Introduction
Primitive neuroectodermal tumors (PNETs) are rare,
highly malignant neoplasms consisting of small round cells of
neural crest origin (1). PNETs may be
further subdivided into central and peripheral PNETs (pPNETs),
which arise outside the central and sympathetic nervous system.
pPNETs often occur in the soft tissues of thoracopulmonary region
(Askin tumor), retroperitoneum and abdomen, and, more rarely, in
the bones (1–3). There is no consensus with regard to the
guidelines for the treatment of pPNET at present, due to its rare
occurrence. As PNET exhibits similarities to Ewing sarcoma,
surgical resection followed by adjuvant radiotherapy, as well as
multiagent chemotherapy if possible, is considered necessary to
improve patient survival. There have been few studies published
exclusively on the osseous pPNET (3),
particularly regarding its radiological and clinical features.
Therefore, the present study aimed to document the radiological and
clinical features of osseous pPNET by conducting a retrospective
radiological and clinical review of 15 patients with surgically or
bioptically confirmed osseous pPNET.
Materials and methods
Patients
The database of the Second Affiliated Hospital of
Zhejiang University School of Medicine (Hangzhou, China) was
searched for the records of patients with pPNET who were treated
between January, 2011 and January, 2014. A total of 17 patients
with pPNET were identified; 2 patients with extraosseous pPNET were
excluded and 15 patients with osseous pPNET were finally included
in the study. An Institutional Review Board exemption and a waiver
for the requirement of written informed consent were obtained to
perform this retrospective study.
Imaging
All 15 patients had undergone computed tomography
(CT) and 11 had also undergone magnetic resonance imaging (MRI). CT
imaging was performed using a Somatom Sensation 16 helical scanner
(Siemens Healthcare, Erlangen, Germany). The scanning parameters
