when the aggressive behaviors of carcinoma were compared
with nuclear p-P70S6K expression (31). Zhang et al (32) identified that the expression level of
p-mTOR and p-ribosomal protein S6 kinase β 1 (RPS6KB1) was
significantly higher in NSCLC tumor specimens compared with
adjacent non-cancerous normal lung tissues. In this study, a high
expression level of p-mTOR or p-RPS6KB1 in NSCLC was associated
with a shorter overall survival time, and multivariate analysis
indicated that a high level of p-mTOR expression was an independent
prognostic factor in patients with NSCLC (32). In the present study, it was found that
overexpression of the p-P70S6K and p-mTOR proteins was
significantly associated with the degree of differentiation,
occurrence of distant metastasis, TNM stage and occurrence of lymph
node metastasis. These findings suggest that the mTOR signal
pathway performs an important role in colorectal tumorigenesis. To
the best of our knowledge, the present study is the first to
investigate the association between p-mTOR and p-P70S6K
overexpression in colorectal cancer tissues and the
clinicopathological characteristics of colorectal cancer patients.
The present results also indicated that the mTOR signaling pathway
was frequently activated and that overexpression of mTOR may be an
important step in the carcinogenesis and progression of human
colorectal cancer.
At present, it is hypothesized that targeting mTOR
with small interfering (si)RNA may inhibit the proliferation of
cancer cell proliferation. Ji et al reported that targeting
the expression of p-mTOR with specific siRNA reduced the growth and
overall survival rate of Hela cervical cancer cells in vitro
(33). The present study also
indicates that downregulating the mTOR signaling pathway may be a
promising novel molecular target for designing novel therapeutic
strategies to control colorectal cancer. This hypothesis is
supported by a previous study that reported targeting mTOR complex
2 inhibits colon cancer cell proliferation in vitro and
tumor formation in vivo (34).
In summary, the present study indicated that p-P70S6K and p-mTOR
are overexpressed in human colorectal carcinoma, and also indicated
that the overexpression of p-P70S6K and p-mTOR is associated with
certain clinical characteristics. This suggests that p-P70S6K and
p-mTOR may play a important role in colorectal cancer and may be
employed to indicate the biological behaviors of colorectal
carcinoma in clinicopathological practice.
Acknowledgements
The present study was supported by a grant from the
National Science and Technology Special Foundation for Major
Infectious Diseases Prevention and Control (grant no. 2008Ex
10002019).
References
|
1
|
World Health Organization, . The Global
Burden of Disease: 2004 Update. World Health Organization; Geneva:
2008
|
|
2
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Otake S, Takeda H, Fujishima S, et al:
Decreased levels of plasma adiponectin associated with increased
risk of colorectal cancer. World J Gastroenterol. 16:1252–1257.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buck E, Eyzaguirre A, Brown E, et al:
Rapamycin synergizes with the epidermal growth factor receptor
inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and
breast tumors. Mol Cancer Ther. 5:2676–2684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pene F, Claessens YE, Muller O, et al:
Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase
pathways in the proliferation and apoptosis in multiple myeloma.
Oncogene. 21:6587–6597. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foster DA: Phosphatidic acid signaling to
mTOR: Signals for the survival of human cancer cells. Biochim
Biophys Acta. 1791:949–955. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang BH and Liu LZ: Role of mTOR in
anticancer drug resistance: Perspectives for improved drug
treatment. Drug Resist Updat. 11:63–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X and Proud CG: The mTOR pathway in
the control of protein synthesis. Physiology (Bethesda).
21:362–369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim EK, Kim HA, Koh JS, et al:
Phosphorylated S6K1 is a possible marker for endocrine therapy
resistance in hormone receptor-positive breast cancer. Breast
Cancer Res Treat. 126:93–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han D, Li SJ, Zhu YT, et al:
LKB1/AMPK/mTOR signaling pathway in non-small-cell lung cancer.
Asian Pac J Cancer Prev. 14:4033–4039. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raymond E, Alexandre J, Faivre S, et al:
Safety and pharmacokinetics of escalated doses of weekly
intravenous infusion of CCI-779, a novel mTOR inhibitor, in
patients with cancer. J Clin Oncol. 22:2336–2347. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O’Donnell A, Faivre S, Burris HA III, et
al: Phase I pharmacokinetic and pharmacodynamic study of the oral
mammalian target of rapamycin inhibitor everolimus in patients with
advanced solid tumors. J Clin Oncol. 26:1588–1595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rizell M, Andersson M, Cahlin C, et al:
Effects of the mTOR inhibitor sirolimus in patients with
hepatocellular and cholangiocellular cancer. Int J Clin Oncol.
