Introduction
Neuroendocrine tumors (NETs) are a group of
carcinomas that secrete various polypeptides with hormonal activity
(1). These tumors arise from the
amine precursor uptake and decarboxylation system, and have been
identified in the gastrointestinal tract (entrochromaffin cells),
the lung (Kulchitsky's cells, bronchus) and the pancreas (islet
cells) (1–3). NETs are slowly growing tumors with an
indolent course. A significant percentage of patients already have
hepatic metastases at the time of initial diagnosis, and 80–90% of
these tumors are inoperable at the time of presentation (4,5). The
presence of distant metastases is associated with a poor prognosis
for neuroendocrine tumors: patients with carcinoid NETs have a
5-year survival rate of only 22% (6).
Liver metastases from NETs are invariably hypervascular, with a
blood supply primarily from the hepatic artery. This feature,
combined with the fact that the liver has a dual vascular supply,
provides a good rationale for the use of hepatic transcatheter
arterial embolization (TAE) to treat these metastases by inducing
tumor ischemia. For higher metastatic load, TAE or
chemoembolization (TACE) is the preferred approach for the
management of symptomatic and local tumor control, as well as for
improvement of the survival rate (7).
Following a study by Moertel et al (8), which demonstrated a higher disease
regression rate and a longer duration of regression with systemic
chemotherapy following hepatic arterial occlusion than with
occlusion alone, TACE (which used chemotherapy in addition to the
embolic material) has often been favored over TAE. There is no
consensus on the most effective chemotherapeutic agent for use in
this procedure. Various chemotherapeutic agents, including
doxorubicin, streptozocin, 5-fluorouracil, mitomycin C, cisplatin,
and a combination of these agents have been used to perform TACE
for hepatic metastases of NETs (9).
However, there were no significant differences in the response rate
to these agents (9). Complications of
TACE are moderate in the majority of patients, with
post-embolization syndrome being common. The present study reported
the case of a patient with neuroendocrine hepatic metastases who
developed acute thrombocytopenia following TACE, and discussed the
hypothetical etiopathogenetic mechanisms involved.
Case report
A 56-year-old male, who had undergone surgical
resection of a NET of the pancreas 17 months earlier, was admitted
to the Fudan University Shanghai Cancer Center (Shanghai, China)
for the treatment of multiple liver metastases from NET. Written
informed consent was obtained from the patient for participation in
the present study. The liver lesions were first observed 16 months
after surgery. TACE was scheduled 1 month after the initial
diagnosis was confirmed by histopathological examination of a
needle biopsy. TACE was performed according to the following
protocol: a selective 5-F catheter was introduced, and visceral
angiography was carried out to assess the arterial blood supply to
the liver and to confirm patency of the portal vein. Selective
proper hepatic angiography revealed innumerable hypervascular
tumors located throughout the liver. In order to treat lesions in
the whole liver, the right and left hepatic arteries were
separately catheterized using a coaxial technique and
microcatheters (2.8 F; Terumo Corporation, Tokyo, Japan). Hepatic
artery infusion chemotherapy was performed using cisplatin 60 mg
and floxuridine 1,000 mg (both from Zhejiang Hisun Pharmaceutical,
Taizhou, China). Next, chemoembolization was performed using drugs
mixed at a 2:1 ratio of epirubicin (60 mg; Zhejiang Hisun
Pharmaceutical) and non-ionic contrast material (5 ml; Ultravist;
Schering, Berlin, Germany) to iodized oil (8 ml; Lipiodol Ultra
Fluide; Laboratoires Guerbet, Paris, France). No adverse events
were noted during the interventional therapy.