were as follows: 5-mm slice thickness reconstructions for viewing,
1-mm slice thickness reconstructions for post-processing, B40s
medium kernel, 20-cm field of view, 120 kV voltage, 200–300 mA
current and 512×512 matrix. MRI was performed using a 3.0T GE Signa
MRI scanner (GE Healthcare, Little Chalfont, UK). The scan
parameters were as follows: T1-weighted fast spin echo (FSE)
sequence [repetition time/echo time (TR/TE), 500/10 msec; slice
thickness, 5.0 mm; field of view, 380–520 mm; and matrix scan,
256×256] and T2-weighted turbo-spin echo sequence (TR/TE, 3,000/75
msec; slice thickness, 3.0 mm; field of view, 300–380 mm; and
matrix scan, 256×256). An intravenous dose of 0.1–0.2 mmol/kg of
contrast agent (Gadolinium-diethylene triamine pentaacetic acid;
Magnevist®; Bayer Schering Pharma AG, Berlin, Germany) was
administered to the patients undergoing contrast-enhanced MRI. A
total of 7 patients underwent chemotherapy (n=3) or
chemoradiotherapy (n=4). For 2 of those cases MRI data were also
available following chemotherapy.
Results
Clinical data
The study group included 9 men and 6 women with a
mean age of 29 years (range, 16–64 years). The tumors were located
in the maxilla (1 case), mandible (1 case), humerus (1 case),
radius (1 case), fibula (1 case), femur (1 case), scapula (1 case),
ilium (1 case), cervical vertebrae (1 case), lumbar vertebrae (1
case), clavicle (2 cases), tibia (2 cases) and ulna (2 cases). One
tumor was located in the tibia and fibula in the same patient
(Table I). A total of 15 patients
presented with varying degrees of pain; of these, 11 patients
presented with local edema and a progressive, growing mass.
 | Table I.Summary of the radiological findings
of 15 patients with osseous peripheral primitive neuroectodermal
tumors. |
Table I.
Summary of the radiological findings
of 15 patients with osseous peripheral primitive neuroectodermal
tumors.
|
|
| Computed tomography
findings | Magnetic resonance
imaging findings |
|---|
|
|
|
|
|
|---|