13:66–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benson AB III, Bekaii-Saab T, Chan E, et
al: National Comprehensive Cancer Network: Localized colon cancer,
version 3.2013: featured updates to the NCCN Guidelines. J Natl
Compr Canc Netw. 11:519–528. 2013.PubMed/NCBI
|
|
16
|
Murayama T, Inokuchi M, Takagi Y, Yamada
H, Kojima K, Kumagai J, Kawano T and Sugihara K: Relation between
outcomes and localisation of p-mTOR expression in gastric cancer.
Br J Cancer. 100:782–788. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herberger B, Puhalla H, Lehnert M, Wrba F,
Novak S, Brandstetter A, Gruenberger B, Gruenberger T, Pirker R and
Filipits M: Activated mammalian target of rapamycin is an adverse
prognostic factor in patients with biliary tract adenocarcinoma.
Clin Cancer Res. 13:4795–4799. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pham NA, Schwock J, Iakovlev V, Pond G,
Hedley DW and Tsao MS: Immunohistochemical analysis of changes in
signaling pathway activation downstream of growth factor receptors
in pancreatic duct cell carcinogenesis. BMC Cancer. 8:432008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dobashi Y, Suzuki S, Matsubara H, Kimura
M, Endo S and Ooi A: Critical and diverse involvement of
Akt/mammalian target of rapamycin signaling in human lung
carcinomas. Cancer. 115:107–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kremer CL, Klein RR, Mendelson J, Browne
W, Samadzedeh LK, Vanpatten K, Highstrom L, Pestano GA and Nagle
RB: Expression of mTOR signaling pathway markers in prostate cancer
progression. Prostate. 66:1203–1212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng W, Duan X, Liu J, Xiao J and Brown
RE: Morphoproteomic evidence of constitutively activated and
overexpressed mTOR pathway in cervical squamous carcinoma and high
grade squamous intraepithelial lesions. Int J Clin Exp Pathol.
2:249–260. 2009.PubMed/NCBI
|
|
23
|
Walsh S, Flanagan L, Quinn C, Evoy D,
McDermott EW, Pierce A and Duffy MJ: mTOR in breast cancer:
Differential expression in triple-negative and non-triple-negative
tumors. Breast. 21:178–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pantuck AJ, Seligson DB, Klatte T, Yu H,
Leppert JT, Moore L, O’Toole T, Gibbons J, Belldegrun AS and Figlin
RA: Prognostic relevance of the mTOR pathway in renal cell
carcinoma: Implications for molecular patient selection for
targeted therapy. Cancer. 109:2257–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung JY, Hong SM, Choi BY, Cho H, Yu E
and Hewitt SM: The expression of phospho-AKT, phospho-mTOR, and
PTEN in extrahepatic cholangiocarcinoma. Clin Cancer Res.
15:660–667. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu G, Wang J, Chen Y, Wang X, Pan J, Li G,
Jia Z, Li Q, Yao JC and Xie K: Overexpression of phosphorylated
mammalian target of rapamycin predicts lymph node metastasis and
prognosis of chinese patients with gastric cancer. Clin Cancer Res.
15:1821–1829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang D, Chen J, Guo F, Chen H, Duan Z, Wei
MY, Xu QM, Wang LH and Zhong MZ: Clinical significance of mTOR and
p-mTOR protein expression in human colorectal carcinomas. Asian Pac
J Cancer Prev. 12:2581–2584. 2011.PubMed/NCBI
|
|
28
|
No JH, Jeon YT, Park IA, Kang D, Kim JW,
Park NH, Kang SB and Song YS: Expression of mTOR protein and its
clinical significance in endometrial cancer. Med Sci Monit.
15:BR301–BR305. 2009.PubMed/NCBI
|
|
29
|
Berven LA, Willard FS and Crouch MF: Role
of the p70(S6K) pathway in regulating the actin cytoskeleton and
cell migration. Exp Cell Res. 296:183–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Contessa JN, Hampton J, Lammering G, et
al: Ionizing radiation activates Erb-B receptor dependent Akt and
p70 S6 kinase signaling in carcinoma cells. Oncogene. 21:4032–4041.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao L, Wang YC, Li WS and Du Y: The role
of mTOR and phospho-p70S6K in pathogenesis and progression of
gastric carcinomas: An immunohistochemical study on tissue
microarray. J Exp Clin Cancer Res. 28:1522009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Ni HJ and Cheng DY: Prognostic
value of phosphorylated mTOR/RPS6KB1 in non- small cell lung
cancer. Asian Pac J Cancer Prev. 14:3725–3728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji J and Zheng PS: Activation of mTOR
signaling pathway contributes to survival of cervical cancer cells.
Gynecol Oncol. 117:103–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roulin D, Cerantola Y, Dormond-Meuwly A,
Demartines N and Dormond O: Targeting mTORC2 inhibits colon cancer
cell proliferation in vitro and tumor formation in vivo. Mol
Cancer. 9:572010. View Article : Google Scholar : PubMed/NCBI
|