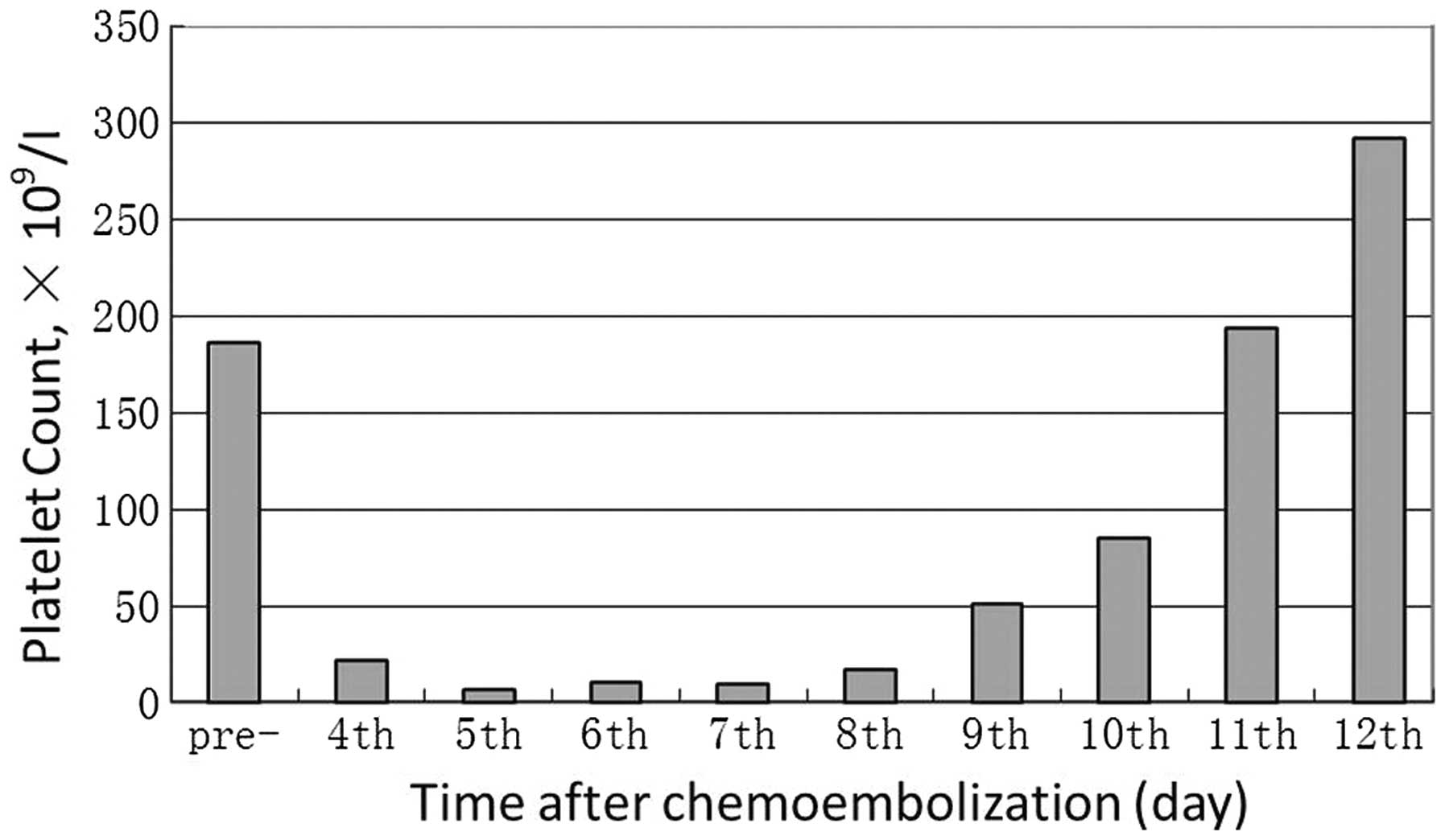
Full blood counts were within normal ranges before
the patient's treatment: hemoglobin, 159 g/l; white blood count
(WBC), 5.2×109/l; and platelet (PLT) count,
186×109/l. Four days after administering
chemoembolization, hematological examination revealed the following
results: hemoglobin, 158 g/l; WBC, 5.1×109/l; and PLT,
22×109/l. Five days after administering
chemoembolization, the PLT count was 7×109/l. Since
grade 4 thrombocytopenia was diagnosed, platelet transfusions were
administered. However, the PLT counts remained at levels below
10×109/l in spite of a total platelet transfusion of 20
units. Anti-nuclear antibody was negative, and no evidence of
disseminated intravascular coagulopathy was observed. The
platelet-associated immunoglobulin-G (IgG) level was 734 ng/dl. A
clinical diagnosis of autoimmune thrombocytopenia was made. Therapy
with 40 mg/day dexamethasone and 20 g/day immunoglobulin for five
days were started on the seventh day after performing
chemoembolization. The number of platelets increased immediately,
with the PLT count being 51×109/l and
85×109/l on the second and third days after starting the
steroid. Subsequent PLT counts remained stable at levels over
150×109/l (Fig. 1).
Discussion
The complications observed with TACE and TAE in the
treatment of neuroendocrine hepatic metastases vary in the
literature. Associated problems include liver abscesses, transient
hepatorenal failure, pleural effusion, sepsis, bowel ischemia
requiring surgery, septicemia requiring antibiotic therapy and
hepatic infarction. Post-embolization syndrome is a further less
severe adverse reaction, and is observed in the majority of
patients. It may also cause a fever that subsides within a few
days, leukocytosis, abdominal pain that may require morphine, and a
transient increase in liver enzymes, particularly transaminases and
lactate dehydrogenase, which tend to return to normal within a few
days to 2–3 weeks. Increased bilirubin levels have also been noted.
Ischemia of the biliary tree has been reported occasionally
following embolization. In addition, elevation of alkaline
phosphatase has been observed. The most appropriate means of
reducing post-embolization syndrome is by keeping the patient well
hydrated and in supportive care (10).
In the case reported in the present study, four days
after administering chemoembolization, hematological examination
revealed an abnormal PLT count (22×109/l).