| Case no. | Location | Density | Calcification | Periosteal
reaction | T1WI | T2WI | Enhanced T1WI |
|---|
| 1 | Radius (L) | Homogeneous | – | – | Isointense | Hetero-, iso- or
hyperintense |
Heteroenhancementb |
| 2 | Ulna (L) | Heterogeneous | – | + | Isointense | Hetero-, iso- or
hyperintense |
Heteroenhancementb |
| 3 | Maxilla (R) | Heterogeneous | + | + | Isointense | Hetero-, iso- or
hyperintense |
Heteroenhancementb |
| 4 | Tibia (R) | Heterogeneous | – | + | N/A | N/A | N/A |
| 5 | Lumbar vertebrae | Heterogeneous | – | – | Isointense | Hetero-, iso- or
hyperintense |
Heteroenhancementc |
| 6 | Tibia/fibula (R) | Heterogeneous | – | – | N/A | N/A | N/A |
| 7 | Femur (L) | Heterogeneous | – | – | Isointense | Hetero-, iso- or
hyperintense |
Heteroenhancementb |
| 8 | Scapula (L) | Homogeneous | – | – | Isointense | Hetero-, iso- or
hyperintense |
Heteroenhancementb |
| 9 | Mandible (R) | Heterogeneous | – | + | Isointense | Hetero-, iso- or
hyperintense |
Heteroenhancementb |
| 10 | Ilium (R) | Homogeneous | + | – | N/A | N/A | N/A |
| 11 | Clavicle (R) | Homogeneous | – | – | N/A | N/A | N/A |
| 12 | Clavicle (R) | Homogeneous | – | + |
Hyperintensea | Hetero-, iso- or
hyperintense |
Heteroenhancementb |
| 13 | Ulna (L) | Homogeneous | – | – |
Hyperintensea | Hetero-, iso- or
hyperintense |
Heteroenhancementb |
| 14 | Humerus (R) | Heterogeneous | – | – |
Hyperintensea | Hetero-, iso- or
hyperintense |
Heteroenhancementb |
| 15 | Cervical
vertebrae | Homogeneous | – | – | Isointense | Hetero-, iso- or
hyperintense |
Heteroenhancementb |
CT findings
The radiological findings from the 15 cases are
summarized in Table I. A CT scan was
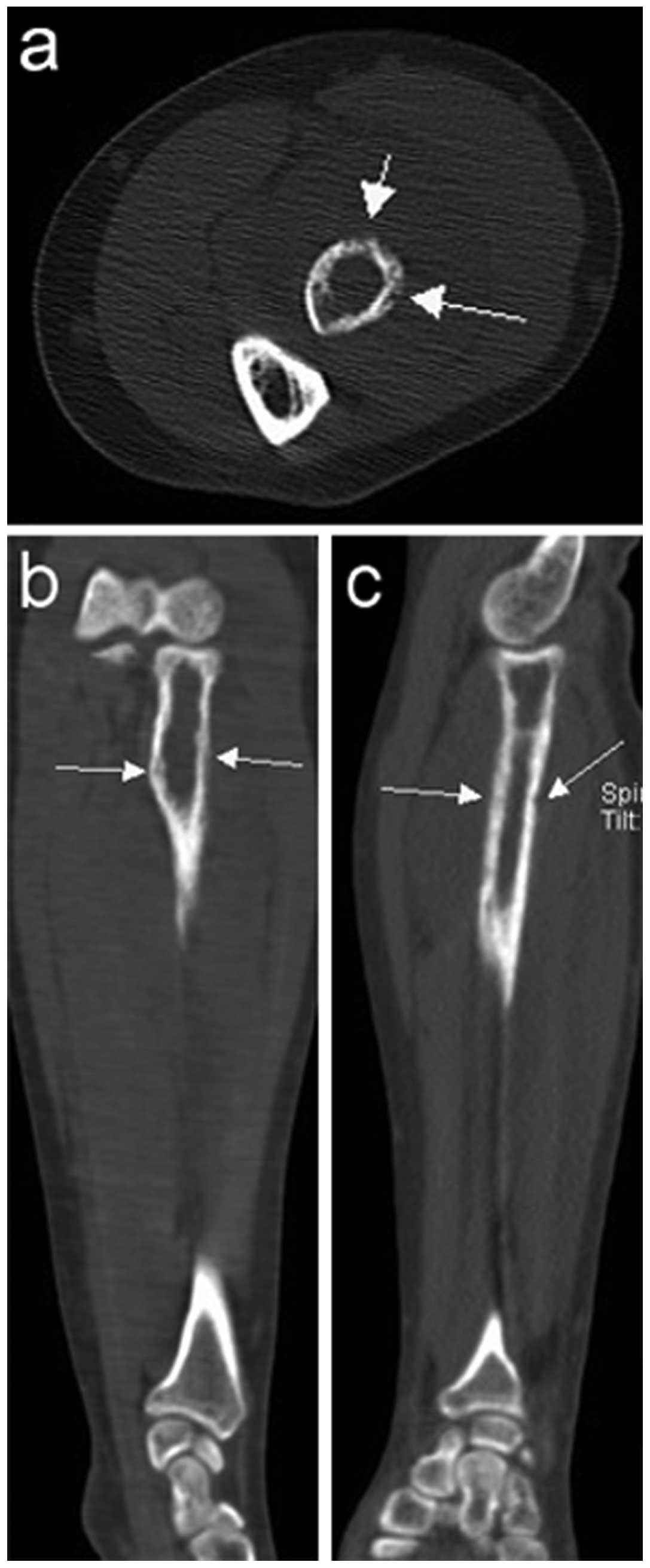
performed in all 15 cases. A total of 13 cases exhibited lytic bone
lesions (Figs. 1 and 2) and the remaining 2 cases exhibited lytic
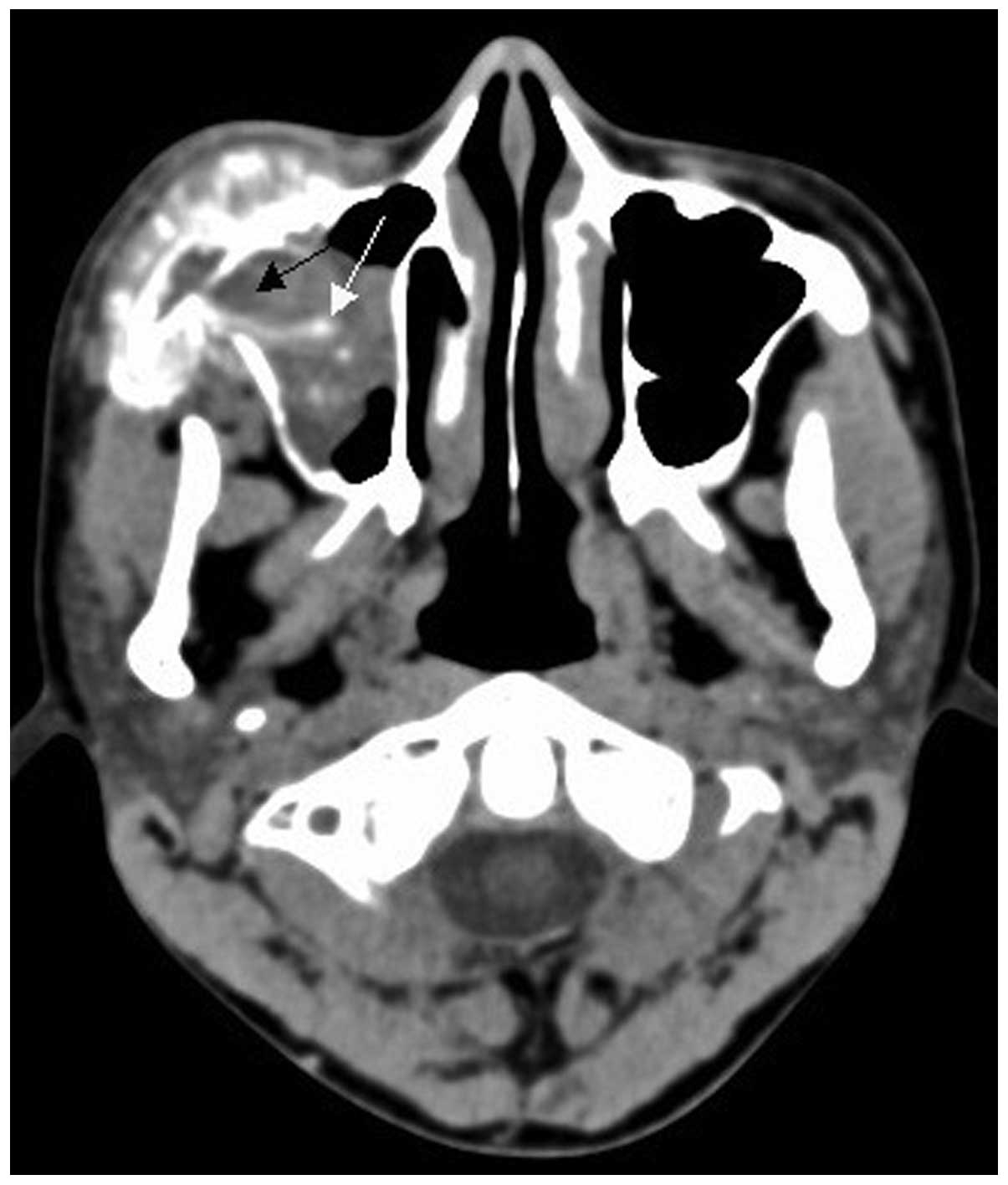
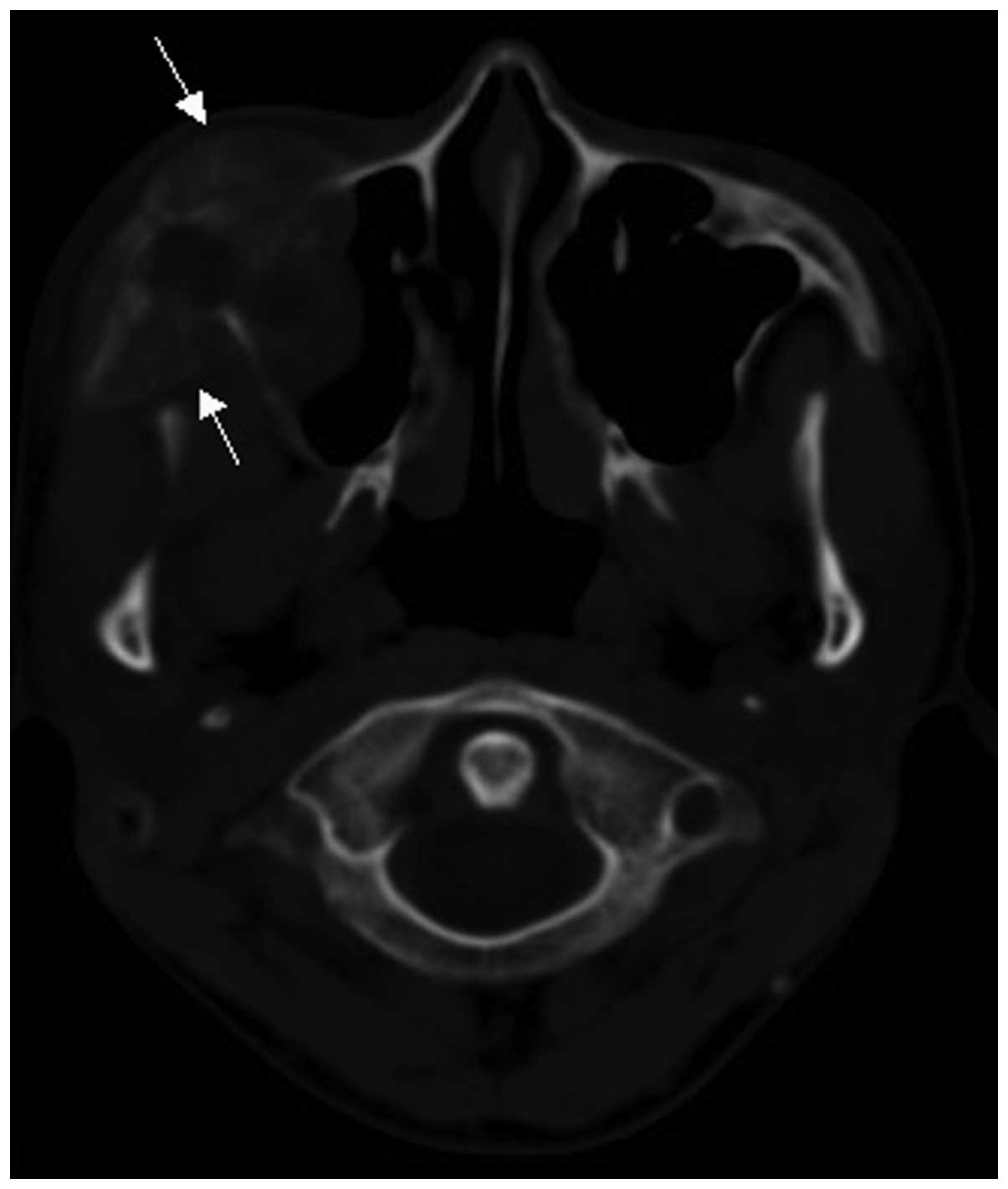
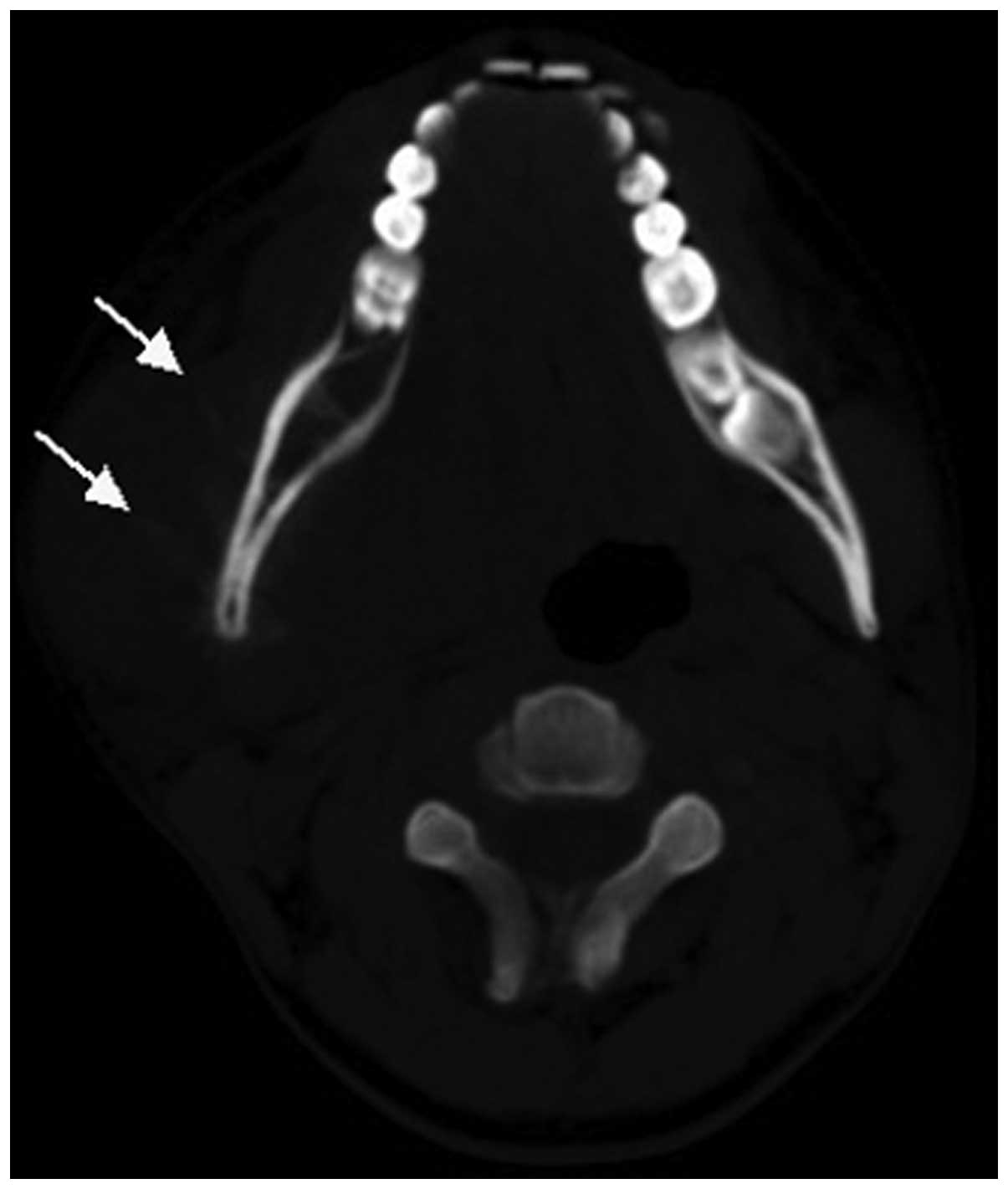
bone lesions with bone expansion (Figs.
3 and 4). All 15 cases exhibited
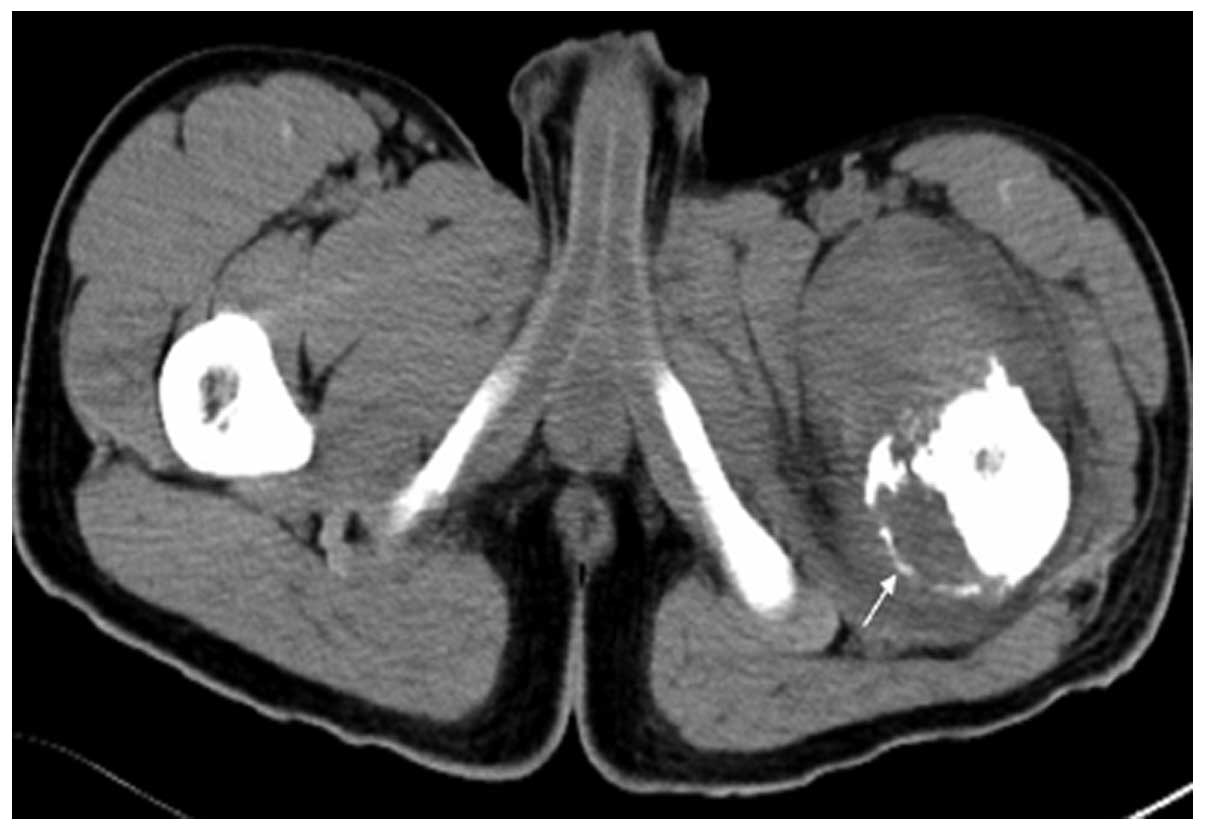
surrounding soft tissue mass formation; of the soft tissue masses,
9 cases were heterogeneous, with different sizes of lower-density
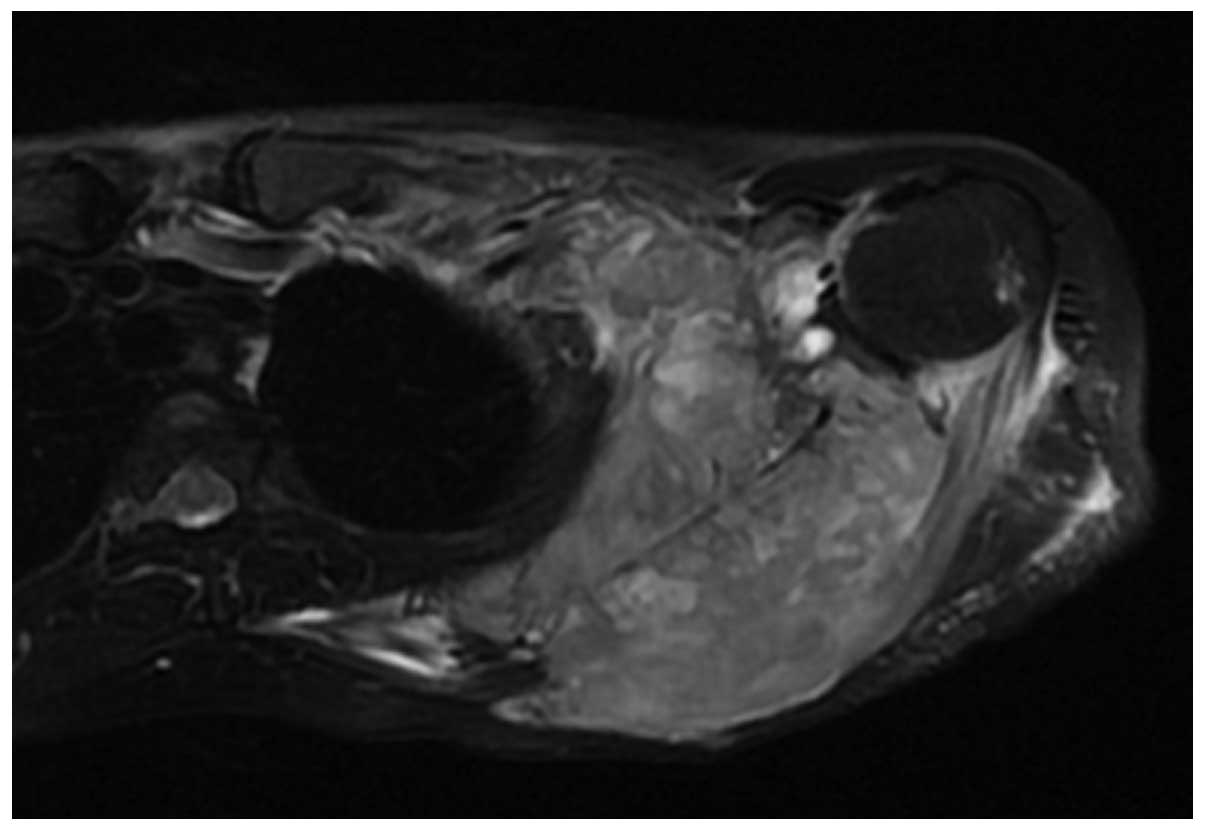
necrotic areas (Fig. 5). The CT value
of solid sections of the tumors was 40–65 HU. All 15 cases
exhibited a relatively limited extent of bone cortical destruction,
with surrounding soft tissue mass formation (Figs. 1 and 2).