Thrombocytopenia was initially believed to have been caused by
prolonged myelosuppression induced by chemotherapy. However,
despite extensive repeated transfusions, thrombocytopenia was not
improved, and there was no leukopenia during chemotherapy. It is
unlikely that other factors may have caused thrombocytopenia. The
diagnosis of immune thrombocytopenia was made based on the results
of the elevated platelet-associated IgG level and the response to
dexamethasone and immunoglobulin therapy.
Immune-mediated thrombocytopenia resembling
idiopathic thrombocytopenic purpura accompanied by additional
morbidities is known as secondary autoimmune thrombocytopenia
(11). Common causes of
thrombocytopenia in solid tumors are myelosuppression secondary to
chemotherapy, bone marrow infiltration with malignant cells and
increased peripheral utilization or destruction of platelets due to
disseminated intravascular coagulation. Immune-mediated platelet
destruction as a cause of thrombocytopenic syndromes in patients
with solid tumors was first described by Kim et al (12). However, no antibody or kinetic
verification tests were performed in this study. Schwartz et
al (13) reported on eight
patients with solid tumors having immune-mediated thrombocytopenia,
and revealed evidence of antibodies attached to autologous
platelets by radioimmune and release antibody assays. Further
studies reveal that the frequency of accompanying solid tumors in
patients with autoimmune thrombocytopenia is 5.6–12.9% (12,13).
Autoimmune thrombocytopenia has been observed in a number of solid
tumors, including breast, lung, prostate, testis, kidney, skin and
ovarian cancers. However, elevated platelet-associated IgG levels
may also be induced by drugs (14).
Therefore, in the case presented here, it was concluded that the
elevated platelet-associated IgG may have been associated not only
with tumors but also with drugs.
In conclusion, we have presented a case of immune
thrombocytopenia in a patient with neuroendocrine hepatic
metastases, which developed following TACE, and which was difficult
to differentiate from thrombocytopenia caused by myelosuppression.
We recommend that immune thrombocytopenia is considered in the
differential diagnosis of thrombocytopenia in cancer patients.
Acknowledgements
This study was supported by grants from the Science
Technology Commission of Shanghai Municipality (nos. 0952nm03400,
11nm0504000 and 124119a0100) and the National Natural Science
Foundation of China (nos. 81301218 and 81301262).
References
|
1
|
Loewe C, Schindl M and Cejna M: Permanent
transarterial embolization of neuroendocrine metastases of the
liver using cyanoacrylate and lipiodol: assessment of mid- and
long-term results. AJR Am J Roentgenol. 180:1379–1384. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venook AP: Embolization and
chemoembolization therapy for neuroendocrine tumors. Curr Opin
Oncol. 11:39–44. 1999. View Article : Google Scholar
|
|
3
|
Kress O, Wagner HJ, Wied M, et al:
Transarterial chemoembolization of advanced liver metastases of
neuroendocrine tumors: a retrospective single centre analysis.
Digestion. 68:94–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Proye C: Natural history of liver
metastasis of gastroenteropancreatic neuroendocrine tumors: place
for chemoembolization. World J Surg. 25:685–688. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blonski WC, Reddy KR, Shaked A, et al:
Liver transplantation for metastatic neuroendocrine tumor: a case
report and a review of literature. World J Gastroenterol.
11:7676–7683. 2005.PubMed/NCBI
|
|
6
|
Modlin IM and Sandor A: An analysis of
8305 cases of carcinoid tumors. Cancer. 79:813–829. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogl TJ, Naguib NN, Zangos S, et al: Liver
metastases of neuroendocrine carcinomas: interventional treatment
via transarterial embolization, chemoembolization and thermal
ablation. Eur J Radiol. 72:517–528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moertel CG, Johnson CM, McKusick MA, et
al: The management of patients with advanced carcinoid tumors and
islet cell carcinomas. Ann Intern Med. 120:302–309. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madoff DC, Gupta S, Ahrar K, Murthy R and
Yao JC: Update on the management of neuroendocrine hepatic
metastases. J Vasc Interv Radiol. 17:1235–1249; quiz 1250. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Touzios JG, Kiely JM, Pitt SC, et al:
Neuroendocrine hepatic metastases: does aggressive management
improve survival. Ann Surg. 241:776–783. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bussel JB: Autoimmune thrombocytopenic
purpura. Hematol Oncol Clin N Am. 4:179–191. 1990.
|
|
12
|
Kim HD and Boggs DR: A syndrome resembling
idiopathic thrombocytopenic purpura in 10 patients with diverse
forms of cancer. Am J Med. 67:371–377. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwartz KA, Slichter SJ and Harker LA:
Immune-mediated platelet destruction and thrombocytopenia in
patients with solid tumors. Br J Haematol. 51:17–24. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kelton JG, Meltzer D, Moore J, et al: Drug
induced thrombocytopenia is associated with increased binding of
IgG to platelets both in vivo and in vitro. Blood. 58:524–529.
1981.PubMed/NCBI
|