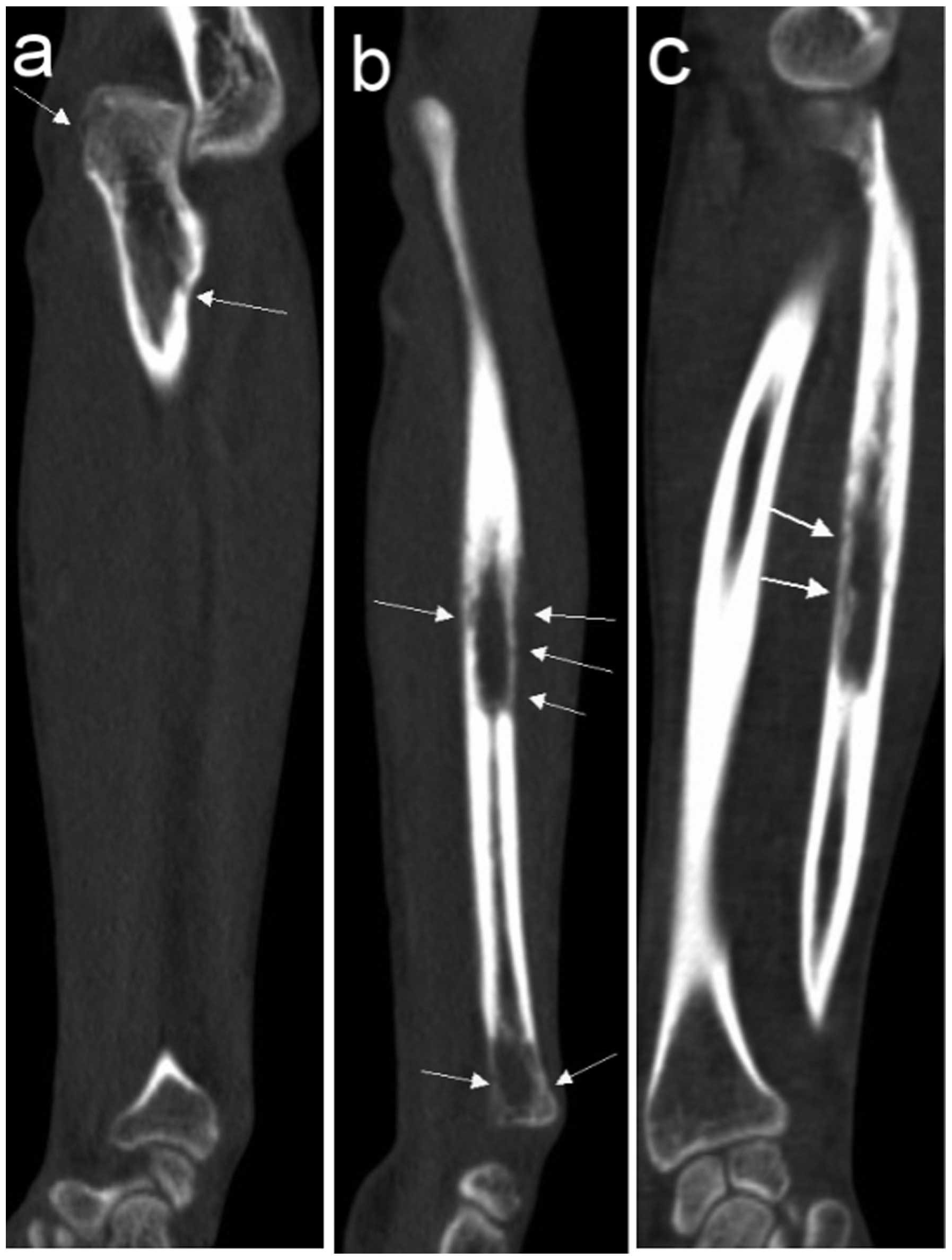
Two cases of soft tissue mass exhibited calcification (Figs. 4 and 5)
and 5 cases exhibited periosteal reaction, including clear
sunburst-like periosteal reactions in 3 cases (Figs. 6 and 7).
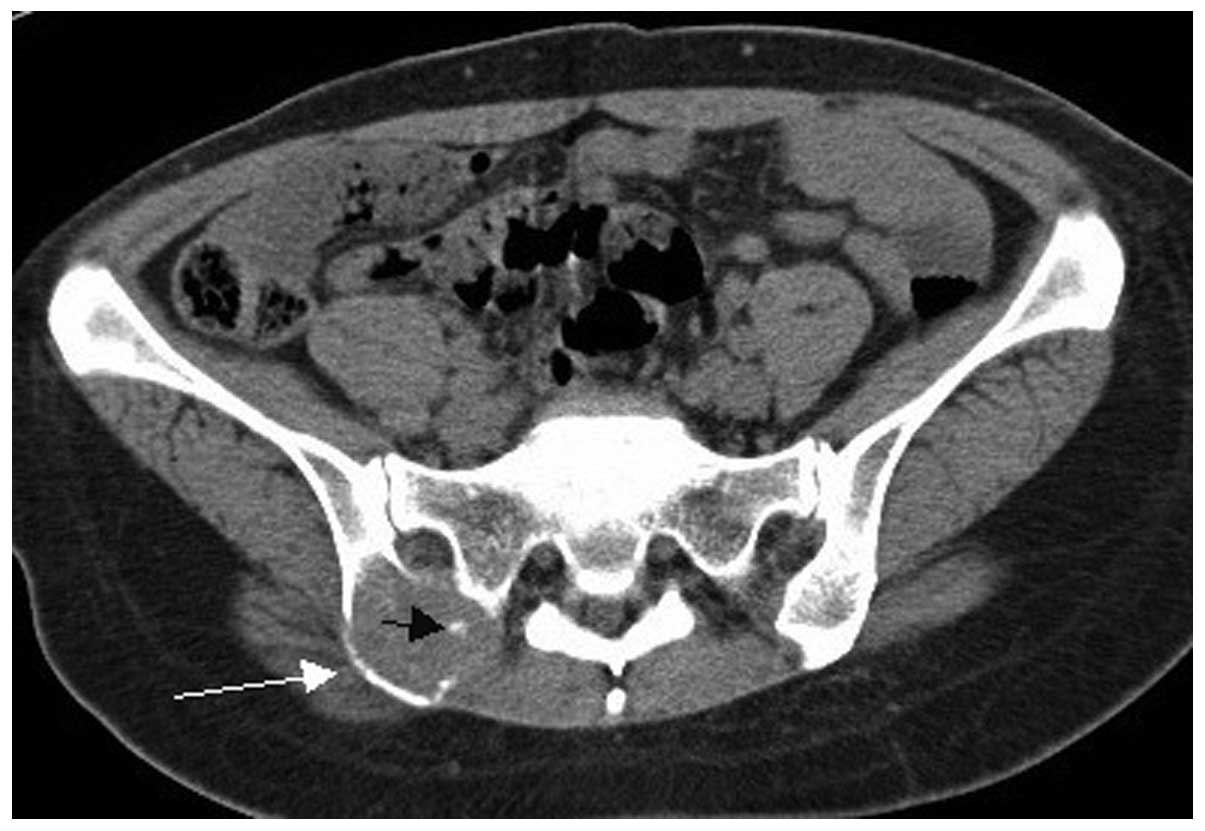
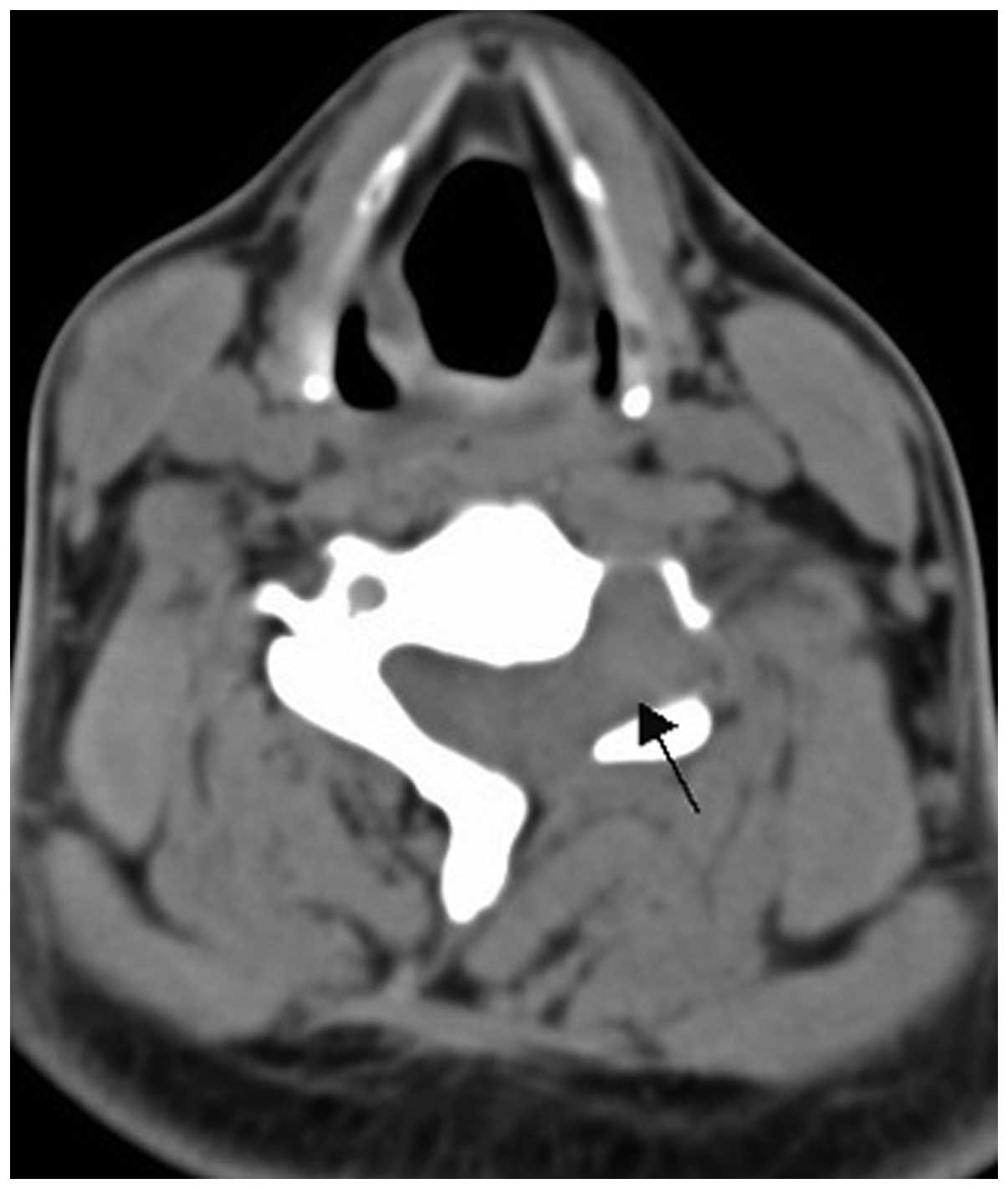
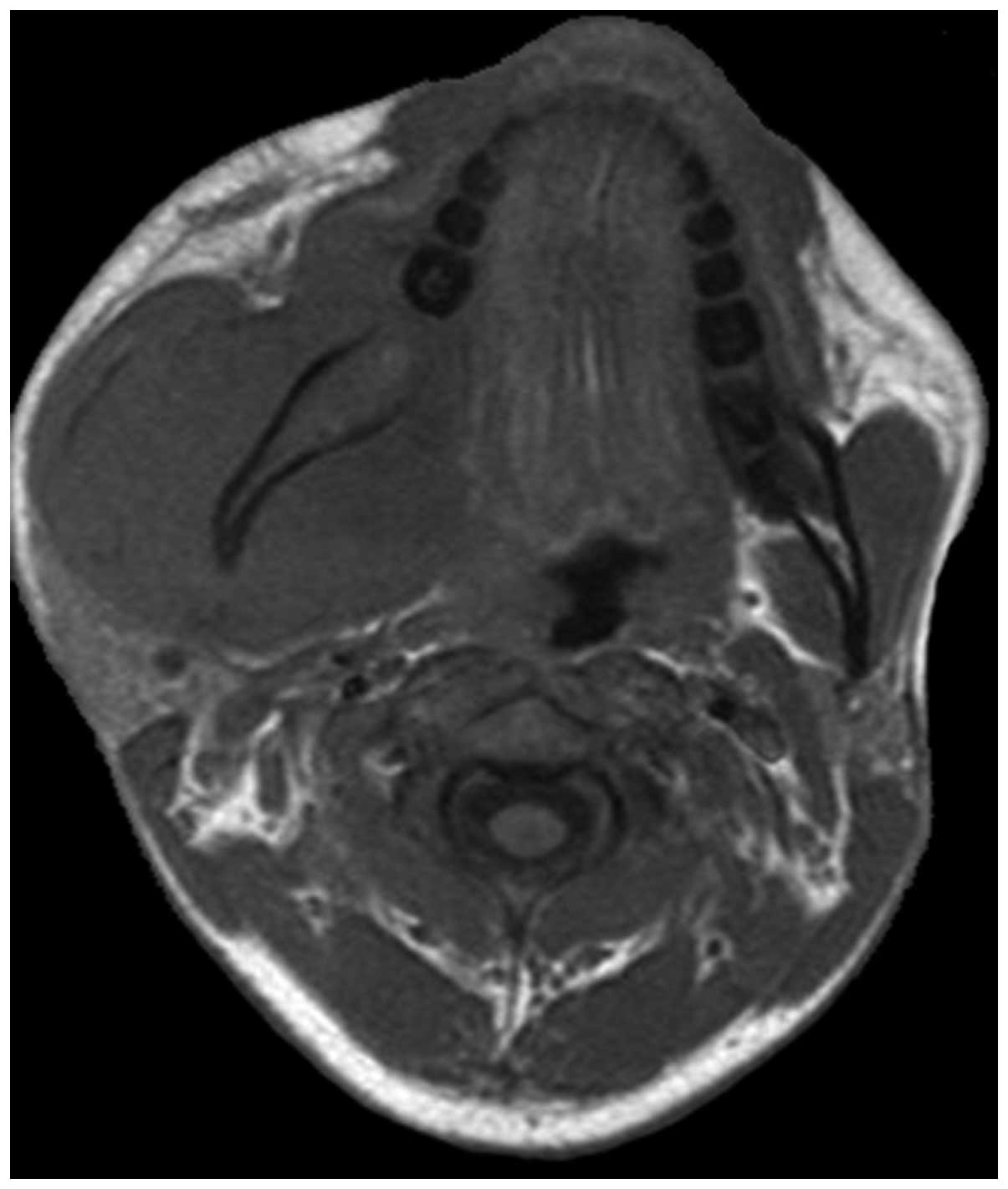
Two cases of vertebral pPNET exhibited a soft tissue mass convex to
the spinal canal, causing spinal cord compression (Fig. 8).
MRI findings
Contrast medium-enhanced MRI scanning was performed
in 11 cases. All 11 cases exhibited osseous signal abnormalities
and the confines of the lesions were wider compared with those
identified on CT. All 11 cases exhibited a surrounding soft tissue
mass. On T1-weighted images (WI), a soft tissue mass with
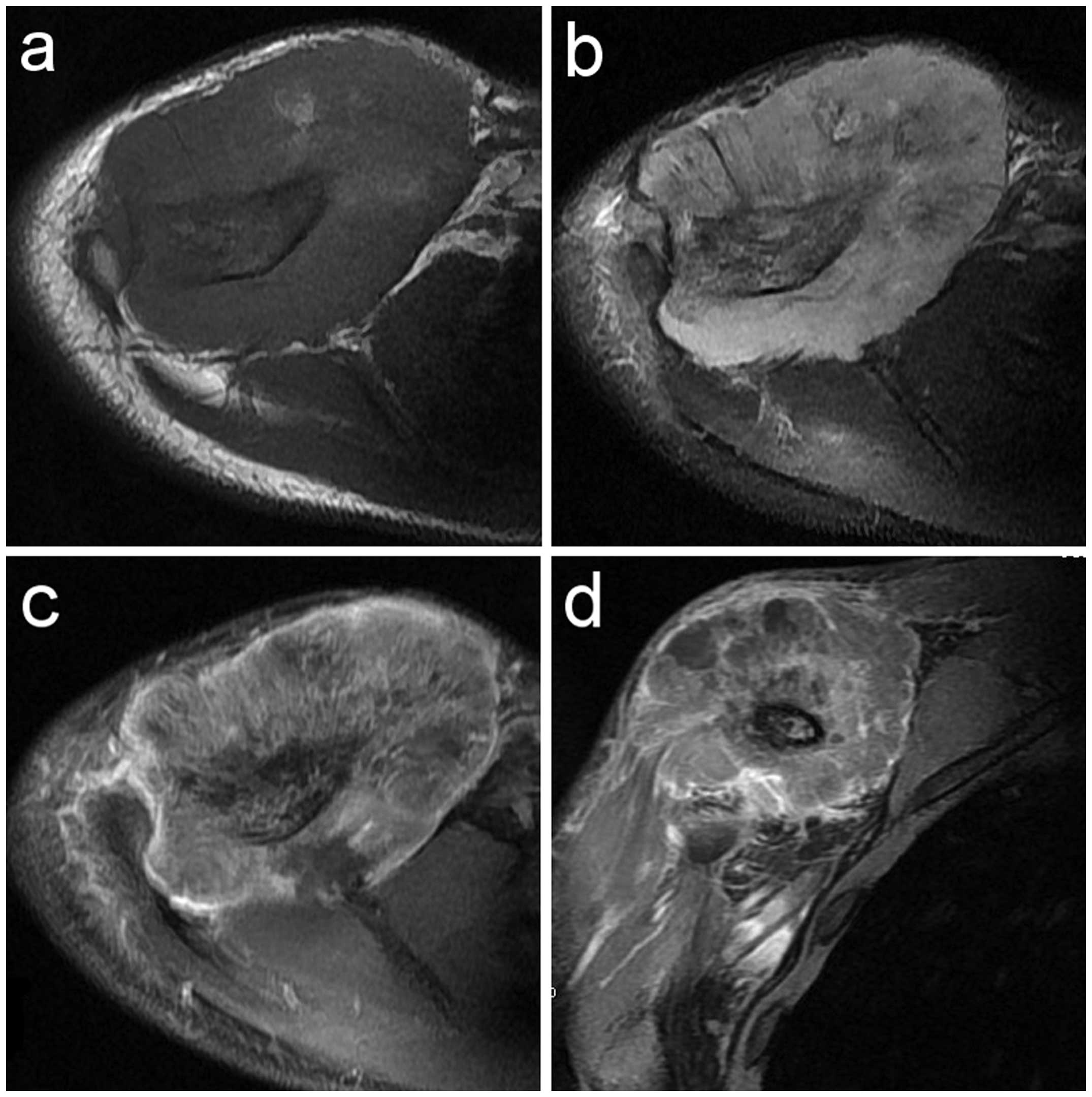
isointensity (8 cases) (Figs. 9a and
10) and marginal hyperintensity (3
cases) was detected, whereas in 7 cases the signal of the soft
tissue mass was heterogeneous. On T2WI, an aggressive soft tissue
mass with heterogeneous iso- or hyperintensity (11 cases) (Figs. 9b and 11) was detected. On contrast-enhanced T1WI,
marked heterogeneous enhancement (Fig. 9c
and d) was present in 10 cases and intermediate heterogeneous
enhancement in 1 case.
Follow-up
The clinical course of the 15 patients is summarized
in Table II. Of the 15 patients, 14
developed no distant metastasis and the remaining patient exhibited
lung and hepatic metastasis at the time of diagnosis. Of the 15
patients, 7 underwent surgical treatment, 3 received chemotherapy
alone and 4 received chemoradiotherapy alone. One patient received
no treatment and succumbed to the disease 6 months after hospital
discharge. Of the 7 surgical patients, 6 developed local recurrence
and distant metastasis subsequent to surgery during the follow-up
period, of whom 2 patients eventually succumbed to the disease,
whereas the remaining patient did not exhibit local recurrence or
metastasis. Of the 7 patients who underwent chemotherapy or
chemoradiotherapy alone, 3 achieved a remission during the
follow-up period, whereas the remaining 4 patients succumbed to
lymph node, pulmonary, hepatic, osseous or meningeal
metastasis.
 | Table II.General data and clinical course of
15 patients with osseous peripheral primitive neuroectodermal
tumors. |
Table II.
General data and clinical course of
15 patients with osseous peripheral primitive neuroectodermal
tumors.
| Case no./ age
(years)/ gender | Treatment
modalities | Follow-up
(months) |
Recurrence/metastasis during
follow-up | Status |
|---|
| 1/38/F | Surgical resection,
postoperative chemotherapy | 7 | Local recurrence
and distant metastasis | Alive |
| 2/22/F | Chemotherapy | 24 | Remission | Alive |
| 3/16/M | Chemotherapy,
radiotherapy | 23 | Remission | Alive |
| 4/64/F | No treatment | 6 | Distant
metastasis | Deceased |
| 5/19/M | Radiotherapy,
chemotherapy | 5 | Distant metastasis
at the time of diagnosis | Deceased |
| 6/25/M | Chemotherapy | 19 | Remission | Alive |
| 7/17/M | Preoperative
chemotherapy, surgical resection | 23 | Local recurrence
and distant metastasis | Deceased |
| 8/54/F | Preoperative
chemotherapy, surgical resection | 6 | Local recurrence
and distant metastasis | Alive |
| 9/16/M | Chemotherapy,
radiotherapy | 18 | Distant
metastasis | Deceased |
| 10/43/F | Radiotherapy,
chemotherapy | 12 | Distant
metastasis | Deceased |
| 11/17/F | Surgical resection,
postoperative chemotherapy | 18 | Local recurrence
and distant metastasis | Alive |
| 12/19/M | Surgical resection,
postoperative chemotherapy | 22 | Local recurrence
and distant metastasis | Alive |
| 13/28/M | Surgical resection,
postoperative chemotherapy | 6 | No local recurrence
and distant metastasis | Alive |
| 14/30/M | Chemotherapy | 8 | Distant
metastasis | Deceased |
| 15/25/M | Surgical resection,
postoperative chemotherapy | 14 | Local recurrence
and distant metastasis | Deceased |
Discussion
The first case of pPNET, occurring in the ulnar
nerve, was reported by Stout in 1918 and the tumor was composed of
small round cells, focally arranged as rosettes (4). Ewing reported an undifferentiated,
diffuse, small round-cell tumor occurring in the diaphysis of long
bones in 1921; that type of tumor was eventually named Ewing's
sarcoma (ES) (5). In 1984, Jaffe
(6) analyzed the pathological
sections of 4 cases that had been diagnosed as bone ES by previous
clinical, X-ray and pathological examinations and identifed
Homer-Wright rosettes by microscopy and neurospecific enolase (NSE)
expression by immunohistochemical staining; thus, these cases were
diagnosed as neuroectodermal tumors, rather than ES, and were the
first reported cases of osseous pPNET. It was recently demonstrated
that pPNET and ES may be further differentiated using
characteristics observed on electron microscopic and
immunohistochemical examination. For example, neurotubules,
neurofilaments and neurosecretory granules may be observed under an
electron microscope, whereas O13, CD99, S100-protein, vimentin,
chorionic gonadotropin α (CgA) and NSE may be identified by
immunohistochemical staining in PNETs (7–12).
The PNETs, a subtype of the family of small
round-cell malignancies, are mainly found in the central nervous
system (CNS). Rarely, however, PNETs may be found outside the CNS.
These pPNETs are most common in the thoracopulmonary region,
followed by the abdomen, pelvic cavity and retroperitoneum. Bone is
a rare location and, to date, there have been few reports of
osseous pPNET in the literature (3).
To the best of our knowledge, this is the first English language
study exclusively investigating the CT and MRI findings of osseous
pPNETs. Osseous pPNETs may occur in any bone in every age group,
but mainly affect children and adolescents and exhibit a marginal
male predominance. Patients often present with a rapidly enlarging
mass and associated compression symptoms (3,12).
Consistent with previous reports (9,11), osseous
pPNET mainly affected males, adolescents and young adults in the
present study (Table II). The 15
patients presented with varying degrees of pain and 11 patients
presented with local edema and a progressively growing mass. The
tumors were located in the limb bones (7 cases), vertebrae (2
cases), clavicle (2 cases), scapula (1 case), ilium (1 case),
maxilla (1 case) and mandible (1 case). It should be noted that
pPNETs of the maxilla and mandible are extremely rare. Following a
review of the literature, only 16 pPNET cases of the mandible
(13) and 13 of the maxilla (14) have been reported.
The CT findings of osseous pPNETs include
destructive lesions with a sizeable soft tissue mass and,
occasionally, with periosteal reaction (3,13). The
soft tissue mass is usually isodense or marginally hypodense
compared with normal muscle (1,3,15). When the tumor is smaller, its density
is often homogeneous. However, when the tumor is larger, it often
exhibits isodensity with patchy hypodense necrotic areas. Tumor
calcification is uncommon (11,12). In
the present study, 13 of the 15 cases exhibited lytic bone lesions;
all 15 cases exhibited surrounding soft tissue mass formation and 5
cases exhibited associated periosteal reaction. In 9 cases the soft
tissue mass was heterogeneous, with different sizes of
lower-density necrotic areas. Two cases of soft tissue mass
exhibited calcification. These findings were consistent with the CT
findings of osseous pPNET. Of note, osseous pPNET is a highly
invasive malignant tumor and, therefore, it theoretically lacks the
time required for calcification or ossification. Thus, the 2 cases
exhibiting calcifications may be due to the pressure exerted on
normal bone tissue, which may be observed among the CT findings of
primary bone lymphoma (formation of sequestra) (16).
On T1WI, the majority of pPNETs are isointense or
marginally hyperintense compared with normal muscle, and may
contain hyperintense hemorrhagic areas. On T2WI, the majority of
pPNETs are heterogeneous iso- or hyperintense. On contrast-enhanced
T1WI, the tumor is often homogeneous when it is smaller and
heterogeneous when it grows larger and exhibits areas of hemorrhage
or necrosis (8,9). In the present study, 11 cases exhibited
a surrounding soft tissue mass on MRI. On T1WI, a soft tissue mass
exhibiting isointensity (8 cases) or marginal hyperintensity (3
cases) was identified. On T2WI, a soft tissue mass exhibiting
heterogeneous iso- or hyperintensity (11 cases) was identified. On
contrast-enhanced T1WI, marked heterogeneous enhancement was
present in 10 cases and intermediate heterogeneous enhancement in 1
cases. These findings were consistent with those of previous
reports (3,4). MRI is a sensitive method for displaying
and accurately evaluating the extent of the lesions, evaluating
treatment effectiveness and detecting the presence of distant
metastases during the follow-up period. The two cases of the
present study and those in other literature reports demonstrated
that the ability of MRI to detect change is superior to that of CT,
even following chemotherapy (11).
pPNETs are often associated with distant metastases
and local recurrence following treatment, as well as poor
prognosis. The most common recurrence is characterized by localized
soft tissue masses and distant metastases, often occurring in the
lung, bone, liver, adrenal gland, brain and retroperitoneum
(15). In the present study, 1
patient had pulmonary and hepatic metastases at the time of
diagnosis, 6 patients developed local recurrence and distant
metastasis following surgery during the follow-up period and 4
patients developed distant metastasis following chemotherapy or
chemoradiotherapy. A total of 7 patients succumbed to lymph node,
pulmonary, hepatic, osseous or meningeal metastasis during the
follow-up period.
In conclusion, osseous pPNETs mainly affect males
aged <30 years. The patients often present with varying degrees
of pain, local edema and a progressively growing mass. The CT
findings of osseous pPNET include destructive lesions with a
sizeable soft tissue mass and, occasionally, with periosteal
reaction. Tumor calcification is uncommon. The MRI findings include
aggressive soft tissue mass with isointensity on T1WI and iso- or
hyperintensity on T2WI and markedly heterogeneous tumors following
enhancement. The CT and MRI findings demonstrated that the tumor
originated in the bone marrow cavity and exhibited a bone-centric
growth pattern. Although the imaging characteristics of pPNETs may
be non-specific, CT and MRI may be useful in delineating the extent
of the tumor, identifying distant metastases, predicting
resectability and monitoring treatment.
References
|
1
|
Schulman H, Newman-Heinman N, Kurtzbart E,
Maor E, Zirkin H and Laufer L: Thoracoabdominal peripheral
primitive neuroectodermal tumors in childhood: radiological
features. Eur Radiol. 10:1649–1652. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong J, Zhang Y, Zuo M, et al: Imaging
findings of abdominal peripheral primitive neuroectodermal tumor:
report of four cases with pathological correlation. Clin Imaging.
33:196–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ibarburen C, Haberman JJ and Zerhouni EA:
Peripheral primitive neuroectodermal tumors. CT and MRI evaluation.
Eur J Radiol. 21:225–232. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stout AP: A tumor of the ulnar nerve. Proc
NY Pathol Soc. 12:2–12. 1918.
|
|
5
|
Ewing J: Diffuse endothelioma of bone.
Proc NY Pathol Soc. 21:17–24. 1921.
|
|
6
|
Jaffe R: The neuroectodermal tumor of
bone. Am J Surg Pathol. 8:885–898. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Linnoila RI, Tsokos M, Triche TJ, Marangos
PJ and Chandra RS: Evidence for neural origin and PAS-positive
variants of the malignant small cell tumor of thoracopulmonary
region (‘Askin tumor’). Am J Surg Pathol. 10:124–133. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carvajal R and Meyers P: Ewing's sarcoma
and primitive neuroectodermal family of tumors. Hematol Oncol Clin
North Am. 19:501–525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Alava E and Gerald WL: Molecular
biology of the Ewing's sarcoma/primitive neuroectodermal tumor
family. J Clin Oncol. 18:204–213. 2000.PubMed/NCBI
|
|
10
|
Jones JE and McGill T: Peripheral
primitive neuroectodermal tumors of the head and neck. Arch
Otolaryngol Head Neck Surg. 121:1392–1395. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dick EA, McHugh K, Kimber C and Michalski
A: Imaging of non-central nervous system primitive neuroectodermal
tumours: diagnostic features and correlation with outcome. Clin
Radiol. 56:206–215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khong PL, Chan GC, Shek TW, Tam PK and
Chan FL: Imaging of peripheral PNET: common and uncommon locations.
Clin Radiol. 57:272–277. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeh CH, Yeow KM, Chu SY, et al: Imaging
findings in mandibular primitive neuroectodermal tumour: a report
of a rare case and review of the literature. Dentomaxillofac
Radiol. 40:451–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hormozi AK, Ghazisaidi MR and Hosseini SN:
Unusual presentation of peripheral primitive neuroectodermal tumor
of the maxilla. J Craniofac Surg. 21:1761–1763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang WD, Chen YF, Li CX, Zhang L, Xu ZB
and Zhang FJ: Computed tomography and magnetic resonance imaging
findings of peripheral primitive neuroectodermal tumors of the head
and neck. Eur J Radiol. 80:607–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mulligan ME and Kransdorf MJ: Sequestra in
primary lymphoma of bone: prevalence and radiologic features. AJR
Am J Roentgenol. 160:1245–1248. 1993. View Article : Google Scholar : PubMed/NCBI
|